Abstract
Dengue Fever (DF) and Dengue Hemorrhagic Fever/Dengue Shock Syndrome (DHF/DSS) are considered the most important arthropod-borne viral diseases in terms of morbidity and mortality. The emergency and severity of dengue (Den) infections increase the necessity of an early, quick and effective dengue laboratory diagnostic. Viral isolation is considered a gold standard for diagnosis dengue infection using monoclonal antibodies (mAbs) as a tool for determining serotype specificity. Alternatives have been used to improve sensitivity and time in dengue diagnosis. Based on the early expression of dengue C protein in the life cycle, we focused our study in the application of an anti-dengue 2 virus capsid protein mab in dengue diagnosis. The kinetic expression of dengue-2 capsid in mosquito cells and its immuno-localization in experimentally infected suckling albin Swiss (OF-1) mice brain tissues was established. The results demonstrate the utility of this mAb in significant early dengue diagnosis in traditional isolation. On the other hand, a preliminary study of an enzyme immunoassay method using 8H8 mab for specific detection of dengue C protein antigen was performed making possible recombinant C protein quantification. The results suggest that detection of dengue capsid protein could be useful in the diagnosis of early dengue infection.
Introduction
Dengue fever (DF) and dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) are considered the most important arthropod-borne viral diseases in terms of morbidity and mortality.Citation1 It is estimated that more than 100 million people are infected with dengue virus every year.Citation2 This situation enhances the need for an early, fast and efficient dengue laboratory diagnostic.
Dengue diagnosis based on antibody identification has become the most practical approach due to a variety of commercial and “in-house” immunoassays for dengue diagnosis that have been developed.Citation3–Citation6 The IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) is the assay of preference for the serological diagnosis of acute dengue virus infection, and is a very useful tool for sero-epidemiological dengue surveillance.Citation7,Citation8 The detection of both anti-dengue IgM and IgG by ELISA permits classification of the dengue infection type (primary or secondary), thereby contributing to a better understanding of the pathogenesis of DHF.Citation8,Citation9 On the other hand, molecular biology techniques detecting specific sequences of nucleic acid as well as viral proteins are valuable for diagnosis. However, the sample and RNA must be processed carefully to avoid RNA degradation.Citation9
Although the process is time-consuming, isolation and viral identification from infectious samples are considered the gold standard of dengue virus infection diagnosis. Viral isolation allows follow-up virological study and biological characterizations of the agent.Citation9–Citation11 In order to reduce the time to diagnosis, a variety of modifications, novel methods and tools have been introduced.Citation11–Citation14
Monoclonal antibodies (mAbs) are efficient analytic tools for detection, screening and characterization of biomolecules, and have wide application as diagnostics and immunotherapies. mAbs have been successfully used to identify serotype specificity of dengue viruses in conventional diagnosis as viral isolation. Dengue envelope (E) and non structural 1 (NS1) proteins have been targets of these mAbs in different assays.Citation15–Citation17 Dengue capsid (C) protein has been used for therapeutic and vaccinology purposes, but not in diagnostic research. Studies of this protein have established its intracellular localization in different cellular lines, and its early expression in BHK-21 cell lines has been reported.Citation18–Citation20 In our studies, we evaluated the usefulness of an anti-C mAb in the establishment of the kinetic expression of Den-2 capsid protein in Aedes albopictus cells, demonstrated the efficacy of this anti-C mAb in the early identification of dengue virus from viremic sera in conventional diagnosis, and determined its utility in the immuno-localization of Den-2 capsid protein in suckling mice brain by immunohistochemistry assay. Additionally, 8H8 anti-C mAb was used in an antigen-capture Enzyme-Linked Immunosorbent Assay (ELISA) test to quantify a recombinant C protein, suggesting a potential future application in the early diagnosis of dengue infection.
Results
Kinetic expression of the dengue capsid protein.
C protein was detected in infected cells during the entire study interval from 6 h to 96 h after infection. At 6 h and 8 h pi a slight positive staining was observed in the cytoplasm of infected cells ( and B). After 12 h pi the signal became more intense at the cytoplasm. At 48 h and 72 h pi the signal appeared as spots in the cytoplasm and in the nuclei ( and E). The highest intensity was observed at 72 h pi (). No immunofluorescence was observed in non-infected C6/36-HT cells (). Anti-E H3–6 mAb was used as control assay and it was able to detect the expression of E protein of the Den-2 strain from 24 h pi.
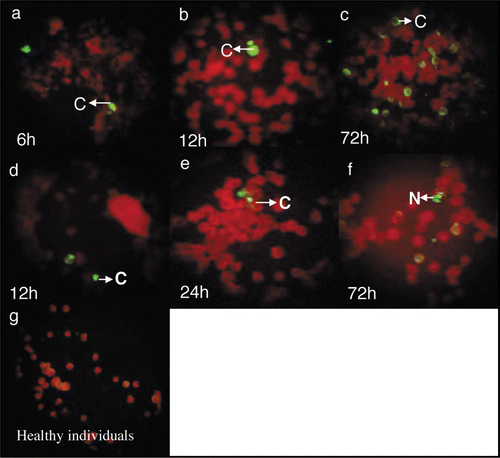
In addition, the kinetic expression of the C protein of Den-2 isolates in C6/36-HT cells from two sera collected from dengue patients was also studied (). In the two cases, an increase in the intensity of the immunofluorescence was observed over time during infection. The C protein of the 57 and 59 isolates began to express at the same time (6 h p.i.) as the Dengue-2 A15 strain ().
Expression of dengue capsid protein in suckling mice brain.
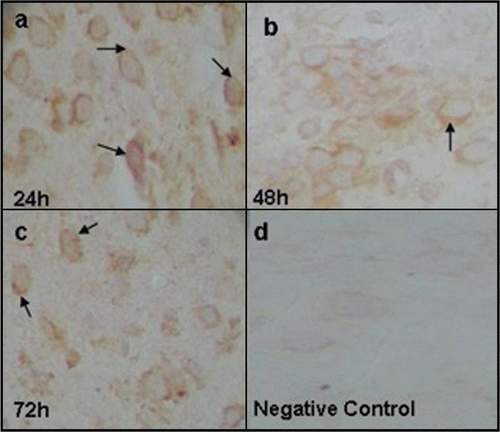
Capsid protein expression was demonstrated in the brain of suckling OF-1 mice from 24 h until 72 h pi. C protein antigen was immunolocalized in the cytoplasm (). Nevertheless, no histopathological findings were observed in the hematoxylin-eosin stained tissue sections.
Detection of dengue C protein antigens by ELISA.
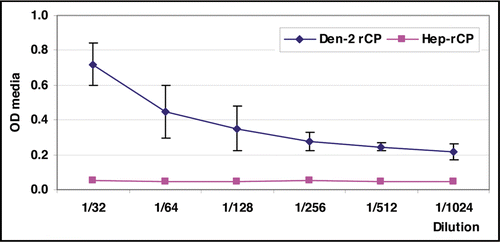
Based on the utility of the anti-capsid 8H8 mAb in the early detection of the C protein by immunofluorescence assay, an ELISA for the detection and quantification of the Den-2 recombinant C protein was standardized. The antigen-capture assay using 8H8 mAb might allow viral identification after short incubation times under the recombinant protein up to 1/1,024 dilution which corresponded to 1.67 µg/mL ().
Discussion
mAbs have previously been used to locate intracellular viral antigens.Citation20 The C protein of Kunjin and Japanese encephalitis viruses (JE) has been detected in the cytoplasm, the nucleus and nucleolus of infected mammalian and mosquito cells.Citation21 In addition, mAbs have been used to demonstrate the probable significance of the nuclear localization of the West Nile virus C protein in replication and pathogenesis.Citation22 Dengue C protein has also been detected in the cytoplasm, nucleusCitation19,Citation23 and in the nucleolus of infected cells.Citation18 Nevertheless, the kinetic expression of dengue C protein has been studied only in BHK-21 cells.Citation20
To our knowledge, the present study is the first report of kinetic expression of the Den-2 C protein in C6/36-HT cells, leading to detection of the protein at a very early stage (6 h pi). We observed similar findings when C6/36-HT cells were infected with acute sera from dengue patients. Dengue diagnosis by traditional procedures (viral isolation) requires at least 6–10 daysCitation24 and viral identification is done using anti-E mAbs.Citation10,Citation11,Citation25 Therefore, the use of anti-capsid 8H8 mAb can allow the viral identification in minor incubation time under epidemiological conditions where serotype 2 is circulating. The specificity and sensitivity of the 8H8 mAb was demonstrated, since the acute sera from dengue patients were positive by isolation and RT-PCR.
mAbs are also considered valuable tools for dengue diagnosis from fatalities through use of immunochemistry studies.Citation26–Citation29 Many researchers use this method to determine pathological changes in infected tissues related to the presence of Den antigen.Citation30–Citation32 Although we did not find histopathological changes in the mice brain infected tissues, 8H8 mAb was able to detect C protein from the same paraffin-embedded sections. This suggests that 8H8 mAb could also be helpful for the diagnosis of dengue in fatal cases occurring under similar epidemiological conditions.
High titers of viremia are present in patients at the early stage of dengue infection, suggesting that the detection of viral antigens is appropriate for early diagnosis.Citation33,Citation34 The antigen capture ELISA test has demonstrated utility in the detection of viral antigens from blood.Citation35 This assay has been used recently for the detection of non-structural protein 1 (NS1) protein dengue virus.Citation16,Citation17,Citation36 C protein has also been used as a target in the antigen capture ELISA for some viruses, such as Rift Valley Virus,Citation37 Hepatitis B virus,Citation38 Hepatitis C virus,Citation39 MarburgCitation40 and EbolaCitation41 using monoclonal antibodies. However, it has not been used for this purpose in dengue diagnosis.
Saijo et al. developed an antigen capture ELISA to detect recombinant nucleoprotein of Marburg virus (MARV). The test could detect 40 ng/mL and the authentic MARV nucleoprotein at a level equivalent from 3 × 103 PFU/mL to 1.2 × 104 PFU/mL.Citation42 The use of polyclonal antibody against nucleoprotein of MARV as detector contributed to an increase the sensitivity of the assay.
The preliminary results of our study indicate that 8H8 mAb could successfully quantify a C recombinant dengue protein. In order to improve the sensitivity of capture ELISA assay the application of polyclonal antibody or another dengue anti-capsid mAb should be applied since the use of mAbs offer higher specificity, but less sensitivity, to the assay.Citation43,Citation44
Possible improvements in detection of C dengue protein in the ELISA assay requires a more extensive study, including use of a detergent cocktail in pre-treatment of samples, a kinetic study of clinical samples, and increases in the sensitivity of the test.Citation45
The applicability of 8H8 mAb in dengue diagnosis is limited because its specificity to C protein of Den-2 serotype. Nevertheless, it provides the possibility of earlier identification of this serotype in viral isolation performed during dengue diagnosis. Our results suggest that generation of monoclonal antibodies against consensus regions of C dengue protein will be a useful tool for early dengue laboratory diagnosis.
Materials and Methods
Cells.
Aedes albopictus cell line (clone C6/36-HT) was grown at 33°C in MEM medium containing 2 mmol/L glutamine and 10% of heat-inactivated fetal bovine serum (FBS). These cells were maintained in the same medium with 2% of FBS.
Viruses.
Den-2 A15 Cuban strainCitation46 with a history of four passages in suckling mouse brain (4pSMB) and seven passages in C6/36-HT cells (7pC6/36) with a titer of 2.05 × 105 PFU/mL was employed.Citation47
Two dengue acute sera from patients confirmed as Den-2 by isolation in C6/36-HT cell culture and RT-PCR were included in the study. These sera were identified as number 57 and 59.
Monoclonal antibody (mAb).
Anti E dengue protein H3/6 mAb was produced by Hermida et al. and is able to recognize the 4 dengue serotype, it is an IgG2 immunoglobulin subclass capable to neutralize in vitro.Citation48 This mAb was used as a control for detecting of viral presence in immunofluorescence assay.
Anti-capsid 8H8 mAb was generated by Pupo et al. It was characterized as IgG1 immunoglobulin subclass, specific to Den-2 serotype as stated by immunofluorescence and immunoassay. It was unable to inhibit the hemagglutination and neutralize the virus.Citation49
Both hybridoma cells producing 8H8 and H3/6 mAbs were maintained in RPMI 1640 medium supplemented with 10% FBS, non essential amino acids, and antibiotics (streptomycin and penicillin). A suspension of cells (2 × 106) was injected intraperitoneally in ten Pristane primed mice, collected ascetic fluids were clarified by centrifugation at 1,500 rpm for 10 min at 4°C.
8H8 mAb purification was achieved by immunochromatographic methods using protein A conjugated to Sepharose 4 Fast Flow (Pharmacia, Uppsala, Sweden). Purified antibodies were conjugated with horseradish peroxidase enzyme (Sigma) following the periodate method.
Immunofluorescence staining.
To determine C dengue protein expression, C6/36-HT cells were inoculated with Den-2A15 (4pSMB, 7pC6/36) (m.o.i 0.01) following the shell vial method described elsewhere.Citation50 The cells were harvested every 12 h from 6 h to 96 h post-infection (pi) to be fixed on slides with cold acetone for 20 min. Fixed cells were covered with the appropriate antibodies and examined under a fluorescence microscope (Leitz Wetzler Germany). The capsid protein expressed in infected cells was detected by immunofluorescence staining using 8H8 mAb at 1:50 dilution in phosphate buffered saline (PBS) as the primary antibody and FITC-conjugate goat anti-mouse IgG (Sigma) diluted 1:40 in PBS as the secondary antibody.
Similarly, acute sera collected from two cases of Den-2 infections were used for C6/36-HT inoculation. These patients had been classified as dengue infection clinically and by RT-PCR assay in the laboratory. Serum from the blood of a healthy donor was included as a negative control.
Envelope (E) protein was also detected using the anti-E monoclonal antibody H3–6.Citation48
Immunohistochemistry study.
Suckling OF-1 mice were inoculated by intracranial route (ic) with 0.02 ml of a 1:10 dilution in MEM medium of Den-2A15 strain. Brain tissues samples were obtained during three days at 24 h interval and paraffin embedded in a routine fashion for histopathology studies described elsewhere.Citation51
Hematoxylin and eosin staining was performed for histopathology studies. The C protein antigen presence was assayed by indirect immunoperoxidase method using the 8H8 mAb as primary antibody and anti-mouse rabbit immunoglobulins conjugated with horseradish peroxidase (Dako Cytomation) as a secondary antibody. The reaction was detected as a golden brown stain when 3,3′-diaminobenzidine (Sigma) was used as the chromogen. Uninfected suckling mouse brains collected at the same time as the infected tissues samples were employed as negative control.Citation28
ELISA for detection of dengue capsid protein.
An ELISA was performed using 8H8 mAb for the capture and the revelation of C antigens. Microtiter plates (Nuncs) were coated with 100 µL per well containing 5 µg/well of 8H8 mAb in carbonate-bicarbonate buffer pH 9.6 (coating buffer) and incubated overnight at 4°C. Afterward, the wells were washed, blocked by 2% skimmed milk solution and dried. Serial dilutions of a Den-2 recombinant capsid proteinCitation52 (100 µL) were then added. Plates were incubated for 2 h at 37°C and washed three times. A 100 µL of a 1/3,000 peroxidase-conjugated 8H8 mAb dilution was added to each well and incubated for 1 h at 37°C followed by three washes; 100 µl of the substrate solution (o-phenylendiamine and H2O2 in 0.1 M citrate buffer, pH 5.0) were added and incubated for 30 min at room temperature. The stop reagent, 12% H2SO4, was added and the plates were read at 492 nm. Serial dilutions of a recombinant hepatitis C virus (HCV) capsid antigen (from UMELISA HCV kit, Immunoassay Center, Cuba) were used as negative control. Samples were considered positive when optical density (OD) was twice OD of negative control. The assay was performed five times for duplication, and the graphic was achieved using OD arithmetic mean and standard deviation.
Abbreviations
| DF | = | dengue fever |
| DHF/DSS | = | dengue hemorrhagic fever/dengue shock syndrome |
| C | = | capsid |
| Den | = | dengue |
| Mab | = | monoclonal antibodies |
| RNA | = | ribonucleic acid |
| ELISA | = | enzyme-linked immunosorbent assay |
| FBS | = | fetal bovine serum |
| SMB | = | suckling mouse brain |
| FITC | = | fluorescein isotiocyanate |
| E | = | envelope |
| pi | = | post infection |
| ic | = | intracranial route |
| MEM | = | minimal essential medium |
| HCV | = | hepatitis C virus |
| OD | = | optical density |
| JE | = | Japanese encephalitis |
| BHK | = | baby hamster kidney |
| RT-PCR | = | reverse transcriptase-polymerase chain reaction |
| MARV | = | marburg virus |
Figures and Tables
Figure 1 Immunofluorescence staining with 8H8 mAb of dengue infected cells with Den-2 A15 strain. C6/36-HT cells were infected with Den-2 A15. At 6 (a), 8 (b), 12 (c), 48 (d) and 72 (e) h p.i, the infected cells were reacted 8H8 mAb followed with an FITC-conjugated goat anti-mouse antibody and then examined under a fluorescence microscope. Cytoplasmatic and nuclear staining of infected cells are indicated as C and N, respectively. C6/36-HT cells infected with Den-2A15 reacted with anti-Den-2 polyclonal antibody at 72 h p.i was used as a positive control (f) and non-infected C6/36-HT cells were used as negative control (g).
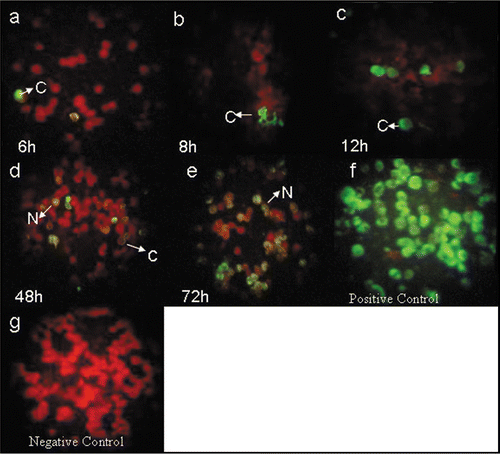
Figure 2 Immunofluorescence staining with 8H8 mab of dengue infected cells. C6/36-HT cells were infected with samples 57 (a–c) and 59 (d–f) from dengue acute patients. At 6 (a), 12 (b), 72 (c) h pi, for sample 57 and 12 (d), 24 (e), 72 (f) h pi for sample 59, the infected cells were reacted 8H8 mab followed with an FITC-conjugated goat anti-mouse antibody and then examined under a fluorescence microscope. Cytoplasmatic and nuclear staining of infected cells is indicated as C and N, respectively. Samples from healthy individuals as negative control (g).
Acknowledgements
We want to thanks Didye Ruiz, Naifi Calzada, Maria Caridad Lopez Quevedo and Ledys Lopez Fuentes from Pedro Kourè Tropical Medicine Institute for their expert technical assistance.
References
- Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis 2002; 2:33 - 42
- Halstead S. Dengue. Seminar. Lancet 2007; 370:1644 - 1652
- Groen J, Koraka P, Velzing J, Copra C, Osterhaus AD. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin Diagn Lab Immunol 2000; 7:867 - 871
- Anandarao R, Swaminathan S, Fernando S, Jana A, Khanna N. Recombinant multiepitope protein for early detection of dengue infections. Clin Vaccine Immunol 2006; 59 - 67
- Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin Diagn Lab Immunol 2003; 10:622 - 630
- Schilling S, Ludolfs D, Van An L, Schmitz H. Laboratory diagnosis of primary and secondary dengue infection. J Clin Virol 2004; 31:179 - 184
- Vazquez S, Valdes O, Pupo M, Delgado I, Alvarez M, Pelegrino JL. MAC-ELISA and ELISA inhibition methods for detection of antibodies after yellow fever vaccination. J Virol Methods 2003; 110:179 - 184
- Guzman MG, Kouri G. Dengue diagnosis, advances and challenges. Int J Infect Dis 2004; 8:69 - 80
- Kao CL, King CC, Chao DY, Wu HL, Chang GJ. Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J Microbiol Immunol Infect 2005; 38:5 - 16
- Gubler DJ, Kuno G, Sather GE, Velez M, Oliver A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am J Trop Med Hyg 1984; 33:158 - 165
- Henchal EA, Gentry MK, McCown JM, Brandt WE. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg 1982; 31:830 - 836
- Rodriguez-Roche R, Alvarez M, Guzman MG, Morier L, Kouri G. Comparison of rapid centrifugation assay with conventional tissue culture method for isolation of dengue 2 virus in C6/36-HT cells. J Clin Microbiol 2000; 38:3508 - 3510
- Chen WJ, Chen SL, Fang AH, Wang MT. Detection of dengue virus antigens in cultured cells by using protein A-gold-silver staining (pAgs) method. Microbiol Immunol 1993; 37:359 - 363
- Kao CL, Wu MC, Chiu YH, Lin JL, Wu YC, Yueh YY. Flow cytometry compared with indirect immunofluorescence for rapid detection of dengue virus type 1 after amplification in tissue culture. J Clin Microbiol 2001; 39:3672 - 3677
- Hogrefe WR, Moore R, Lape-Nixon M, Wagner M, Prince HE. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J Clin Microbiol 2004; 42:4641 - 4648
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol 2002; 40:376 - 381
- Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol 2000; 38:1053 - 1057
- Tadano M, Makino Y, Fukunaga T, Okuno Y, Fukai K. Detection of dengue 4 virus core protein in the nucleus I. A monoclonal antibody to dengue 4 virus reacts with the antigen in the nucleus and cytoplasm. J Gen Virol 1989; 70:1409 - 1415
- Bulich R, Aaskov JG. Nuclear localization of dengue 2 virus core protein detected with monoclonal antibodies. J Gen Virol 1992; 73:2999 - 3003
- Wang SH, Syu WJ, Huang KJ, Lei HY, Yao CW, King CC, et al. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J Gen Virol 2002; 83:3093 - 3102
- Westaway EG, Khromykh AA, Kenney MT, Mackenzie JM, Jones MK. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology 1997; 234:31 - 41
- Yang J-S, Ramanathan M, Muthumani K, Choo AY, Jin S-H, Yu Q-C, et al. Induction of Inflammatino by West Nile Virus Capsid through the Caspase-9 Apoptotic Pathway. Emerg Infect Dis 2002; 8
- Makino Y, Tadano M, Anzai T, Ma SP, Yasuda S, Fukunaga T. Detection of dengue 4 virus core protein in the nucleus. II. Antibody against dengue 4 core protein produced by a recombinant baculovirus reacts with the antigen in the nucleus. J Gen Virol 1989; 70:1417 - 1425
- Kumarasamy V, Wahab AH, Chua SK, Hassan Z, Chem YK, Mohamad M, et al. Evaluation of a commercial dengue NS1 antigen-capture ELISA for laboratory diagnosis of acute dengue virus infection. J Virol Methods 2007; 140:75 - 79
- Henchal EA, McCown JM, Seguin MC, Gentry MK, Brandt WE. Rapid identification of dengue virus isolates by using monoclonal antibodies in an indirect immunofluorescence assay. Am J Trop Med Hyg 1983; 32:164 - 169
- Ramos C, Sanchez G, Pando RH, Baquera J, Hernandez D, Mota J. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J Neurovirol 1998; 4:465 - 468
- Jessie K, Fong MY, Devi S, Lam SK, Wong KT. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J Infect Dis 2004; 189:1411 - 1418
- Limonta D, Capo V, Torres G, Perez A, Guzman M. Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol 2007; 40:50 - 54
- Miagostovich MP, Ramos RG, Nicol AF, Nogueira RM, Cuzzi-Maya T, Oliveira AV. Retrospective study on dengue fatal cases. Clin Neuropathol 1997; 16:204 - 208
- de Macedo FC, Nicol AF, Cooper LD, Yearsley M, Pires AR, Nuovo GJ. Histologic, viral and molecular correlates of dengue Fever infection of the liver using highly sensitive immunohistochemistry. Diagn Mol Pathol 2006; 15:223 - 228
- Suksanpaisan L, Cabrera-Hernandez A, Smith DR. Infection of human primary hepatocytes with dengue virus serotype 2. J Med Virol 2007; 79:300 - 307
- Sriurairatna S, Bhamarapravati N, Phalavadhtana O. Dengue virus infection of mice: morphology and morphogenesis of dengue type-2 virus in suckling mouse neurones. Infect Immun 1973; 8:1017 - 1028
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue in the early febrile phase: viremia and antibody responses. J Infect Dis 1997; 176:322 - 330
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S. Dengue viremia titer, antibody response pattern and virus serotype correlate with disease severity. J Infect Dis 2000; 181:2 - 9
- Saijo M, Niikura M, Maeda A, Sata T, Kurata T, Kurane I, et al. Characterization of monoclonal antibodies to Marburg virus nucleoprotein (NP) that can be used for NP-capture enzyme-linked immunosorbent assay. J Med Virol 2005; 76:111 - 118
- Koraka P, Burghoorn-Maas CP, Falconar A, Setiati TE, Djamiatun K, Groen J. Detection of Immune-Complex-Dissociated Nonstructural-1 Antigen in Patients with Acute Dengue Virus Infections. J Clin Microbiol 2003; 41:4154 - 4159
- Zaki A, Coudrier D, Yousef A, Falkeek M, Bouloy M, Billecocq A. Production of monoclonal antibodies against Rift Valley fever virus Application for rapid diagnosis tests (virus detection and ELISA) in human sera. J Virol Methods 2005; 12
- Kimura T, Rokuhara A, Matsumoto A, Sagi Y, Tanak E, Kiyosawa K. New enzyme Immunoassay for detection of hepatitis B cirus core antigen (HBcAg) and relation between levels of HBcAg and HBV DNA. J Clin Microbiol 2003; 41:1901 - 1906
- Fabrizi F, Vecchi Ad, Como G, Lunghi G, Martin P. De novo HCV infection among dialysis patients: a prospective study by HCV core antigen ELISA assay. Aliment Pharmacol Ther 2005; 21:861 - 869
- Saijo M, Niikura M, Maeda A, Sata T, Kurata T, Kurane I. Characterization of monoclonal antibodies to Marburg virus nucleoprotein (NP) that can be used for NP-capture enzyme-linked immunosorbent assay. J Med Virol 2005; 76:111 - 118
- Saijo M, Niikura M, Ikegami T, Kurane I, Kurata T, Morikawa S. Laboratory diagnostic systems for Ebola and Marburg hemorrhagic fevers developed with recombinant proteins. Clin Vaccine Immunol 2006; 13:444 - 451
- Saijo M, Georges-Courbot MC, Fukushi S, Mizutani T, Philippe M, Georges AJ, et al. Marburgvirus nucleoprotein-capture enzyme-linked immunosorbent assay using monoclonal antibodies to recombinant nucleoprotein: detection of authentic Marburgvirus. J Infect Dis 2006; 59:323 - 325
- Goding JW. Monoclonal antibodies: Principles and Practice 1986; 2 da Orlando Academic Press
- Goding JW. Monoclonal antibodies: Principles and Practice 1996; 3era San Diego, CA Academic Press
- Kobayashi M, Tanaka E, Matsumoto A, Yoshizawa K, Imai H, Sodeyama T, et al. Clinical application of hepatitis C virus core protein in early diagnosis of acute hepatitis C. J Gastroenterol Hepatol 1998; 33:508 - 511
- Kouri G, Mas P, Guzman MG, Soler M, Goyenechea A, Morier L. Dengue hemorrhagic fever in Cuba, 1981: rapid diagnosis of the etiologic agent. Bull Pan Am Health Organ 1983; 17:126 - 132
- Alvarez M, Rodriguez-Roche R, Bernardo L, Vazquez S, Morier L, Gonzalez D, et al. Dengue Hemorrhagic Fever Caused by Sequential Dengue 1–3 Virus Infections over a Long Time Interval: Havana Epidemic, 2001–2002. Am J Trop Med Hyg 2006; 75:1113 - 1117
- Hermida Diaz C, Pupo M, Guzman Tirado MG, Gonzalez Garriga M, Marcet Sanchez R. Use of a dengue anti-complex monoclonal antibody in viral purification. Rev Cubana Med Trop 1992; 44:171 - 176
- Pupo Antunez M, Rodriguez H, Vazquez S, Vilaseca JC, Alvarez M, Otero A. Monoclonal antibodies raised to the dengue-2 virus (Cuban: A15 strain) which recognize viral structural proteins. Hybridoma 1997; 16:347 - 353
- Rodrèguez-Roche R, Alvarez M, Guzmán MG, Morier L, Kourè G. Comparison of rapid centrifugation assay with conventional tissue culture method for isolation of dengue 2 virus in C6/36-HT cells. J Clin Microbiol 2000; 38:3508 - 3510
- Luna L. Manual of Histologic Methods of the Army Force Institute of Pathology 1968; New York
- Lazo L, Hermida L, Zulueta A, Sanchez J, Lopez C, Silva R, et al. A recombinant capsid protein from Dengue-2 induces protection in mice against homologous virus. Vaccine 2007; 25:1064 - 1070