Abstract
Approximately 30 therapeutic monoclonal antibodies have already been approved for cancers and inflammatory diseases, and monoclonal antibodies continue to be one of the fastest growing classes of therapeutic molecules. Because aberrant signaling by receptor tyrosine kinases (RTKs) is a commonly observed factor in cancer, most of the subclasses of RTKs are being extensively studied as potential targets for treating malignancies. The first two RTKs that have been targeted by antibody therapy, with five currently marketed antibodies, are the growth factor receptors EGFR and HER2. However, due to systemic side effects, refractory patients and the development of drug resistance, these treatments are being challenged by emerging therapeutics. This review examines current monoclonal antibody therapies against RTKs. After an analysis of agents that have already been approved, we present an analysis of antibodies in clinical development that target RTKs. Finally, we highlight promising RTKs that are emerging as new oncological targets for antibody-based therapy.
Introduction
Antibodies represent an ideal approach for selectively interfering with a single target molecule, because of their high specificity. Therefore, therapeutic monoclonal antibodies (mAbs) have one of the most active and promising pipelines in the pharmaceutical industry, especially in the field of oncology. During the past 30 y, antibody cancer therapeutics have been developed and used clinically in an effort to realize the potential of targeted therapy. Therapeutic mAbs can lead to tumor cell death by different mechanisms of action. First, mAbs can block ligand-receptor growth and survival pathways by targeting antigens that are differentially expressed in tumor cells (tumor-associated antigens) and consequently can be used to block molecules involved in cancer progression or angiogenesis. Second, in cancer therapy, the innate immune effector mechanisms of mAbs lead to antibody-dependent cell-mediated cytotoxicity (ADCC), complement-mediated cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP) of targeted cells.Citation1 Medical advances during the past 10 y have led to the introduction of 9 antibodies for the treatment of diverse cancers, with the antibodies targeting different tumor-associated antigens including surface glycoproteins associated with clusters of differentiation (CD), CTLA-A, or pathways regulated by growth factor receptors.Citation2 Five of these antibodies specifically and selectively target and block a receptor tyrosine kinase (RTK). RTKs are high-affinity cell surface receptors for many polypeptide growth factors, cytokines, and hormones that usually contain an intracellular kinase domain. This kinase function is the first element involved in the activation of one or several downstream intracellular signal transduction pathways. By targeting the extracellular part of the receptor protein kinase, the mAb is able to block the binding of the natural ligand, avoid conformational rearrangement essential to the activation of the kinase and thus the activation of the downstream signaling pathways, or activate immune effector functions. As key regulators of normal cellular processes and the development and progression of many types of cancer, RTKs are ideal cancer targets. In this review, we focus on non-conjugated mAb therapies developed against RTKs, with a brief analysis of agents that are already approved and used clinically, followed by a list of recent clinical and preclinical efforts toward new targets to develop novel immunotherapy approaches for cancers.
Currently Marketed Monoclonal Antibodies Targeting Receptor Tyrosine Kinases are Directed Against the Human Epidermal Growth Factor Receptor Family
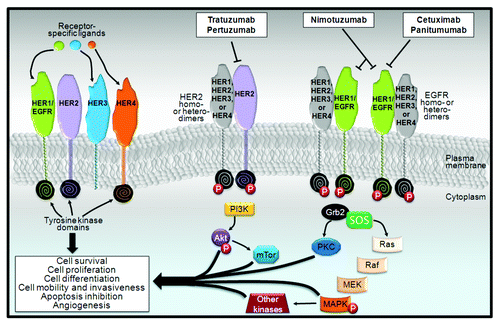
Currently, the five antibodies on the market that target RTKs are directed against the human epidermal growth factor receptor family (). The human epidermal growth factor receptor (HER) family members are structurally related and include EGFR (ERBB1), HER2/neu (ERBB2), HER3 (ERBB3), and HER4 (ERBB4), and all except HER3 contain an intracellular tyrosine kinase domain. All of the HER members other than HER2 are able to bind natural extracellular ligands. Seven ligands bind to EGFR including epidermal growth factor and transforming growth factor α; two ligands bind to HER3; and seven ligands bind to HER4. Activation of EGFR and HER2 (by its heterodimerization with HER3) induces a cascade of downstream signaling through several pathways, such as MAPK and PI3K/Akt/mTOR, resulting in cellular proliferation, differentiation, survival, motility, adhesion, and repair ().Citation3 Mutations, overexpression or abnormal activation of receptors in the HER family are common features in several epithelial malignancies including lung, breast, stomach, colorectal, and head and neck cancers, pancreatic carcinoma and glioblastoma.Citation4
Table 1. Monoclonal antibodies targeting human receptor tyrosine kinases that are currently approved in oncology
Figure 1. Overview of human epidermal growth factor receptor (HER) family signaling. Although the morphology of the extracellular domains of the four HERs are almost identical, their functional activity varies considerably. HER3 lacks inherent kinase function, but can heterodimerize with other HER receptors. Indeed, the HER2-HER3 dimer, which is considered to be the most active HER signaling dimer, is fundamental for HER2-mediated signaling in tumors with HER2 amplification. After ligand binding, the HER receptor becomes activated by dimerization between two identical receptors (i.e., homodimerization) or between different receptors of the same family (i.e., heterodimerization). Dimerization leads to the phosphorylation of several intracellular catalytic substrates, including members of the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway, the phosphatidyl-3-kinase (PI3K)/Akt/PTEN family, and other important signaling pathways that regulate apoptosis, protein synthesis, and cellular proliferation. The monoclonal antibodies trastuzumab and pertuzumab are directed against HER2 members, with both leading to an inhibition of the HER2 signaling pathway but by two distinct mechanisms. Cetuximab and pertuzumab bind to the EGFR protein.
ANTI-HER2
Trastuzumab
Trastuzumab (Herceptin®; Genentech, Inc.) was the first approved anti-RTK mAb. This approval has paved the way and proved the concept of targeting kinases with mAbs in cancer therapy. Trastuzumab is a humanized mAb that binds to the extracellular domain of the receptor tyrosine kinase HER2.Citation5,Citation6 Trastuzumab was first approved in 1998 to treat early stage HER2-positive breast cancer, or metastatic breast cancer that substantially overexpresses HER2, and the approval was extended in 2010 to include HER2-positive metastatic cancer of the stomach or gastresophageal junction. The mechanisms underlying the anticancer activity of trastuzumab have not been completely elucidated.Citation7 However, several mechanisms have been proposed and there are generally accepted basic principles. First, trastuzumab does not block the dimerization of HER2 but its binding to its targeted receptor induces an inhibition of the intracellular signaling pathways (up to 50% over 5 d).Citation8,Citation9 Second, trastuzumab downregulates the overall levels of HER2 on the cell surface.Citation10 Third, trastuzumab, by its binding to HER2, presents the cells to the immune system to allow ADCC of tumor cells, but the CDC process is not involved.Citation11,Citation12 Through these three global mechanisms, treatment with trastuzumab leads to the inhibition of proliferation and the death of cells that overexpress HER2, induction of cell cycle arrest, and effects on cell adhesion, angiogenesis and the metastatic potential of tumor cells.Citation13-Citation15
Pertuzumab
With the understanding that HER3 is a necessary partner for HER2-mediated oncogenic activity in HER2-overexpressing tumors, the success and approval in 2012 of pertuzumab (Perjeta®/Omnitarg®; Genentech, Inc.), is not surprising.Citation16 Pertuzumab is a first-in-class HER2 dimerization inhibitor that acts by blocking ligand-induced HER2-to-HER3 heterodimerization and inhibiting HER3 signaling.Citation17,Citation18 Pertuzumab is also able to inhibit heterodimerization of HER2 with the two other HER family members, EGFR and HER4, but preclinical observations have demonstrated that the blocking of HER2-HER3 likely represents the most relevant action of pertuzumab.Citation19,Citation20 Other studies have also indicated that interfering with the HER3 component may be more relevant than inhibition of EGFR in HER2-amplified breast cancer cell lines.Citation21 Similarly, high levels of HER3, rather than overexpression of HER2, were correlated with shorter overall survival in patients with ovarian cancer.Citation22 Pertuzumab is not a downregulator of the cell surface expression of HER2, but its binding to the tumor cells recruits the immune system and induces an ADCC process with only minor CDC effects.Citation23,Citation24
Since the initial use of each anti-HER2 antibody alone as monotherapy or associated with chemotherapy, several studies have demonstrated the benefit of using trastuzumab and pertuzumab together. The co-localization of the two HER2 antibodies on distinct epitopes of the HER2 extracellular domain provides more efficient inhibition of tumor growth than one antibody alone.Citation23,Citation25
ANTI-EGFR
Cetuximab
Given that EGFR is a transmembrane glycoprotein receptor that induces tumor cell proliferation and neovascularization, many studies have led to the development of either mAbs or small tyrosine kinase inhibitors as new agents targeting EGFR.Citation26,Citation27 Studies have demonstrated that blocking EGFR signaling decreases growth and metastasis and also enhances the effect of chemotherapy.Citation28 Cetuximab (Erbitux®; Imclone Systems, Inc.) is a chimeric human-murine monoclonal IgG1 antibody that binds to the extracellular domain of EGFR and inhibits its dimerization.Citation29 The significant antitumor effect of cetuximab is mediated, in part, by inhibition of angiogenesis.Citation30,Citation31 Cetuximab may elicit immunological responses such as ADCC or complement activation.Citation32-Citation34 Several studies have demonstrated the in vivo anti-tumoral activity of cetuximab in diverse types of cancer and elucidated its mechanism of action.Citation35-Citation37 For example, treatment with cetuximab influences cellular proliferation, apoptosis, and radiosensitivity in carcinomas of the head and neck.Citation38 Cetuximab is approved in many countries all over the world for treating patients with squamous cell carcinoma of the head and neck (SCCHN) and metastatic colorectal Cancer (mCRC). Continuous experimental and clinical investigations to better understand the activity of cetuximab have led to the discovery that a real benefit of treatment is obtained in patients with EGFR-expressing, RAS wild-type metastatic colorectal cancer.Citation39,Citation40 The RAS mutation status is now used clinically to stratify patients likely to benefit from this therapy.
Panitumumab
In 2006, panitumumab (Vectibix®; Amgen, Inc.), a human antibody against EGFR, was approved by the US. Food and Drug Administration (FDA) for the treatment of patients with EGFR-expressing mCRC. By competing with endogenous EGF and TGFα ligand binding, panitumumab inhibits receptor phosphorylation and activation of cell signaling leading to blocking of EGF-dependent tumor growth mechanisms and also resulting in receptor internalization, apoptosis induction, inhibition of angiogenesis and autophagy.Citation41,Citation42 In vivo, panitumumab not only blocks the formation of new tumors but also mediates therapeutic elimination of established tumors and acts cooperatively with chemotherapeutics in mediating tumor regression.Citation43 With an IgG2 isotype, panitumumab is not able to mediate ADCC or CDC.Citation44,Citation45 Notably, panitumumab is not effective at recruiting NK cells and inducing NK cell-mediated ADCC but is effective at recruiting ADCC by myeloid effector cells (i.e., neutrophils and monocytes).Citation46,Citation47 Based on the importance of RAS status for stratifying patients for treatment with cetuximab, several studies have demonstrated that the specific RAS mutation also determines the responsiveness to panitumumab treatment.Citation48,Citation49 Further data are still needed to clarify the role of each specific RAS mutation for anti-EGFR-based treatments in mCRC and other types of cancers and this information should be considered for further clinical uses.Citation50
Nimotuzumab
Nimotuzumab (h-R3), a humanized murine mAb, was developed at the Center of Molecular Immunology, Havana, Cuba. It has demonstrated antitumor activity similar to that of other anti-EGFR mAbs and shows promise as a single agent and as an adjunct to radiation. The mechanism of action of nimotuzumab involves not only direct targeting of EGFR in epithelial cancers and in vascular cells but also ADCC and CDC.Citation51 Surprisingly, the typical severe dermatological toxicities thus far associated with anti-EGFR therapy have not been described with nimotuzumab.Citation52 Experimental observations suggest that nimotuzumab requires bivalent binding leading to a more selective binding to cells that express moderate to high EGFR levels, rather than healthy cells expressing low levels of the RTK.Citation53 Nimotuzumab is presently approved in more than 20 countries for various indications including squamous cell carcinoma of the head and neck (SCCHN) in India, Cuba, Argentina, Colombia, Ivory Coast, Gabon, Ukraine, Peru, and Sri Lanka; for glioma (pediatric and adult) in Cuba, Argentina, Philippines, and Ukraine; and for nasopharyngeal cancer in China. Nimotuzumab has been designated as an “orphan drug” for the treatment of glioma by the FDA and for the treatment of glioma and pancreatic cancer by the European Medicines Agency (EMA). Currently, nimotuzumab is still being evaluated in Phase 3 clinical trials.Citation54,Citation55
Continuous Efforts To Target Members of the HER Family: New Anti-HER Monoclonal Antibodies in Clinical Development
While trastuzumab, pertuzumab, cetuximab, panitumumab and nimotuzumab are still under investigation in clinical trials for different types of cancer, new agents directed against the same targets (EGFR and HER2) are entering clinical trials (). In parallel with such novel antibodies, next-generation versions of already approved products emerged. For example, CetuGEXTM (GT-MAB 5.2-GEXTM) and TrasGEXTM (GT-Mab 7.3-GEXTM) are antibodies produced by human glycoengineered cell lines (leukemic cells in suspension) that differ in their glycosylation capabilities and lead to the expression of biotherapeutics with human optimized glycosylation. CetuGEXTM and TrasGEXTM, glyco-optimized versions of cetuximab and trastuzumab, respectively, promised improved bioactivity and bioavailability and low/no immunogenicity. The increase in ADCC due to superior glycosylation in turn renders the improved antibodies suitable for treatment of patient sub-populations that were not responsive to the original product.
Table 2. Monoclonal antibodies targeting human receptor tyrosine kinases that are currently in clinical trials in oncology
With the success of pertuzumab, which proved that targeting HER3 is a good way to treat cancer, some agents directed against HER3 are now being tested in clinical trials.Citation56 Today, HER4 remains the only member of the HER family for which no antibody is being developed clinically.
ANTI-EGFR
Still, the clinical interest in EGFR-targeting approaches is not decreasing, for two reasons: most, if not all, FDA approved EGFR-targeting agents have been associated with the development of chemoresistance in a consistent fraction of patients; and severe skin, renal, and gastrointestinal mucosa toxicities are commonly associated with the EGFR-targeting antibodies cetuximab and panitumumab. Furthermore, despite promising preclinical data, some anti-EGFR antibodies that entered in clinical trials failed to meet primary endpoints. In this way, the development of matuzumab (EMD72000), zalutumumab (HUMAX-EGFR), and imgatuzumab (RO5083945; GA-201; RG7160) was halted either in Phase 2 or Phase 3 because they did not demonstrate any benefit on predefined clinical endpoints of activity. For this reason, another challenge in discovering new EGFR targeted therapies is finding new biomarkers for efficient translation of preclinical data into clinical outcomes. Necitumumab (IMC-11F8/LY3012211) is a human IgG1 that has demonstrated similar antitumor potency to cetuximab, and had been presented as a safer therapeutic alternative.Citation57,Citation58 Necitumumab is currently in Phase 3 clinical trials and the submission of the first marketing approval for necitumumab is expected before the end of 2014. Humanized mAb 806 (ABT-806) targets an EGFR deletion variant as well as a wild-type EGFR expressed in cells overexpressing the receptor.Citation59 ABT-806 has been shown to be superior to other anti-EGFR antibodies and tyrosine kinase inhibitors in treating tumors with specific deletion mutations or EGFR kinase domain mutations in vivo. As demonstrated in a Phase 1 first-in-human clinical trial, ABT-806 lacks normal tissue uptake or toxicity in cancer patients.Citation60 Sym004 is a mixture of two synergistic full-length anti-EGFR antibodies (futuximab and zatuximab), which bind to two separate non-overlapping epitopes on EGFR.Citation61 Both antibodies are involved in extensively cross-linking EGFRs on the cell surface leading to improved receptor elimination and induction of secondary effector functions. Sym004 has proved to be superior to available mAbs in several established animal models and has shown promising results in early human clinical trials.Citation62 MM-151 consists of a mixture of three human mAbs designed to bind to three non-overlapping sites on EGFR to maximize inhibition of ligand-dependent and independent signaling. The use of three antibodies not only results in maximal receptor inhibition but also addresses resistance to EGFR-targeted therapies that may develop through compensatory activity of other EGFR ligands. Currently, a Phase 1 clinical study is being conducted with MM-151 in patients with solid tumors, with a focus on colorectal cancer, non-small cell lung cancer and triple negative breast cancer.
ANTI-HER2
In contrast to the enthusiasm for the discovery of new and improved anti-EGFR therapeutic antibodies, there is less of a frenzy to develop new specific anti-HER2 antibodies. However, the limited efficacy of trastuzumab for cancer therapy, problems of acquired resistance and the potential effect of novel anti-HER2 antibodies as therapeutic partners or alternative therapeutics should justify more investment in HER2 targeting with novel mAbs. In addition to TrasGEXTM, the only other anti-HER2 mAb that has entered clinical trials is margetuximab (MGAH22). Margetuximab is a chimeric antibody with specificity and affinity similar to trastuzumab, with an Fc domain engineered to enhance ADCC.Citation63
ANTI-HER3
Antibodies directly targeting the HER3 extracellular domain are currently under clinical investigation. Seribantumab (MM-121/SAR256212), patritumab (U3–1287/AMG888) and LJM716 are human mAbs that target the HER3 receptor, inhibiting HER3 signaling and activation of associated survival pathways.Citation64-Citation67 Other candidates that inhibit both ligand-dependent and ligand-independent HER3 signaling have been designed (AV-203, GSK2849330, REGN1400, KTN3379).Citation68 Therapeutic inhibitors of HER3 are currently being tested in several Phase 1 and Phase 2 clinical trials spanning various patient populations, and there is strong evidence that these agents have limited potential for single agent activity and should be considered as part of multi-drug combinations.Citation69,Citation70 In this context, RG7116/RO5479599 could be a highly potent HER3-targeting single agent because it was designed not only to block HER3 activation and induce HER3 downregulation but also to mediate enhanced ADCC by glycoengineering.Citation71
Clinically Investigated Monoclonal Antibodies Directed Against New Receptor Tyrosine Kinase Targets
New growth factor receptors with kinase domains, including c-MET, RON, IGF1R and EPHA2, EPHA3, are targeted by antibodies in cancer patients ().Citation72,Citation73 To prevent tumor growth, two pathways are also targeted by new mAbs: the formation of new microvessels, and more generally, the angiogenesis process is repressed by inhibiting VEGFR signaling; and stromal cells and the tumor microenvironment are altered using PDGFR, CSF1R and FGFR as targets.
Antibodies directed against the MET family
ANTI-MET
c-MET (mesenchymal-epithelial transition; HGFR [hepatocyte growth factor receptor]) is a membrane-spanning receptor tyrosine kinase. Upon binding with its only known ligand, hepatocyte growth factor (HGF), c-MET autophosphorylates and recruits several downstream effectors activating several biological processes including motility, proliferation, survival, invasion, and morphogenesis.Citation74,Citation75 Consequently, the HGF/c-MET axis is implicated in a wide variety of epithelial, mesenchymal, and hematological malignancies, rendering c-MET an attractive target for cancer therapies.Citation74,Citation76 There are currently several HGF/c-MET inhibitors under clinical evaluation, with four antibodies directed against the extracellular part of c-MET. Onartuzumab (MetMab/OA-5D5) is a one-armed monovalent humanized antibody designed to prevent receptor oligomerization and initiation of signaling frequently observed with antibodies against c-MET. Onartuzumab reduces c-MET phosphorylation and diminishes c-MET density on the cell surface and is currently the most advanced of the anti-MET antibodies, with Phase 3 clinical trials in progress.Citation77,Citation78 However, a Phase 3 study (METLung study), evaluating onartuzumab in combination with erlotinib (Tarceva®; Roche), a small chemical inhibitor of EGFR tyrosine kinase activity, compared with erlotinib alone has recently been stopped due to a lack of clinically meaningful efficacy of ornatuzumab.Citation79 Even if some data from this study have yet to be extensively analyzed and reviewed, these disappointing findings challenge the future development of onartuzumab and revive the race to find clinical benefits of using anti-MET antibodies. Although almost all major human cancers seem to harbor some dysregulation of the MET pathway, only a minority of tumors are ‘addicted to MET’ mainly because tumor cells develop many concomitant or staggered resistance pathways.Citation80 Greater benefits would probably be observed if clinical trials are designed with prospective evaluation or better targeting of the specific subpopulation of drug-resistant patients, or by staggering treatment. Other humanized mAbs with high affinity for c-MET are ABT-700 (h224G11) and LY2875358 (LA480), which inhibit the major functions of c-MET, including not only cell proliferation, migration, and invasion, but also cell scattering and angiogenesis.Citation81,Citation82 By inducing c-MET downregulation and triggering ADCC functions, both are promising candidates for the treatment of ligand-dependent and ligand-independent tumors as single or combined therapies.Citation83 ARGX-111 is a human mAb that potently neutralizes both ligand-dependent and independent receptor signaling of c-MET with enhanced ADCC by Fc engineering.
ANTI-RON
Given the increasing interest in developing MET inhibitors to fight cancer, it is not surprising that new therapeutics are now being developed to target RON, another member of the MET family.Citation84 RON (recepteur d’origine nantais) or MS1R (macrophage stimulating 1 receptor) shares many common structural and biochemical features with MET. Ligand-dependent or independent activation of RON results in cell proliferation, migration, and matrix invasion, collectively known as invasive growth, as occurs with activation of c-MET.Citation85 Intensive study has led to the preclinical evaluation of several anti-RON antibodies, with narnatumab (IMC-RON8) being the first to enter clinical trials targeting RON overexpression. Narnatumab is a human mAb that prevents binding of RON ligand, downregulates RON expression, hinders ligand-induced cell migration and reduces cell transformation.Citation86
Antibodies directed against the Insulin receptor family
Similar to the EGFR pathway, the insulin-like growth factor receptor (IGFR) signaling system is comprised of multiple circulating ligands, such as IGF-I, IGF-II, and insulin, which interact with membrane-bound receptors, such as type I IGF receptor (IGF1R) and insulin receptor (IR).Citation87 Most anti-IGFR pathway agents in clinical development are targeted against the IGF1R, which contains an intracellular tyrosine kinase domain capable of recruiting insulin-receptor substrates (IRS) or Src homology 2 domain-containing (Shc) proteins. IGF1R activation has been identified as playing a major role in the development, maintenance, and progression of cancer.Citation88,Citation89 There are several IGF1R-targeting agents undergoing clinical testing; all of these agents have a similar mechanism of action, with binding to IGF1R that causes receptor internalization, and thereby prevents binding of ligand to receptor by removing receptors from the cell surface.Citation90 Such anti-IGF1R mAbs have demonstrated broad preclinical antitumor activities.Citation91,Citation92 However, their effectiveness in clinical trials has been limited and none have been approved yet by the FDA for general oncological use.Citation93 In the past, several agents failed to demonstrate a statistically significant improvement in the primary end point in clinical trials. For example, figitumumab (CP-751871) and teprotomumab (R1507) failed in Phase 3 for NSCLC; ganitumumab (AMG-479) failed to show a benefit in pancreatic adenocarcinoma patients; cixutumumab (IMC-A12/LY3012217) presented mixed results in Phase 2 and has not progressed to Phase 3; robatumumab (Sch717454) in advanced NSCLC or AVE1642 in multiple myeloma have demonstrated insufficient activity to deserve further development in this subgroup of patients. The development of BIIB022, in contrast, was terminated due to a non scientific decision of the sponsor company. Some of these antibodies, such as ganitumab and cixutumumab, are still being evaluated in Phase 1 and Phase 2 trials for oncological indications. Clinical trials (Phase 2) are ongoing with a fourth anti-IGF1R mAb. Dalotuzumab (MK-0646; h7C10), a recombinant humanized IgG1 mAb, acts by inhibiting IGF1R ligand-mediated tumor cell proliferation, and has proven preclinical efficacy in multiple cancer cell lines and in mouse xenograft models.Citation94
Antibodies directed against the Ephrin receptor family
The 16 members of the Ephrin receptor superfamily of RTKs are key players in various signaling pathways and mediate critical steps in a wide variety of physiological and pathological processes. The members of the Ephrin Receptors superfamily are divided into two groups, EPHA and EPHB, based on the types of ligands (ephrins) they bind. EPH receptors are associated with tumor growth, invasiveness and metastasis.Citation95 The function of an EPH receptor in oncogenesis can be a consequence of either its upregulation or overexpression or its downregulation.Citation96 Despite the considerable complexity of EPH signaling, EPH receptors have emerged as potential therapeutic targets.Citation97,Citation98 The development of several antibodies targeting EPHA2 (agonist), EPHB2 (antagonist) or EPHB4 (antagonist) was prematurely stopped due to a lack of antitumor activity or a limited therapeutic window in preclinical models. Only two antibodies targeting an EPH receptor are currently in clinical trials (Phase 1): an anti-EPHA2 (DS-8895a) and an anti-EPHA3 (KB004/IIIA4). Little is known about DS-8895a because no public data are available. In contrast, published data has described encouraging preclinical results, mainly in hematological malignancies, for KB004, a non-fucosylated human IgG1 derived from the agonistic anti-EPHA3 IIIA4 antibody.Citation73
Antibodies directed against the VEGFR family
The first observation, by Folkman in the 1970s, that tumors are unable to grow beyond 2 mm3 unless they are supported by neovascularization, resulted in intensive study to understand the angiogenesis mechanism in tumor growth. In this context, vascular endothelial growth factors and receptors (VEGF, VEGFR) emerged as principal angiogenesis actors and have been validated as targets in cancer therapy.Citation99 The VEGF family is comprised of the ligands placental growth factor (PlGF), VEGF-A, VEGF-B, VEGF-C, and VEGF-D, and the receptors VEGFR1, VEGFR2, and VEGFR3. Several anti-VEGFR mAbs are now in clinical development ().
ANTI-VEGFR1
Although VEGF-A is known to bind and activate both VEGFR1 and VEGFR2, VEGFR1 is the only known receptor for the ligands PlGF and VEGF-B. In human cancer, VEGFR1 mediated signaling is responsible for several cancer pathogenesis mechanisms including direct tumor activation, activation of nonmalignant supporting cells and angiogenesis.Citation100 Icrucumab (IMC-18F1/LY3012212) is a human IgG1 mAb that was designed to target VEGFR1. This antibody potently inhibits ligand-dependent phosphorylation of VEGFR1 by blocking the binding of VEGF-A, VEGF-B and PlGF and downstream signaling.Citation101
ANTI-VEGFR2
VEGF-A interaction with VEGFR2 has been demonstrated to be an essential modulator of tumor angiogenesis based on the results of an extensive range of human and preclinical investigations. Ramucirumab (IMC-1121B/ LY3009806) is a human IgG1 specific for the VEGFR2 receptor. By antagonizing endogenous VEGF signaling, ramucirumab inhibits the most prominent interactions between neoplastic and stromal cells, i.e., neo-angiogenesis.Citation102,Citation103 A decision from the FDA on ramucirumab as a second-line single-agent treatment option for patients with advanced gastric cancer is anticipated in the spring of 2014. Ramucirumab has also been explored in Phase 3 trials as a treatment for colorectal cancer, hepatocellular carcinoma, and lung cancer and is currently under evaluation for other oncological indications.Citation104,Citation105 Tanibirumab (TTAC-0001) is a human mAb that specifically binds to VEGFR2 and prevents the binding of its ligand. Tanibirumab has demonstrated antitumor activity against various cancer models, including lung, breast, colorectal and glioblastoma models, and is being studied in Phase 1 clinical trials.Citation106
ANTI-VEGFR3
VEGFR3 and its ligands, VEGF-C and VEGF-D, control tumor lymphangiogenesis by enhancing proliferation and survival of lymphatic endothelial cells. VEGFR3 levels are upregulated in tumor vessels, and inhibitors blocking VEGFR3 homodimerization, VEGFR3/VEGFR2 heterodimerization, or VEGF-C binding inhibit tumor angiogenesis in culture and in mice.Citation107 Based on these observations, IMC-3C5 (hF4–3C5), a human IgG1 mAb, was designed to bind VEGFR3 and block the binding of VEGF-C and VEGF-D ligands to the receptor, thereby inhibiting subsequent signaling.Citation108 However, with results from the literature suggesting that a dual blockade of both VEGFR2 and VEGFR3 simultaneously may represent a more potent approach to effective cancer therapy than inhibiting VEGFR3 alone, the clinical efficacy of IMC-3C5 should probably be investigated in association with an anti-VEGFR2 or an anti-VEGF therapy.Citation108,Citation109
Antibodies directed against the KIT family
The KIT family of receptor tyrosine kinases includes c-Kit, Flt3 and fms/CSF1R. The c-Kit receptor plays a key role in tumor occurrence, development, migration and recurrence.Citation110 Although targeted drugs have emerged to inhibit the kinase activity of this receptor, biotherapeutics are mainly directed against CSF1R. Also known as macrophage colony-stimulating factor receptor (M-CSFR), CSF1R is a cell-surface receptor for its ligands, colony-stimulating factor 1 (CSF1) and Interleukin-34 (IL-34). CSF1R plays a vital role in monocyte-macrophage generation, survival, and function and as a regulator of tumor-associated macrophages (TAMs) and tumoral cell growth. Two anti-CSF1R antibodies are being investigated in Phase 1 clinical trials. IMC-CS4 was shown to trigger in vitro depletion of TAMs, and RG7155 (RO5509554) was demonstrated to enhance the efficacy of chemotherapy.
Antibodies directed against the PDGFR family
The PDGF family consists of five isoforms that are homodimers of A-, B-, C-, and D-polypeptide chains, and a heterodimer AB. The five PDGF ligands act via binding to PDGFRα and PDGFRβ. PDGFRα and PDGFRβ associate as homo- or heterodimers, each inducing similar, but not identical, cellular effects. PDGFR has attracted increasing attention as a potential target of anti-tumor therapy for several types of cancer.Citation111 PDGFRα is expressed frequently by tumor-associated stromal cells and by cancer cells in a subset of tumors. Olaratumab, IMC-3G3, a human IgG1 mAb, specifically binds to the human PDGFRα with high affinity, which blocks ligand binding and receptor activation.Citation112 Olaratumab has already been evaluated in several types of cancer in preclinical studies and is currently being evaluated in Phase 2 clinical trials for patients with several types of solid malignancies.Citation113 After a Phase 2 stage on glioblastoma multiforme and NSCLC, the development of tovetumab (MEDI-575), a humanized IgG2 mAb directed against the PDGFRα, was stopped with undisclosed reasons.
Antibodies directed against the FGFR family
The fibroblast growth factor/fibroblast growth factor receptor (FGF/FGFR) signaling pathway plays a fundamental role in many physiological processes by orchestrating angiogenesis. The FGFR cascades play crucial roles in tumor cell proliferation, angiogenesis, migration, and survival, thereby providing a strong rationale for the development of anti-FGF/FGFR agents.Citation114,Citation115 Until the discovery of the different mutation and expression status of each of the FGFR isoforms in cancer, a plethora of small molecule kinase inhibitors, either selective or pan-FGFR (FGFR1–4), are being investigated in clinical trials.Citation116,Citation117 Although anti-FGFR mAbs have been assessed and their efficacy has been demonstrated in preclinical cancer models, little information is available on the tolerability profile of any of these agents. The first FGFR antibody to enter clinical development was the anti-FGFR3 antibody RG7444/MFGR1877S (but its development seems to be paused because the Phase 1 clinical trial was terminated) followed by the anti-FGFR2 antibody BAY 1179470.
Future Emerging Promising Targets in the Preclinical Stage
Because aberrant signaling by RTKs is an etiologic factor in cancer, most (if not almost all) of the other subclasses of RTKs are being studied as potential targets for treating malignancies. For example, the angiopoietin/Tie ligand/receptor system (especially angiopoietin2/Tie-2) emerged as an anti-angiogenic alternative approach to anti-VEGF pathway therapies.Citation118,Citation119 Receptor tyrosine kinase-like orphan receptor (ROR) targeting is anticipated as a safe approach to selectively attack a tumor much more forcefully than healthy tissue.Citation120,Citation121 Anaplastic lymphoma kinase (ALK)-targeted immunotherapy was proposed as a promising therapeutic strategy for cancer therapy.Citation122,Citation123 Currently, the most promising anti-cancer targets appear to be the members of the TAM receptor tyrosine kinase family (Axl, Tyro3 and Mer).Citation124,Citation125 Even if Tyro-3 is an interesting target, in this family, Axl and Mer are particularly under the spotlight,Citation126 and many studies have demonstrated their crucial role in cancer as major players in the tumor microenvironment.Citation127-Citation130 Moreover, both are involved in the drug-resistance process and their inhibition clearly enhances sensitivity of cancer cells to cytotoxic agents.Citation131-Citation134 In this context, joint efforts have been made to preclinically validate the efficiency of anti-Axl antibodies.Citation135-Citation137 Potent therapeutic mAbs targeting Axl are currently in the discovery and preclinical stages of development and demonstrate highly promising results (unpublished in-house data).
Emerging New Approaches to Enhance Treatment Efficacy
Given that most of patients develop resistance within one year after initiation of anti-HER2 or anti-EGFR treatment, there is still a great need for alternative treatments to overcome this acquired resistance.Citation138,Citation139 The molecular mechanisms by which tumor cells may adapt and escape after HER2 or EGFR inhibition are similar: appearance of mutations, upregulation of downstream signaling pathways or activation of alternative pathways.Citation80,Citation140 Extensive crosstalk among receptor kinases suggests that blocking signaling from more than one family member or from different families may be essential to effectively treating cancer and limiting drug resistance. Strategies that should prevent and overcome resistance include targeting several RTKs with the same antibody (bispecific antibodies), targeting the same RTK with different antibodies, or combined targeting of two or more different RTKs.
Bispecific antibodies
The clinical efficacy of bispecific antibodies is currently being investigated (). Among these promising types of antibodies, we can cite the dual EGFR/HER3 duligotumab (MEHD7945A) that presents a greater antitumor potency than cetuximab and retained potent activity in tumors refractory to EGFR inhibitors; the dual HER2/HER3 MM-111 antibody that could prevent the development of HER3-mediated resistance to currently existing anti-HER2 therapies; and the dual IGF1R/HER3 MM-141 antibody that displays enhanced in vitro and in vivo activity relative to inhibitors of either pathway in isolation, and synergizes with targeted and chemotherapies.Citation141-Citation143 Ertumaxomab is a trifunctional antibody (trAb) that bivalently targets HER2 and the T cell specific CD3 antigen, and its Fc region is selectively recognized by immune cells. Ertumaxomab is a promising novel anticancer biologic that links innate with adaptive immunity.Citation144,Citation145 Treatment with ertumaxomab might complement the therapeutic activity of chemotherapy and other anti-HER2/anti-EGFR receptor reagents.Citation146,Citation147
Table 3. Bispecific antibodies targeting at least one human receptor tyrosine kinase that are currently in clinical trials in oncology
Antibody combination
Preclinical data have shown that the use of two or more inhibitors against the same EGFR family member leads to synergistic anti-tumor activity.Citation148,Citation149 This dual-targeting approach has been successfully translated to the clinic with the combination of trastuzumab, pertuzumab and docetaxel for HER2-positive breast cancer. The Sym004 or MM-151 mixtures, previously described in this review, are the lead examples of this category of therapeutics. The synergistic anti-tumoral effect observed by associating antibodies able to target non-overlapping epitopes of a defined RTK is most probably due to their ability to increase the RTK downregulation.Citation150 Although the receptor cell surface expression is reduced, combination therapy seems to be able to further improve the ADCC process as demonstrated in the trastuzumab and pertuzumab combination.Citation151 This approach is currently being evaluated on other RTK family members, including the combination of two antibodies directed against IGF1R.Citation152 Even if this strategy will increase efficacy and overcome acquired resistance due directly to the targeted RTK pathway, it may lack efficacy when the resistance appears by indirect activation of an alternative pathway. Consequently, one of the most promising strategies to fight resistance is to combine antibodies targeting distinct RTK families. IGF1R, MET and AXL pathways are described as the main alternative RTK pathways leading the tumor to become resistant.Citation140 Given that, we could expect higher activity and less resistance combining current anti-EGFR or anti-HER2 therapies with anti-MET, anti-IGF1R or anti-AXL antibodies. With this aim in mind, ganitumab used in combination with panitumumab is currently under evaluation in a Phase 1 clinical trial.
Antibody-drug conjugates
The majority of unconjugated mAbs are used in combination with conventional chemotherapy drugs and radiotherapy. As an example, it is well known that cetuximab enhances the sensitivity of tumor cells to chemotherapy and to radiation. However, this concurrent administration results in interplay and exacerbation between the adverses effects of the individual agents.Citation153 Therefore, significant effort has been devoted to improving mAbs through various modifications to achieve the efficacy of combination treatments while minimizing adverse effects. One idea was to conjugate mAbs either with a radioactive substance (radioimmunotherapy), drug (chemolabeled mAbs), or a cytotoxin (immunotoxins) to generate conjugated mAbs or antibody-drug conjugates (ADCs) capable of antigen-specific delivery of highly potent cytotoxic molecules to tumor cells. ADCs are a niche class of drugs that offer promise, particularly as oncology drugs.Citation154 Several ADCs are already in clinical development for cancer treatment. Ado-trastuzumab-emtansine (Kadcyla®), a chemolabeled antibody, is the first approved ADC targeting a receptor tyrosine kinase (approved in 2013 by the FDA and the EMA). Ado-trastuzumab-emtansine is indicated for the treatment of patients with refractory HER2-positive locally advanced or metastatic breast cancer. Given all of the advantages previously described of targeting RTKs in oncology and the high clinical value of ado-trastuzumab-emtansine, it is not surprising that other ADCs in this domain are being developed (). Until now, the clinical success of immuno-conjugates in oncology has been very limited compared with naked mAbs. However, with the evolution of the research on ADCs, we speculate that the number of ADCs entering clinical trials will rapidly accelerate in the coming years.
Table 4. ADCs targeting human receptor tyrosine kinases that are currently in clinical trials in oncology
Conclusion
In the last decade, due to intensive innovative discovery of novel cancer therapies, high-profile approved agents entered the market. Advances in molecular biology have led to an explosion in the number of potential cancer targets and a rich pipeline of anticancer drugs. However, cancer is an extremely complex set of diseases, both anatomically and molecularly, and with only 15–20% of clinical candidates reaching the market, cancer continues to be an area of high unmet medical need, with many potential cancer targets still lacking drugs and drug resistance remaining as an enduring problem.
Despite the recognized success of the five clinical antibodies currently directed against the receptor tyrosine kinases EGFR and HER2, there are still many remaining issues. Overcoming systemic side effects and the generation of resistance would be a significant improvement for the pharmaceutical industry and for patients. Thus, in addition to new anti-HER antibodies in development, new RTKs involved in tumor progression, angiogenesis or stromal cells are emerging as oncological targets for antibody therapies.
In this review, we decided to focus on unconjugated antibodies that directly target RTKs. However, with the success of bevacizumab (an anti-VEGF antibody developed by Genentech and marketed under the name Avastin®), inhibition of some of these RTKS can also be indirectly accomplished by ligand trapping (for example, antibodies are being developed against HGF, FGF, IGF, and others).Citation155,Citation156 Moreover, although the efficacy of unconjugated antibodies has been demonstrated, molecular genetics and chemical modifications to mAbs have advanced their clinical utility. Derivatives of mAbs, produced by modification of immune effector engagement or by conjugating cytotoxic agents to mAbs, have improved pharmacokinetic profiles and enhanced targeted therapeutic delivery to tumors. The increasing abilities of antibody modifications will continue to make it possible to construct more diverse and efficacious mAb-based therapeutics.
| Abbreviations: | ||
| mAb | = | monoclonal antibody |
| ADCC | = | antibody-dependent cell-mediated cytotoxicity |
| CDC | = | complement-dependent cytotoxicity |
| RTK | = | receptor tyrosine kinase |
| HER | = | human epidermal growth factor receptor |
| FDA | = | U.S. Food and Drug Administration |
| EMA | = | European Medicines Agency |
| INN | = | International Nonproprietary Names |
| ADC | = | antibody-drug conjugate |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jiang X-R, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov 2011; 10:101 - 11; http://dx.doi.org/10.1038/nrd3365; PMID: 21283105
- Sharkey RM, Goldenberg DM. Targeted therapy of cancer: new prospects for antibodies and immunoconjugates. CA Cancer J Clin 2006; 56:226 - 43; http://dx.doi.org/10.3322/canjclin.56.4.226; PMID: 16870998
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127 - 37; http://dx.doi.org/10.1038/35052073; PMID: 11252954
- Roskoski R Jr.. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res 2014; 79:34 - 74; http://dx.doi.org/10.1016/j.phrs.2013.11.002; PMID: 24269963
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007; 357:39 - 51; http://dx.doi.org/10.1056/NEJMra043186; PMID: 17611206
- Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther 1999; 21:309 - 18; http://dx.doi.org/10.1016/S0149-2918(00)88288-0; PMID: 10211534
- Nahta R. Molecular Mechanisms of Trastuzumab-Based Treatment in HER2-Overexpressing Breast Cancer. ISRN Oncol 2012; 2012:428062; http://dx.doi.org/10.5402/2012/428062; PMID: 23227361
- McLarty K, Cornelissen B, Cai Z, Scollard DA, Costantini DL, Done SJ, Reilly RM. Micro-SPECT/CT with 111In-DTPA-pertuzumab sensitively detects trastuzumab-mediated HER2 downregulation and tumor response in athymic mice bearing MDA-MB-361 human breast cancer xenografts. J Nucl Med 2009; 50:1340 - 8; http://dx.doi.org/10.2967/jnumed.109.062224; PMID: 19617342
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther 2011; 11:263 - 75; http://dx.doi.org/10.1586/era.10.226; PMID: 21342044
- Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, Dennis PA, Lipkowitz S. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res 2001; 61:4892 - 900; PMID: 11406568
- Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism?. Br J Cancer 2006; 94:259 - 67; http://dx.doi.org/10.1038/sj.bjc.6602930; PMID: 16404427
- Prang N, Preithner S, Brischwein K, Göster P, Wöppel A, Müller J, Steiger C, Peters M, Baeuerle PA, da Silva AJ. Cellular and complement-dependent cytotoxicity of Ep-CAM-specific monoclonal antibody MT201 against breast cancer cell lines. Br J Cancer 2005; 92:342 - 9; PMID: 15655555
- Kumar R, Mandal M, Vadlamudi R. New insights into anti-HER-2 receptor monoclonal antibody research. Semin Oncol 2000; 27:Suppl 11 84 - 91, discussion 92-100; PMID: 11236033
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res 2002; 62:4132 - 41; PMID: 12124352
- Emlet DR, Brown KA, Kociban DL, Pollice AA, Smith CA, Ong BBL, Shackney SE. Response to trastuzumab, erlotinib, and bevacizumab, alone and in combination, is correlated with the level of human epidermal growth factor receptor-2 expression in human breast cancer cell lines. Mol Cancer Ther 2007; 6:2664 - 74; http://dx.doi.org/10.1158/1535-7163.MCT-07-0079; PMID: 17938260
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 2009; 9:463 - 75; http://dx.doi.org/10.1038/nrc2656; PMID: 19536107
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002; 2:127 - 37; http://dx.doi.org/10.1016/S1535-6108(02)00097-1; PMID: 12204533
- Adams CW, Allison DE, Flagella K, Presta L, Clarke J, Dybdal N, McKeever K, Sliwkowski MX. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother 2006; 55:717 - 27; http://dx.doi.org/10.1007/s00262-005-0058-x; PMID: 16151804
- Hughes JB, Berger C, Rødland MS, Hasmann M, Stang E, Madshus IH. Pertuzumab increases epidermal growth factor receptor down-regulation by counteracting epidermal growth factor receptor-ErbB2 heterodimerization. Mol Cancer Ther 2009; 8:1885 - 92; http://dx.doi.org/10.1158/1535-7163.MCT-09-0291; PMID: 19584234
- Metzger-Filho O, Winer EP, Krop I. Pertuzumab: optimizing HER2 blockade. Clin Cancer Res 2013; 19:5552 - 6; http://dx.doi.org/10.1158/1078-0432.CCR-13-0518; PMID: 23942091
- Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 2008; 68:5878 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-08-0380; PMID: 18632642
- Tanner B, Hasenclever D, Stern K, Schormann W, Bezler M, Hermes M, Brulport M, Bauer A, Schiffer IB, Gebhard S, et al. ErbB-3 predicts survival in ovarian cancer. J Clin Oncol 2006; 24:4317 - 23; http://dx.doi.org/10.1200/JCO.2005.04.8397; PMID: 16896008
- Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009; 69:9330 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-08-4597; PMID: 19934333
- Sak MM, Szymanska M, Bertelsen V, Hasmann M, Madshus IH, Stang E. Pertuzumab counteracts the inhibitory effect of ErbB2 on degradation of ErbB3. Carcinogenesis 2013; 34:2031 - 8; http://dx.doi.org/10.1093/carcin/bgt173; PMID: 23698633
- Fuentes G, Scaltriti M, Baselga J, Verma CS. Synergy between trastuzumab and pertuzumab for human epidermal growth factor 2 (Her2) from colocalization: an in silico based mechanism. Breast Cancer Res 2011; 13:R54; http://dx.doi.org/10.1186/bcr2888; PMID: 21600050
- Kao H-F, Lin C-C, Yang JC-H. EGFR inhibitors as the first-line systemic treatment for advanced non-small-cell lung cancer. Future Oncol 2013; 9:991 - 1003; http://dx.doi.org/10.2217/fon.13.56; PMID: 23837762
- Warnault P, Yasri A, Coisy-Quivy M, Chevé G, Boriès C, Fauvel B, Benhida R. Recent advances in drug design of epidermal growth factor receptor inhibitors. Curr Med Chem 2013; 20:2043 - 67; http://dx.doi.org/10.2174/0929867311320160001; PMID: 23410174
- Krawczyk P, Chocholska S, Milanowski J. Anti-HER therapeutic agents in the treatment of non-small-cell lung cancer. Ann Univ Mariae Curie Sklodowska Med 2003; 58:113 - 7; PMID: 15314969
- Patel D, Bassi R, Hooper A, Prewett M, Hicklin DJ, Kang X. Anti-epidermal growth factor receptor monoclonal antibody cetuximab inhibits EGFR/HER-2 heterodimerization and activation. Int J Oncol 2009; 34:25 - 32; PMID: 19082474
- Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1995; 1:1311 - 8; PMID: 9815926
- Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CP. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res 1999; 5:257 - 65; PMID: 10037173
- Kimura H, Sakai K, Arao T, Shimoyama T, Tamura T, Nishio K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci 2007; 98:1275 - 80; http://dx.doi.org/10.1111/j.1349-7006.2007.00510.x; PMID: 17498200
- Hsu Y-F, Ajona D, Corrales L, Lopez-Picazo JM, Gurpide A, Montuenga LM, Pio R. Complement activation mediates cetuximab inhibition of non-small cell lung cancer tumor growth in vivo. Mol Cancer 2010; 9:139; http://dx.doi.org/10.1186/1476-4598-9-139; PMID: 20529262
- Roda JM, Joshi T, Butchar JP, McAlees JW, Lehman A, Tridandapani S, Carson WE 3rd. The activation of natural killer cell effector functions by cetuximab-coated, epidermal growth factor receptor positive tumor cells is enhanced by cytokines. Clin Cancer Res 2007; 13:6419 - 28; http://dx.doi.org/10.1158/1078-0432.CCR-07-0865; PMID: 17962339
- Ashraf SQ, Nicholls AM, Wilding JL, Ntouroupi TG, Mortensen NJ, Bodmer WF. Direct and immune mediated antibody targeting of ERBB receptors in a colorectal cancer cell-line panel. Proc Natl Acad Sci U S A 2012; 109:21046 - 51; http://dx.doi.org/10.1073/pnas.1218750110; PMID: 23213241
- Eller JL, Longo SL, Hicklin DJ, Canute GW. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery 2002; 51:1005 - 13, discussion 1013-4; PMID: 12234411
- Hotz B, Keilholz U, Fusi A, Buhr HJ, Hotz HG. In vitro and in vivo antitumor activity of cetuximab in human gastric cancer cell lines in relation to epidermal growth factor receptor (EGFR) expression and mutational phenotype. Gastric Cancer 2012; 15:252 - 64; http://dx.doi.org/10.1007/s10120-011-0102-9; PMID: 22011788
- Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res 1999; 59:1935 - 40; PMID: 10213503
- Modest DP, Stintzing S, Laubender RP, Neumann J, Jung A, Giessen C, Haas M, Aubele P, Schulz C, Boeck S, et al. Clinical characterization of patients with metastatic colorectal cancer depending on the KRAS status. Anticancer Drugs 2011; 22:913 - 8; http://dx.doi.org/10.1097/CAD.0b013e3283493160; PMID: 21795973
- Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne C-H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012; 48:1466 - 75; http://dx.doi.org/10.1016/j.ejca.2012.02.057; PMID: 22446022
- Yang B-B, Lum P, Chen A, Arends R, Roskos L, Smith B, Pérez Ruixo JJ. Pharmacokinetic and pharmacodynamic perspectives on the clinical drug development of panitumumab. Clin Pharmacokinet 2010; 49:729 - 40; http://dx.doi.org/10.2165/11535970-000000000-00000; PMID: 20923247
- Giannopoulou E, Antonacopoulou A, Matsouka P, Kalofonos HP. Autophagy: novel action of panitumumab in colon cancer. Anticancer Res 2009; 29:5077 - 82; PMID: 20044619
- Foon KA, Yang X-D, Weiner LM, Belldegrun AS, Figlin RA, Crawford J, Rowinsky EK, Dutcher JP, Vogelzang NJ, Gollub J, et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys 2004; 58:984 - 90; http://dx.doi.org/10.1016/j.ijrobp.2003.09.098; PMID: 14967460
- Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, Lammerts van Bueren JJ, Bleeker WK, Parren PWHI, van de Winkel JGJ, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res 2008; 68:4998 - 5003; http://dx.doi.org/10.1158/0008-5472.CAN-07-6226; PMID: 18593896
- Patel D, Guo X, Ng S, Melchior M, Balderes P, Burtrum D, Persaud K, Luna X, Ludwig DL, Kang X. IgG isotype, glycosylation, and EGFR expression determine the induction of antibody-dependent cellular cytotoxicity in vitro by cetuximab. Hum Antibodies 2010; 19:89 - 99; PMID: 21178280
- Schneider-Merck T, Lammerts van Bueren JJ, Berger S, Rossen K, van Berkel PHC, Derer S, Beyer T, Lohse S, Bleeker WK, Peipp M, et al. Human IgG2 antibodies against epidermal growth factor receptor effectively trigger antibody-dependent cellular cytotoxicity but, in contrast to IgG1, only by cells of myeloid lineage. J Immunol 2010; 184:512 - 20; http://dx.doi.org/10.4049/jimmunol.0900847; PMID: 19949082
- Koch T, Derer S, Staudinger M, Rossen K, Glorius P, Peipp M, Kellner C, Kunzendorf U, Valerius T, Dechant M. Antibody-dependent cellular cytotoxicity in patients on chronic hemodialysis. Am J Nephrol 2013; 38:379 - 87; http://dx.doi.org/10.1159/000355972; PMID: 24157422
- Doi T, Tahara M, Yoshino T, Yamazaki K, Tamura T, Yamada Y, Yang B-B, Oliner KS, Otani S, Asahi D. Tumor KRAS status predicts responsiveness to panitumumab in Japanese patients with metastatic colorectal cancer. Jpn J Clin Oncol 2011; 41:210 - 6; http://dx.doi.org/10.1093/jjco/hyq229; PMID: 21169348
- Hocking CM, Price TJ. Panitumumab in the management of patients with KRAS wild-type metastatic colorectal cancer. Therap Adv Gastroenterol 2014; 7:20 - 37; http://dx.doi.org/10.1177/1756283X13498660; PMID: 24381645
- Kumar SS, Price TJ, Mohyieldin O, Borg M, Townsend A, Hardingham JE. KRAS G13D Mutation and Sensitivity to Cetuximab or Panitumumab in a Colorectal Cancer Cell Line Model. Gastrointest Cancer Res 2014; 7:23 - 6; PMID: 24558511
- Crombet-Ramos T, Rak J, Pérez R, Viloria-Petit A. Antiproliferative, antiangiogenic and proapoptotic activity of h-R3: A humanized anti-EGFR antibody. Int J Cancer 2002; 101:567 - 75; http://dx.doi.org/10.1002/ijc.10647; PMID: 12237899
- Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther 2009; 9:1199 - 206; http://dx.doi.org/10.1517/14712590903110709; PMID: 19624281
- Garrido G, Tikhomirov IA, Rabasa A, Yang E, Gracia E, Iznaga N, Fernández LE, Crombet T, Kerbel RS, Pérez R. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther 2011; 11:373 - 82; http://dx.doi.org/10.4161/cbt.11.4.14097; PMID: 21150278
- Teresa Solomon M, Miranda N, Jorrín E, Chong I, Marinello JJ, Alert J, Lorenzo-Luaces P, Crombet T. Nimotuzumab in combination with radiotherapy in high grade glioma patients: A single institution experience. Cancer Biol Ther 2014; 15:504 - 9
- Su D, Jiao S-C, Wang L-J, Shi W-W, Long Y-Y, Li J, Bai L. Efficacy of nimotuzumab plus gemcitabine usage as first-line treatment in patients with advanced pancreatic cancer. Tumour Biol 2014; 35:2313 - 8; http://dx.doi.org/10.1007/s13277-013-1306-x; PMID: 24142531
- Gala K, Chandarlapaty S. Molecular Pathways: HER3 Targeted Therapy. Clin Cancer Res Off J Am Assoc Cancer Res 2014
- Li S, Kussie P, Ferguson KM. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure 2008; 16:216 - 27; http://dx.doi.org/10.1016/j.str.2007.11.009; PMID: 18275813
- Dienstmann R, Tabernero J. Necitumumab, a fully human IgG1 mAb directed against the EGFR for the potential treatment of cancer. Curr Opin Investig Drugs 2010; 11:1434 - 41; PMID: 21154125
- Garrett TPJ, Burgess AW, Gan HK, Luwor RB, Cartwright G, Walker F, Orchard SG, Clayton AHA, Nice EC, Rothacker J, et al. Antibodies specifically targeting a locally misfolded region of tumor associated EGFR. Proc Natl Acad Sci U S A 2009; 106:5082 - 7; http://dx.doi.org/10.1073/pnas.0811559106; PMID: 19289842
- Gan HK, Burgess AW, Clayton AHA, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res 2012; 72:2924 - 30; http://dx.doi.org/10.1158/0008-5472.CAN-11-3898; PMID: 22659454
- Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70:588 - 97; http://dx.doi.org/10.1158/0008-5472.CAN-09-1417; PMID: 20068188
- Iida M, Brand TM, Starr MM, Li C, Huppert EJ, Luthar N, Pedersen MW, Horak ID, Kragh M, Wheeler DL. Sym004, a novel EGFR antibody mixture, can overcome acquired resistance to cetuximab. Neoplasia 2013; 15:1196 - 206; PMID: 24204198
- Nordstrom JL, Gorlatov S, Zhang W, Yang Y, Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res 2011; 13:R123; http://dx.doi.org/10.1186/bcr3069; PMID: 22129105
- Schoeberl B, Faber AC, Li D, Liang M-C, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res 2010; 70:2485 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-09-3145; PMID: 20215504
- Garrett JT, Sutton CR, Kuba MG, Cook RS, Arteaga CL. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clin Cancer Res 2013; 19:610 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-12-2024; PMID: 23224399
- Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, Sineshchekova O, Saxena P, Sutton CR, Chen D, et al. An antibody that locks HER3 in the inactive conformation inhibits tumor growth driven by HER2 or neuregulin. Cancer Res 2013; 73:6024 - 35; http://dx.doi.org/10.1158/0008-5472.CAN-13-1198; PMID: 23928993
- Garrett JT, Sutton CR, Kurupi R, Bialucha CU, Ettenberg SA, Collins SD, Sheng Q, Wallweber J, Defazio-Eli L, Arteaga CL. Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers. Cancer Res 2013; 73:6013 - 23; http://dx.doi.org/10.1158/0008-5472.CAN-13-1191; PMID: 23918797
- Zhang L, Luan B, Yang K, Castanaro C, Papadopoulos N, Thurston G, Daly C. Abstract 2718: REGN1400, a fully-human ERBB3 antibody, potently inhibits tumor growth in preclinical models, both as a monotherapy and in combination with EGFR or HER2 blockers. Cancer Res 2012; 72:2718 - 2718; http://dx.doi.org/10.1158/1538-7445.AM2012-2718
- Huang J, Wang S, Lyu H, Cai B, Yang X, Wang J, Liu B. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer 2013; 12:134; http://dx.doi.org/10.1186/1476-4598-12-134; PMID: 24215614
- Wang S, Huang J, Lyu H, Cai B, Yang X, Li F, Tan J, Edgerton SM, Thor AD, Lee C-K, et al. Therapeutic targeting of erbB3 with MM-121/SAR256212 enhances antitumor activity of paclitaxel against erbB2-overexpressing breast cancer. Breast Cancer Res 2013; 15:R101; http://dx.doi.org/10.1186/bcr3563; PMID: 24168763
- Mirschberger C, Schiller CB, Schräml M, Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G, Kolm I, et al. RG7116, a therapeutic antibody that binds the inactive HER3 receptor and is optimized for immune effector activation. Cancer Res 2013; 73:5183 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-13-0099; PMID: 23780344
- Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev 2013; 39:793 - 801; http://dx.doi.org/10.1016/j.ctrv.2013.02.001; PMID: 23453860
- Vearing C, Lee F-T, Wimmer-Kleikamp S, Spirkoska V, To C, Stylianou C, Spanevello M, Brechbiel M, Boyd AW, Scott AM, et al. Concurrent binding of anti-EphA3 antibody and ephrin-A5 amplifies EphA3 signaling and downstream responses: potential as EphA3-specific tumor-targeting reagents. Cancer Res 2005; 65:6745 - 54; http://dx.doi.org/10.1158/0008-5472.CAN-05-0758; PMID: 16061656
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003; 4:915 - 25; http://dx.doi.org/10.1038/nrm1261; PMID: 14685170
- Rosário M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol 2003; 13:328 - 35; http://dx.doi.org/10.1016/S0962-8924(03)00104-1; PMID: 12791299
- Davis IJ, McFadden AW, Zhang Y, Coxon A, Burgess TL, Wagner AJ, Fisher DE. Identification of the receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor, as therapeutic targets in clear cell sarcoma. Cancer Res 2010; 70:639 - 45; http://dx.doi.org/10.1158/0008-5472.CAN-09-1121; PMID: 20068147
- Jin H, Yang R, Zheng Z, Romero M, Ross J, Bou-Reslan H, Carano RAD, Kasman I, Mai E, Young J, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res 2008; 68:4360 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-5960; PMID: 18519697
- Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, Huang A, Yang NY, Nishimura M, Greve J, et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A 2013; 110:E2987 - 96; http://dx.doi.org/10.1073/pnas.1302725110; PMID: 23882082
- Roche provides update on phase III study of onartuzumab in people with specific type of lung cancer [Internet]. 2014. Available from: http://www.roche.com/media/media_releases/med-cor-2014-03-03.htm
- Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med 2013; 19:1389 - 400; http://dx.doi.org/10.1038/nm.3388; PMID: 24202392
- Goetsch L, Broussas M, Fabre-Lafay S, Robert A, Lepecquet A-M, Gonzalez A, Wurch T, Bailly C, Corvaia N. Abstract 2448: h224G11, a humanized whole antibody targeting the c-Met receptor, induces c-Met down-regulation and triggers ADCC functions. Cancer Res 2011; 70:2448 - 2448; http://dx.doi.org/10.1158/1538-7445.AM10-2448
- Zeng W, Yan L, Peek V, Wortinger M, Tetreault J, Xia J, Chow C-K, Manro JR, Stephens JR, Weir SN, et al. Abstract 2734: c-Met antibody LY2875358 (LA480) shows differential antitumor effects in non-small cell lung cancer. Cancer Res 2012; 72:2734 - 2734; http://dx.doi.org/10.1158/1538-7445.AM2012-2734
- Goetsch L, Gonzalez A, Geronimi F, Fabre-Lafay S, Broussas M, Lepecquet A-M, Wurch T. corvaia N, Bailly C. Abstract B127: Single or combined in vivo therapies of cancer with h224G11, a humanized antibody targeting the c-Met receptor. Mol Cancer Ther 2009; 8:B127 - 127; http://dx.doi.org/10.1158/1535-7163.TARG-09-B127
- Yao H-P, Zhou Y-Q, Zhang R, Wang M-H. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer 2013; 13:466 - 81; http://dx.doi.org/10.1038/nrc3545; PMID: 23792360
- Wang M-H, Padhye SS, Guin S, Ma Q, Zhou YQ. Potential therapeutics specific to c-MET/RON receptor tyrosine kinases for molecular targeting in cancer therapy. Acta Pharmacol Sin 2010; 31:1181 - 8; http://dx.doi.org/10.1038/aps.2010.106; PMID: 20694025
- Zou Y, Howell GM, Humphrey LE, Wang J, Brattain MG. Ron knockdown and Ron monoclonal antibody IMC-RON8 sensitize pancreatic cancer to histone deacetylase inhibitors (HDACi). PLoS One 2013; 8:e69992; http://dx.doi.org/10.1371/journal.pone.0069992; PMID: 23922886
- Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther 2007; 6:1 - 12; http://dx.doi.org/10.1158/1535-7163.MCT-06-0080; PMID: 17237261
- Werner H, LeRoith D. The role of the insulin-like growth factor system in human cancer. Adv Cancer Res 1996; 68:183 - 223; http://dx.doi.org/10.1016/S0065-230X(08)60354-1; PMID: 8712068
- Grothey A, Voigt W, Schöber C, Müller T, Dempke W, Schmoll HJ. The role of insulin-like growth factor I and its receptor in cell growth, transformation, apoptosis, and chemoresistance in solid tumors. J Cancer Res Clin Oncol 1999; 125:166 - 73; http://dx.doi.org/10.1007/s004320050259; PMID: 10235470
- Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater?. J Natl Cancer Inst 2012; 104:975 - 81; http://dx.doi.org/10.1093/jnci/djs258; PMID: 22761272
- Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res 2007; 13:5549s - 55s; http://dx.doi.org/10.1158/1078-0432.CCR-07-1109; PMID: 17875788
- Beltran PJ, Mitchell P, Chung Y-A, Cajulis E, Lu J, Belmontes B, Ho J, Tsai MM, Zhu M, Vonderfecht S, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther 2009; 8:1095 - 105; http://dx.doi.org/10.1158/1535-7163.MCT-08-1171; PMID: 19366899
- Gao J, Chang YS, Jallal B, Viner J. Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Res 2012; 72:3 - 12; http://dx.doi.org/10.1158/0008-5472.CAN-11-0550; PMID: 22215692
- Scartozzi M, Bianconi M, Maccaroni E, Giampieri R, Berardi R, Cascinu S. Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer. Curr Opin Mol Ther 2010; 12:361 - 71; PMID: 20521225
- Wykosky J, Debinski W. The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol Cancer Res 2008; 6:1795 - 806; http://dx.doi.org/10.1158/1541-7786.MCR-08-0244; PMID: 19074825
- Xi H-Q, Wu X-S, Wei B, Chen L. Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med 2012; 16:2894 - 909; http://dx.doi.org/10.1111/j.1582-4934.2012.01612.x; PMID: 22862837
- Nievergall E, Saunders T, Lackmann M. Targeting of EPH receptor tyrosine kinases for anticancer therapy. Crit Rev Oncog 2012; 17:211 - 32; http://dx.doi.org/10.1615/CritRevOncog.v17.i2.60; PMID: 22471709
- Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov 2014; 13:39 - 62; http://dx.doi.org/10.1038/nrd4175; PMID: 24378802
- Clarke JM, Hurwitz HI. Targeted inhibition of VEGF receptor 2: an update on ramucirumab. Expert Opin Biol Ther 2013; 13:1187 - 96; http://dx.doi.org/10.1517/14712598.2013.810717; PMID: 23803182
- Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem 2013; 153:13 - 9; http://dx.doi.org/10.1093/jb/mvs136; PMID: 23172303
- Schwartz JD, Rowinsky EK, Youssoufian H, Pytowski B, Wu Y. Vascular endothelial growth factor receptor-1 in human cancer: concise review and rationale for development of IMC-18F1 (Human antibody targeting vascular endothelial growth factor receptor-1). Cancer 2010; 116:Suppl 1027 - 32; http://dx.doi.org/10.1002/cncr.24789; PMID: 20127948
- Krupitskaya Y, Wakelee HA. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr Opin Investig Drugs 2009; 10:597 - 605; PMID: 19513949
- Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs 2009; 23:289 - 304; http://dx.doi.org/10.2165/11317600-000000000-00000; PMID: 19754219
- Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, et al, REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014; 383:31 - 9; http://dx.doi.org/10.1016/S0140-6736(13)61719-5; PMID: 24094768
- Garcia JA, Hudes GR, Choueiri TK, Stadler WM, Wood LS, Gurtler J, Bhatia S, Joshi A, Hozak RR, Xu Y, et al. A phase 2, single-arm study of ramucirumab in patients with metastatic renal cell carcinoma with disease progression on or intolerance to tyrosine kinase inhibitor therapy. Cancer 2014; Forthcoming http://dx.doi.org/10.1002/cncr.28634; PMID: 24577874
- Lee SH. Tanibirumab (TTAC-0001): a fully human monoclonal antibody targets vascular endothelial growth factor receptor 2 (VEGFR-2). Arch Pharm Res 2011; 34:1223 - 6; http://dx.doi.org/10.1007/s12272-011-0821-9; PMID: 21910042
- Tvorogov D, Anisimov A, Zheng W, Leppänen V-M, Tammela T, Laurinavicius S, Holnthoner W, Heloterä H, Holopainen T, Jeltsch M, et al. Effective suppression of vascular network formation by combination of antibodies blocking VEGFR ligand binding and receptor dimerization. Cancer Cell 2010; 18:630 - 40; http://dx.doi.org/10.1016/j.ccr.2010.11.001; PMID: 21130043
- Persaud K, Tille J-C, Liu M, Zhu Z, Jimenez X, Pereira DS, Miao H-Q, Brennan LA, Witte L, Pepper MS, et al. Involvement of the VEGF receptor 3 in tubular morphogenesis demonstrated with a human anti-human VEGFR-3 monoclonal antibody that antagonizes receptor activation by VEGF-C. J Cell Sci 2004; 117:2745 - 56; http://dx.doi.org/10.1242/jcs.01138; PMID: 15150322
- Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmökel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J 2007; 21:1003 - 12; http://dx.doi.org/10.1096/fj.06-6656com; PMID: 17210781
- Liang J, Wu Y-L, Chen B-J, Zhang W, Tanaka Y, Sugiyama H. The C-kit receptor-mediated signal transduction and tumor-related diseases. Int J Biol Sci 2013; 9:435 - 43; http://dx.doi.org/10.7150/ijbs.6087; PMID: 23678293
- Ostman A, Heldin C-H. PDGF receptors as targets in tumor treatment. Adv Cancer Res 2007; 97:247 - 74; http://dx.doi.org/10.1016/S0065-230X(06)97011-0; PMID: 17419949
- Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer 2010; 116:Suppl 1018 - 26; http://dx.doi.org/10.1002/cncr.24788; PMID: 20127943
- Chiorean EG, Sweeney C, Youssoufian H, Qin A, Dontabhaktuni A, Loizos N, Nippgen J, Amato R. A phase I study of olaratumab, an anti-platelet-derived growth factor receptor alpha (PDGFRα) monoclonal antibody, in patients with advanced solid tumors. Cancer Chemother Pharmacol 2014; 73:595 - 604; http://dx.doi.org/10.1007/s00280-014-2389-9; PMID: 24452395
- Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov 2013; 3:264 - 79; http://dx.doi.org/10.1158/2159-8290.CD-12-0362; PMID: 23418312
- Liang G, Liu Z, Wu J, Cai Y, Li X. Anticancer molecules targeting fibroblast growth factor receptors. Trends Pharmacol Sci 2012; 33:531 - 41; http://dx.doi.org/10.1016/j.tips.2012.07.001; PMID: 22884522
- Knights V, Cook SJ. De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol Ther 2010; 125:105 - 17; http://dx.doi.org/10.1016/j.pharmthera.2009.10.001; PMID: 19874848
- Tiong KH, Mah LY, Leong C-O. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis 2013; 18:1447 - 68; http://dx.doi.org/10.1007/s10495-013-0886-7; PMID: 23900974
- Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 2013; 73:1649 - 57; http://dx.doi.org/10.1158/0008-5472.CAN-12-4697; PMID: 23467610
- Eroglu Z, Stein CA, Pal SK. Targeting angiopoietin-2 signaling in cancer therapy. Expert Opin Investig Drugs 2013; 22:813 - 25; http://dx.doi.org/10.1517/13543784.2013.793306; PMID: 23621441
- Daneshmanesh AH, Mikaelsson E, Jeddi-Tehrani M, Bayat AA, Ghods R, Ostadkarampour M, Akhondi M, Lagercrantz S, Larsson C, Osterborg A, et al. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int J Cancer 2008; 123:1190 - 5; http://dx.doi.org/10.1002/ijc.23587; PMID: 18546292
- Rebagay G, Yan S, Liu C, Cheung N-K. ROR1 and ROR2 in Human Malignancies: Potentials for Targeted Therapy. Front Oncol 2012; 2:34; http://dx.doi.org/10.3389/fonc.2012.00034; PMID: 22655270
- Carpenter EL, Haglund EA, Mace EM, Deng D, Martinez D, Wood AC, Chow AK, Weiser DA, Belcastro LT, Winter C, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene 2012; 31:4859 - 67; http://dx.doi.org/10.1038/onc.2011.647; PMID: 22266870
- Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer 2013; 13:685 - 700; http://dx.doi.org/10.1038/nrc3580; PMID: 24060861
- Linger RMA, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res 2008; 100:35 - 83; http://dx.doi.org/10.1016/S0065-230X(08)00002-X; PMID: 18620092
- Nguyen K-Q, Tsou W-I, Kotenko S, Birge RB. TAM receptors in apoptotic cell clearance, autoimmunity, and cancer. Autoimmunity 2013; 46:294 - 7; http://dx.doi.org/10.3109/08916934.2013.794515; PMID: 23662598
- Demarest SJ, Gardner J, Vendel MC, Ailor E, Szak S, Huang F, Doern A, Tan X, Yang W, Grueneberg DA, et al. Evaluation of Tyro3 expression, Gas6-mediated Akt phosphorylation, and the impact of anti-Tyro3 antibodies in melanoma cell lines. Biochemistry 2013; 52:3102 - 18; http://dx.doi.org/10.1021/bi301588c; PMID: 23570341
- Linger RMA, Keating AK, Earp HS, Graham DK. Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets 2010; 14:1073 - 90; http://dx.doi.org/10.1517/14728222.2010.515980; PMID: 20809868
- Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther 2011; 10:1763 - 73; http://dx.doi.org/10.1158/1535-7163.MCT-11-0116; PMID: 21933973
- Cook RS, Jacobsen KM, Wofford AM, DeRyckere D, Stanford J, Prieto AL, Redente E, Sandahl M, Hunter DM, Strunk KE, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. J Clin Invest 2013; 123:3231 - 42; http://dx.doi.org/10.1172/JCI67655; PMID: 23867499
- Cummings CT, Deryckere D, Earp HS, Graham DK. Molecular pathways: MERTK signaling in cancer. Clin Cancer Res 2013; 19:5275 - 80; http://dx.doi.org/10.1158/1078-0432.CCR-12-1451; PMID: 23833304
- Linger RMA, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DHG, Lu X, Barón AE, Franklin WA, Merrick DT, et al. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene 2013; 32:3420 - 31; http://dx.doi.org/10.1038/onc.2012.355; PMID: 22890323
- Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK, Yoon S-J, Park BM, Park E, Bae JH, Choi C-M, et al. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res 2014; 74:253 - 62; http://dx.doi.org/10.1158/0008-5472.CAN-13-1103; PMID: 24165158
- Dufies M, Jacquel A, Belhacene N, Robert G, Cluzeau T, Luciano F, Cassuto JP, Raynaud S, Auberger P. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget 2011; 2:874 - 85; PMID: 22141136
- Hong C-C, Lay J-D, Huang J-S, Cheng A-L, Tang J-L, Lin M-T, Lai G-M, Chuang S-E. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 2008; 268:314 - 24; http://dx.doi.org/10.1016/j.canlet.2008.04.017; PMID: 18502572
- Li Y, Ye X, Tan C, Hongo J-A, Zha J, Liu J, Kallop D, Ludlam MJC, Pei L. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 2009; 28:3442 - 55; http://dx.doi.org/10.1038/onc.2009.212; PMID: 19633687
- Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, Weimer R, Wu Y, Pei L. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 2010; 29:5254 - 64; http://dx.doi.org/10.1038/onc.2010.268; PMID: 20603615
- Leconet W, Larbouret C, Chardès T, Thomas G, Neiveyans M, Busson M, Jarlier M, Radosevic-Robin N, Pugnière M, Bernex F, et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene 2013; Forthcoming http://dx.doi.org/10.1038/onc.2013.487; PMID: 24240689
- Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 2010; 7:493 - 507; http://dx.doi.org/10.1038/nrclinonc.2010.97; PMID: 20551942
- Pohlmann PR, Mayer IA, Mernaugh R. Resistance to Trastuzumab in Breast Cancer. Clin Cancer Res 2009; 15:7479 - 91; http://dx.doi.org/10.1158/1078-0432.CCR-09-0636; PMID: 20008848
- Thery J-C, Spano J-P, Azria D, Raymond E, Penault Llorca F. Resistance to human epidermal growth factor receptor type 2-targeted therapies. Eur J Cancer 2014; 50:892 - 901; http://dx.doi.org/10.1016/j.ejca.2014.01.003; PMID: 24462377
- Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20:472 - 86; http://dx.doi.org/10.1016/j.ccr.2011.09.003; PMID: 22014573
- Huang S, Li C, Armstrong EA, Peet CR, Saker J, Amler LC, Sliwkowski MX, Harari PM. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res 2013; 73:824 - 33; http://dx.doi.org/10.1158/0008-5472.CAN-12-1611; PMID: 23172311
- Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, Rimkunas V, Xu L, Kohli N, Rennard R, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther 2014; 13:410 - 25; http://dx.doi.org/10.1158/1535-7163.MCT-13-0255; PMID: 24282274
- Stemmler HJ, Salat C, Lindhofer H, Menzel H, Untch M, Kahlert S, Konecny G, Sauer H, Ledderose G, Heinemann V, et al. Combined treatment of metastatic breast cancer (MBC) by high-dose chemotherapy (HDCT) and bispecific antibodies: a pilot study. Anticancer Res 2005; 25:3047 - 54; PMID: 16080564
- Hess J, Ruf P, Lindhofer H. Cancer therapy with trifunctional antibodies: linking innate and adaptive immunity. Future Oncol 2012; 8:73 - 85; http://dx.doi.org/10.2217/fon.11.138; PMID: 22149036
- Diermeier-Daucher S, Ortmann O, Buchholz S, Brockhoff G. Trifunctional antibody ertumaxomab: Non-immunological effects on Her2 receptor activity and downstream signaling. MAbs 2012; 4:614 - 22; http://dx.doi.org/10.4161/mabs.21003; PMID: 22820509
- Schroeder P, Lindemann C, Dettmar K, Brieger J, Gosepath J, Pogorzelski B, Seimetz D, Atz J. Trifunctional antibodies induce efficient antitumour activity with immune cells from head and neck squamous cell carcinoma patients after radio-chemotherapy treatment. Clin Transl Oncol 2011; 13:889 - 98; http://dx.doi.org/10.1007/s12094-011-0751-5; PMID: 22126733
- Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer 2013; 13:663 - 73; http://dx.doi.org/10.1038/nrc3559; PMID: 23949426
- Emde A, Pradeep C-R, Ferraro DA, Ben-Chetrit N, Sela M, Ribba B, Kam Z, Yarden Y. Combining epitope-distinct antibodies to HER2: cooperative inhibitory effects on invasive growth. Oncogene 2011; 30:1631 - 42; http://dx.doi.org/10.1038/onc.2010.547; PMID: 21132012
- Spangler JB, Neil JR, Abramovitch S, Yarden Y, White FM, Lauffenburger DA, Wittrup KD. Combination antibody treatment down-regulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl Acad Sci U S A 2010; 107:13252 - 7; http://dx.doi.org/10.1073/pnas.0913476107; PMID: 20616078
- Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res 2011; 17:5060 - 70; http://dx.doi.org/10.1158/1078-0432.CCR-10-2927; PMID: 21700765
- Dong J, Demarest SJ, Sereno A, Tamraz S, Langley E, Doern A, Snipas T, Perron K, Joseph I, Glaser SM, et al. Combination of two insulin-like growth factor-I receptor inhibitory antibodies targeting distinct epitopes leads to an enhanced antitumor response. Mol Cancer Ther 2010; 9:2593 - 604; http://dx.doi.org/10.1158/1535-7163.MCT-09-1018; PMID: 20716637
- Cante D, La Porta MR, Franco P, Sciacero P, Girelli GF, Marra A, Numico G, Denaro N, Russi EG, Ricardi U. Management of ‘in-field’ skin toxicity in head and neck cancer patients treated with combined cetuximab and radiotherapy. Oncology 2013; 85:257 - 61; http://dx.doi.org/10.1159/000355579; PMID: 24192693
- Drachman JG, Senter PD. Antibody-drug conjugates: the chemistry behind empowering antibodies to fight cancer. Hematology Am Soc Hematol Educ Program 2013; 2013:306 - 10; http://dx.doi.org/10.1182/asheducation-2013.1.306; PMID: 24319196
- Burgess T, Coxon A, Meyer S, Sun J, Rex K, Tsuruda T, Chen Q, Ho S-Y, Li L, Kaufman S, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res 2006; 66:1721 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-05-3329; PMID: 16452232
- Gao J, Chesebrough JW, Cartlidge SA, Ricketts S-A, Incognito L, Veldman-Jones M, Blakey DC, Tabrizi M, Jallal B, Trail PA, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res 2011; 71:1029 - 40; http://dx.doi.org/10.1158/0008-5472.CAN-10-2274; PMID: 21245093
