Abstract
The Janus-faced roles of macrophages in cancer imply both tumor-suppressive and -stimulating actions of these innate immune cells. Whereas the balance is toward tumor promotion in most epithelial cancers, we have recently shown that osteosarcoma metastasis seems to be inhibited by macrophages. Here we discuss the possible mechanism of this observation.
It is not a coincidence that the ‘Father of Immunotherapy,’ William B. Coley, was a bone sarcoma surgeon. The first successful example of immunotherapy was in 1891 when Coley’s toxins, a mixture of toxins of streptococcal bacteria was injected into an unresectable sarcoma. The resulting immunological reaction led to tumor regression,Citation1 similar to what has been observed in osteosarcoma patients suffering from post-operative infection following resection of their primary tumor. The few known permanent responses to Coley’s toxins in carcinoma were in those cases of mesodermal origin.Citation2 Are sarcomas and other tumors of mesodermal origin more immunogenic than carcinomas? Or do immune cells have an effect that is different between sarcomas and carcinomas?
The tumor promoting effect of macrophages in carcinomas is well established. Epithelial tumors with high numbers of infiltrating immune cells have a poor prognosis as compared with cases with few infiltrating cells. This is attributed to a number of properties of the immune cells, especially macrophages, which have been shown to be involved with tumor initiation, invasion, migration, intravasation and angiogenesis.Citation3 Especially the stimulating effect on tumor invasion and migration of in origin non-motile epithelial cells that are the progenitors of carcinomas can be well comprehended. However, it is different for mesenchymal cells, which are much less dependent on contact with adjacent cells and thus more motile. These cells probably do not need the guidance that immune cells seem to give to carcinoma cells in the circulation. Instead, mesenchymal tumor cells might be inhibited in their motility by macrophages, which then act as impediment, instead of promoter for invasion. This is of course speculative, but it has been reported that macrophage inhibitory factor, MIF, which is produced by macrophages, inhibits migration of mesenchymal stem cells.Citation4
Our recent report describing an expression profiling study in a relatively large series of high grade, central osteosarcomas corroborates a metastasis inhibiting role for macrophages.Citation5 The ‘expression profile’ associated with non-metastatic behavior of osteosarcoma surprisingly consisted of a majority of genes associated with macrophage function, such as antigen processing and presentation or pattern recognition, as well as specific monocyte and macrophage markers such as CD14 and MSR1. Also genes related to general immunological expression were found to be upregulated, including several hematopoietic markers and cytokines. Expression of the macrophage-associated genes was confined to primary tumor tissue and not detected in a panel of 19 osteosarcoma cell line RNA samples, indicating that infiltrating immune cells were responsible for this expression profile. Furthermore the results were confirmed at the protein level by immuno histochemical staining on a larger patient cohort.
Osteosarcoma is an extremely aggressive tumor, mostly affecting adolescents. Neoadjuvant chemotherapy and limb-sparing salvage surgery have improved outcome and quality of life, but the last quarter-century no innovation and no improved survival has been achieved and oncologists are still left with about 40% of patients who die as a result of non-curable metastatic disease.
A role for macrophages to prevent or reduce metastases of osteosarcoma is corroborated by one of the few minor efficacious new therapeutic agents that were tested since the successful introduction of conventional chemotherapy for osteosarcoma, i.e., liposomal muramyl tri-peptide (MTP), also known as Mepact or Mifurmatide. This proprietary drug elicits activation of macrophages. Although the clinical trial that included adjuvant treatment with Mepact was initially denounced because of presumed interaction with one of the chemotherapeutic compoundsCitation6 it eventually appeared to give an improvement from 70 to 78% survival, which was the best achievement in improving outcome in decades.Citation7 Our finding that macrophages are associated with less metastases now provides a valid rationale for the efficacy of this drug. Additional supportive proof for the effectiveness of immune-stimulation is the use of interferon-α as adjuvant therapy in osteosarcomaCitation8 albeit that the positive effect may involve both immunological and direct anti-tumor effects. The recently completed EURAMOS1 clinical will shed more light on the value of this drug, since it was included in one of the randomized arms.Citation9
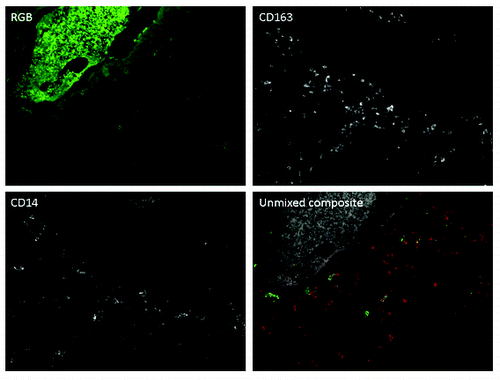
Neither the mechanism of metastasis suppression in osteosarcoma is clarified, nor the contrast with epithelial tumors. It may be sought in the different flavors of macrophages that are distinguishable by specific markers. M1 are tumor suppressive, M2 support invasion, metastasis and angiogenesis of tumor cells. We assessed the nature of the tumor associated macrophages in osteosarcoma clinical samples using HLA-DRα, associated with M1 macrophages and CD163, a marker to distinguish M2. Surprisingly both types of macrophages were present in the tumor tissues analyzed (). Recent perceptions on the good vs. bad macrophages are more nuanced. Macrophages are considered as quite flexible cells that polarize to a certain direction, but are not destined to stay that way.
Figure 1. Osteosarcoma samples are infiltrated with CD14 and CD163 single and double positive macrophages. Spectral imaging was used to reduce autofluorescence of osteosarcoma cells. In the composite image, CD14-positive cells are represented in green, CD163-positive cells are represented in red, and CD14/CD163 double positive cells are represented in yellow. Background autofluorescence of tumor cells is represented in gray.
To complicate things even more, there was a recent report that macrophage infiltration in another primary bone tumor, Ewing sarcoma, predicts a poor prognosis.Citation10
Similar to epithelial cancers, high macrophage infiltration was associated with increased microvessel density in our study, suggesting a similar role for macrophages in the promotion of angiogenesis in osteosarcoma. However, in the case of osteosarcoma, the influx of pro-angiogenic macrophages may be similar to a “Trojan horse.” Perhaps macrophages are attracted by the tumor to support angiogenesis, but following chemotherapeutic treatment, the release of endogenous danger signals by dying tumor cells causes the macrophages to become polarized toward an M1, anti-tumor phenotype. This proposed mechanism is supported by the fact that the survival benefit of high macrophage infiltration in our study was partly dependent on histological response to chemotherapy.
Coley’s toxins were denounced by another famous bone sarcoma expert, the pathologist James Ewing who gave his name to the second aggressive pediatric bone tumor. Ewing was not charmed by the medieval treatment developed by Coley, he was a fervent proponent of radiation therapy, which was effective for many tumors, but ironically not for bone sarcomas.
In conclusion, the relatively good response of sarcomas to immune stimulation and the favorable prognostic effect of tumor associated macrophages as opposed to carcinomas suggests that tumor immunology is different for sarcomas. This does not seem attributable to a particular macrophage subtype, but lies in the nature of this tumor type. Few clinical trials have been conducted on immunotherapy in sarcomas. Given our findings that macrophages are associated with less metastases in osteosarcoma tumor immunotherapy specifically targeted at this tumor type should be seriously evaluated.
References
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 1991; 3 - 11; PMID: 1984929
- Starnes CO. Coley's toxins in perspective. Nature 1992; 357:11 - 2; http://dx.doi.org/10.1038/357011a0; PMID: 1574121
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39 - 51; http://dx.doi.org/10.1016/j.cell.2010.03.014; PMID: 20371344
- Fischer-Valuck BW, Barrilleaux BL, Phinney DG, Russell KC, Prockop DJ, O'Connor KC. Migratory response of mesenchymal stem cells to macrophage migration inhibitory factor and its antagonist as a function of colony-forming efficiency. Biotechnol Lett 2010; 32:19 - 27; http://dx.doi.org/10.1007/s10529-009-0110-6; PMID: 19705068
- Buddingh EP, Kuijjer ML, Duim RA, Burger H, Agelopoulos K, Myklebost O, et al. Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage-activating agents. Clin Cancer Res 2011; 17:2110 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-10-2047; PMID: 21372215
- Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005; 23:2004 - 11; http://dx.doi.org/10.1200/JCO.2005.06.031; PMID: 15774791
- Meyers PA, Schwartz CL, Krailo MD, Healey JH, Bernstein ML, Betcher D, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children's Oncology Group. J Clin Oncol 2008; 26:633 - 8; http://dx.doi.org/10.1200/JCO.2008.14.0095; PMID: 18235123
- Müller CR, Smeland S, Bauer HC, Saeter G, Strander H. Interferon-alpha as the only adjuvant treatment in high-grade osteosarcoma: long term results of the Karolinska Hospital series. Acta Oncol 2005; 44:475 - 80; http://dx.doi.org/10.1080/02841860510029978; PMID: 16118081
- Whelan J, Patterson D, Perisoglou M, Bielack S, Marina N, Smeland S, et al. The role of interferons in the treatment of osteosarcoma. Pediatr Blood Cancer 2010; 54:350 - 4; http://dx.doi.org/10.1002/pbc.22136; PMID: 19902521
- Fujiwara T, Fukushi J, Yamamoto S, Matsumoto Y, Setsu N, Oda Y, et al. Macrophage infiltration predicts a poor prognosis for human ewing sarcoma. Am J Pathol 2011; 179:1157 - 70; http://dx.doi.org/10.1016/j.ajpath.2011.05.034; PMID: 21771572
