Abstract
Mesenchymal stem cells (MSCs) have a capacity to differentiate into the chondrogenic lineage and are a valuable allogenic source for cartilage tissue engineering. However, they still have critical limitations of relatively inefficient chondrogenic differentiation in vitro and of dedifferentiation and/or hypertrophic changes at late stages of differentiation. Numerous approaches using biochemical and mechanical factors have been tried but have so far failed to overcome these problems. Recent studies by other groups and ours have shown that low-intensity ultrasound (LIUS) is an efficient tool for promoting the chondrogenic differentiation of MSCs both in vitro and in vivo. A series of our experiments suggests that LIUS not only induces chondrogenic differentiation of MSCs but also has diverse additional activities that enhance the viability of MSCs, increase possibly the integrity of the differentiated tissues and delays hypertrophic changes during differentiation. Therefore, LIUS could be an innovative and versatile tool for chondrogenic differentiation of MSCs and for cartilage tissue engineering.
Introduction
Mesenchymal stem cells (MSCs) are currently under extensive investigation as an alternative cell source for cartilage tissue engineering.Citation1–Citation3 They can be easily isolated from various tissues such as the bone marrow, adipose tissue and umbilical cord blood, and have high ability to differentiate into chondrogenic lineages in vitro and in vivo. To induce chondrogenic differentiation, MSCs are usually cultured in a chondrogenic defined medium in a three-dimensional (3-D) environment using micromass culture or various scaffold materials. In addition, growth factors, such as transforming growth factor-β (TGFβ) in particular, are utilized to enhance differentiation efficiency.Citation4,Citation5 However, these differentiation protocols have the limitation that they generate undesired tissues different from the native cartilages and that they suffer from hypertrophic changes at late stages of differentiation in vitro and in vivo.Citation6–Citation8 Therefore, it is imperative to develop a well-designed and efficient method for chondrogenic differentiation of MSCs if construction of native cartilage-like tissues is to be achieved.
Recently, diverse mechanical stimulations such as cyclic hydrostatic pressure, cyclic compressive loading and low-intensity ultrasound (LIUS) have been shown to be a promising intervention to enhance the chondrogenic differentiation of MSCs.Citation9–Citation16 Among these mechanical stimulations, LIUS is of particular interest because it is simple, cost-effective and already proven to activate chondrocyte phenotypes in vitroCitation17–Citation22 and improve cartilage repair in animal models.Citation23,Citation24 This review will introduce findings by other groups and ours on the LIUS effect on MSCs during the chondrogenic differentiation and will discuss some of the issues regarding its mechanism and future applications.
Effect of Lius on the Chondrogenic Differentiation of MSCS in Vitro and in Vivo
The activity of LIUS in enhancing chondrogenic differentiation of MSCs was first reported by Ebisawa et al. using pellet culture of human MSCs from bone marrow.Citation12 They used pulsed LIUS at 1 MHz and intensities of 15, 30, 60, 120 mW/cm2 for 20 min a day over 10 days. The pulsed LIUS was shown to increase expression of sulfated proteoglycans in the presence of TGFβ3 in both immunohistochemistry and enzyme-linked immunoabsorbent assays (ELISA). However, the study had some limitations that the LIUS effect was only shown at 30 mW/cm2 and no supportive data was provided such as the expression of type II collagens and other chondrogenic markers. About two years later, Schumann et al. and we reported similar results for the LIUS effect in human and rabbit MSCs.Citation9,Citation14 Schumann et al. cultured human MSCs in aggregates or in a biodegradable composite scaffold of hyaluronan and gelatin (in a ratio of 7:3).Citation9 Their LIUS parameters were similar to those used by Ebisawa et al. except it was applied at 1.5 MHz and a fixed intensity of 30 mW/cm2. Instead, cells were treated for 40 min a day for the first seven days and observed until 21 days in the presence of TGFβ1. The positive effect of LIUS was shown by the expression of proteoglycans and type II collagen, as demonstrated by reverse transcriptase-polymerase chain reaction (RT-PCR) and chemical assays.
We have shown the LIUS effect in vitro and in vivo in more specific and diverse experiments using human and rabbit MSCs from the bone marrow in alginate or polyglycolic acid (PGA) scaffolds.Citation13,Citation14,Citation16,Citation25 In contrast to the two other studies described above, continuous wave LIUS was applied at a frequency of 0.8 or 1 MHz and an intensity of 200 mW/cm2 for 20 min a day or for 10 min every 12 hrs. The LIUS parameters and treatment conditions were optimized based on our studies using chondrocytes.Citation20,Citation21,Citation26,Citation27 In alginate culture of rabbit MSCs, LIUS stimulated synthesis of cartilage matrix, formation of lacunae, and enhanced expression of chondrogenic marker genes such as aggrecan, type II collagen and Sox-9 after one or two weeks of culture.Citation14 Similar results of the LIUS effect were obtained in vitro in alginate culture of human MSCs ()Citation25 and in rabbit MSCs in a PGA scaffold.Citation16 The LIUS effect was also shown in vivo.Citation13 Rabbit MSCs in a PGA scaffold were first implanted in the backs of nude mice and were stimulated with LIUS from the outside for 20 min a day at 0.8 MHz and 200 mW/cm2 in a continuous wave mode. When examined until four weeks the LIUS stimulation clearly increased the compressive strength and the expression of proteoglycans and collagens in the construct. In addition to the LIUS effect to induce chondrogenic differentiation of MSCs, several other noticeable results were observed in our experiments. First, LIUS induced chondrogenic differentiation of MSCs in the absence of added TGFβ both in vitro and in vivo.Citation14,Citation28 This result is inconsistent with those of the other two groups that the LIUS activity was shown only in its presence.Citation9,Citation12 Differences in the mode of LIUS stimulation might be the reason for the apparent discrepancy of the results. Second, LIUS induced expression of tissue inhibitor of metalloproteinases-2 (TIMP-2) but showed no effect on the protein level of metalloproteinase-3 (MMP-3) or mRNA levels of MMP-13, and type I and X collagens.Citation14,Citation16 TIMP-2 functions as an inhibitor of MMPs and an anti-angiogenic factor that can inhibit vascular invasion following hypertrophy of chondrocytes during endochondral ossification.Citation29 Type I and X collagens and MMP-13 are also involved positively in the hypertrophic changes of chondrocytes.Citation30 Therefore, LIUS might inhibit degradation of extracellular matrix (ECM) proteins and/or hypertrophic changes of differentiated MSCs, thereby enhancing the integrity of cartilage constructs. Third, MSCs differentiated using LIUS treatment maintained their chondrogenic phenotypes for a longer time than did the untreated cells, when they were re-plated in monolayer culture.Citation14 Schumann et al. also showed that human MSCs pretreated with pulsed LIUS for one week expressed more proteoglycans and collagens when examined at two and three weeks.Citation9 These results indicate that preconditioning by LIUS for a short period (e.g., for one week) could program MSCs to differentiate well into chondrogenic lineages or to better maintain chondrogenic phenotypes during subsequent culture in the absence of additional LIUS stimulation. These issues will be discussed in more detail below.
Effect of Lius Preconditioning on the Chondrogenic Differentiation of MSCS and the Cartilage Repair in a Rabbit Defect Model
The preconditioning of MSCs with LIUS could be a useful protocol for the application of MSCs to the tissue engineering of cartilage. It could reduce the time and cost for culture of MSCs in vitro as well as enhancing the efficiency of chondrogenic differentiation of MSCs. In addition, it might enable mass production and wide distribution of engineered cartilages with allogenic MSCs. In our experiments, LIUS preconditioning of rabbit MSCs in PGA scaffold for one week in vitro without TGFβ significantly enhanced the chondrogenic differentiation and formation of hyaline cartilage-like tissues, when the construct was implanted into the backs of nude mice ().Citation16 In addition, calcification of tissues with vascular invasion, observed from four weeks of implantation in the untreated group, was apparently delayed by LIUS preconditioning ().Citation16 It appears to be associated with the effect of LIUS on the expression of genes involved in the hypertrophy of chondrocytes and angiogenesis as mentioned above.Citation14,Citation16 Treatment by TGFβ1 alone in vitro showed no significant effect on chondrogenic differentiation and calcification of the implant in nude mice both in the absence and presence of LIUS treatment. The LIUS preconditioning of rabbit MSCs in PGA scaffolds has also been observed to improve repair of the full-thickness cylindrical defect in the femoral trochlea (unpublished data). The implant showed stronger expression of proteoglycans and type II collagen and better integration with host tissue in the LIUS preconditioned group than in the untreated group. In a previous report, De Bari et al. showed that human synovial membrane MSCs, differentiated in vitro, failed to form stable cartilage when implanted subcutaneously in nude mice.Citation31 Noel et al. also showed that C3H10T1/2-derived C9 MSCs injected into the knee joints of CB17-severe combined immunodeficient bg mice did not differentiate into chondrocytes to form cartilage tissue in the absence of bone morphogenic protein-2 (BMP-2) expression.Citation32 Although there are differences in the experimental conditions, our results suggest that the LIUS preconditioning of MSCs is an efficient method for the chondrogenic differentiation of MSCs and cartilage tissue formation in vivo.
Role of TGFβ in the Lius Effect on the Chondrogenic Differentiation of MSCS
It has long been regarded that TGFβ is necessary and sufficient to induce chondrogenic differentiation of MSCs in vitro.Citation4,Citation5 The LIUS effect has been shown in the presence of TGFβ in the reports by the other groups.Citation9,Citation12 They argued that LIUS could increase the synthesis of endogenous TGFβ in MSCs or increase the access of exogenous TGFβ to the center of the constructs.Citation9 In our studies, however, the LIUS effect was consistently observed in diverse experimental conditions in vitro and in vivo without exogenous TGFβ.Citation13,Citation14,Citation16,Citation25 The discrepancy between the results could be caused by differences in the cell source, 3-D culture system and LIUS stimulation mode as described above. We suspect that the LIUS stimulation mode could be a most critical factor because the TGFβ-independent activity of LIUS was reproducibly obtained in our studies using both rabbit and human MSCs and using different scaffold systems such as alginate, PGA and fibrin/hyaluronan composite (unpublished data). The other groups used LIUS of pulsed wave at 30 mW/cm2, while we used continuous wave at 200 mW/cm2. It is not clear, however, why these differences influenced the function of MSCs and the dependency on TGFβ.
We think that LIUS have a unique role and mechanism that is independent of TGFβ during the differentiation of MSCs. First, the role of TGFβ was not always definite in the chondrogenic differentiation of MSCs. TGFβ was not always sufficient to induce chondrogenic differentiation of human MSCs in pellet culture and BMP-1 or BMP-2 were required.Citation33,Citation34 Chondrogenic differentiation occurred efficiently without any added growth factors in bovine MSCs in pellet culture occurredCitation35 and rabbit MSCs in PGA scaffold.Citation13,Citation16 Besides, other mechanical stimulations such as cyclic hydrostatic pressure and cyclic compressive loading also induced robust chondrogenesis of human and rabbit MSCs, respectively, independently of exogenous TGFβ.Citation11,Citation15,Citation36 Second, LIUS must use different cell surface receptors and cellular signaling pathways from those for TGFβ. The TGFβ signal is transmitted via specific cell surface receptors and the Smad pathway,Citation37 whereas the LIUS signal appears to use the mechanotransduction pathway including integrins, stretch-activated ion channels, and interleukin-4 on the cell surface.Citation38–Citation40 In addition, the expression pattern of selected surface markers was different between TGFβ- and LIUS-treated groups during the chondrogenic differentiation of human MSCs (unpublished data).
Effect of Lius on the Hypertrophic Changes of Differentiated MSCS
In contrast to fully differentiated chondrocytes, MSCs under going the chondrogenic differentiation in vitro or in vivo usually lose their differentiated phenotypes, probably by dedifferentiation and/or hypertrophic changes.Citation6,Citation8,Citation16,Citation31 Preconditioning with LIUS in vitro delayed these adverse changes of rabbit MSCs implanted in the back of nude mice, such as the loss of proteoglycans expression, vascular invasion and calcification of tissues in our study.Citation16 These changes are reminiscent of the phenomenon observed during endochondral bone formation.Citation41 Further investigation using rabbit MSCs in alginate or PGA scaffolds showed that LIUS treatment reduced expression of genes involved in the hypertrophy of chondrocytes, such as type I and X collagens, and MMP-13, while induced that of TIMP-2, an inhibitor of angiogenesis, as early as after one week in vitro.Citation14,Citation16 We also observed that LIUS could inhibit vascular invasion, as judged by immunohistochemical analysis for pallet/endothelial cell adhesion molecule-1 (PECAM-1) and could reduce the expression of angiogenic factors such as hypoxia inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF) in rabbit MSCs in vitro or in nude mice (unpublished data). These results suggest that LIUS could inhibit the hypertrophic changes and vascular invasion during the chondrogenic differentiation of MSCs in vitro and in vivo. It is inconsistent with previous reports that LIUS stimulated angiogenesis or hypertrophy of the cartilage during fracture healing and endochondral ossification in vitro.Citation42,Citation43 The differences in the experimental conditions (subcutaneous versus bone) could be a cause of the inconsistent results, and careful and long-term investigations are necessary to fully address the effect of LIUS on the hypertrophic changes of MSC and its value for the cartilage tissue engineering. The mechanism of LIUS to inhibit hypertrophy of cells is not clear but probably independent of its function to enhance chondrogenic differentiation of MSCs. First, hypertrophic change could occur prematurely and often precede the chondrogenic differentiation of MSCs in vitro.Citation8 Therefore, inhibition of hypertrophy by LIUS could be a prerequisite for the efficient chondrogenic differentiation of MSCs rather than its outcome. Second, LIUS was also shown to reduce the expression of type X collagen in the deep zone of the cartilage explants in vitro, where no chondrogenic differentiation is involved.Citation44
Effect of Lius on the Proliferation and Viability of MSCs
The effect of LIUS on the proliferation was rather complicated in chondrocytes depending on the experimental conditions used.Citation17,Citation18,Citation20,Citation21,Citation45 We have previously argued that LIUS enhances the proliferation of chondrocytes in monolayer culture but not in 3-D environment.Citation22 The direct effect of LIUS on the proliferation of MSCs during chondrogenic differentiation has not yet been investigated thoroughly. MSCs differentiated in alginate using LIUS showed higher proliferation rate than that of the untreated controls, when cells were replated in monolayer culture.Citation14 However, MSCs differentiated in a PGA scaffold showed no significant differences in their DNA content in vitro or in vivo.Citation13,Citation16 Therefore, it is likely that LIUS does not have significant effect on the proliferation of MSCs during chondrogenic differentiation in 3-D environments.
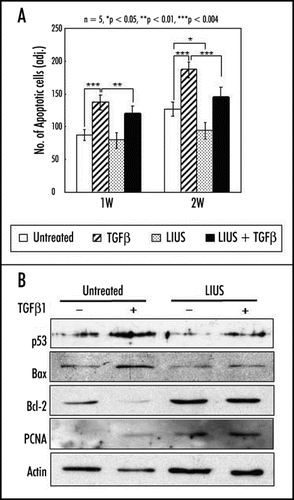
In contrast, LIUS significantly enhanced the viability of both chondrocytes and MSCs in 3-D alginate culture.Citation21,Citation25 Harsh conditions for chondrogenic differentiation of MSCs using 3-D culture and TGFβ appeared to be cytotoxic and induce apoptotic cell death for long time culture. Treatment of LIUS clearly inhibited apoptotic events and reduced expression of apoptosis-related genes such as p53 and bax, while induced that of anti-apoptotic genes such as bcl-2 and proliferating cell nuclear antigen (PCNA) ().Citation14 Interestingly, the protective effect of LIUS was also observed in monolayer culture of MSCs treated with staurosporine to induce apoptosis. Therefore, the anti-apoptotic effect of LIUS appears to be an active process involving specific regulation of related genes but not just simply enhancing the cell proliferation or overall cellular activity.
Summary and Future Perspectives
The results from other groups and ours suggest that LIUS is an efficient tool for promoting the chondrogenic differentiation of MSCs both in vitro and in vivo. LIUS was shown to play not just enhancing but actively inducing roles that direct MSCs to chondrogenic lineages without exogenous growth factors such as TGFβ. LIUS also had diverse additional functions during the chondrogenic differentiation of MSCs in vitro and in vivo. It enhanced (1) viability of MSCs by inhibiting apoptosis of cells, (2) regulated expression of genes involved in the integrity of the differentiated construct, and (3) delayed hypertrophic changes at the late stage of differentiation. Together with its non-invasive and cost-effective properties, the activities of LIUS make it an innovative and versatile tool for the chondrogenic differentiation of MSCs and cartilage tissue engineering. Chondrogenic programming of MSCs by LIUS preconditioning and direct application of LIUS in vivo could also allow diverse approaches for its practical use in the cartilage tissue engineering. Future studies should include (1) further optimization of the LIUS treatment condition such as the LIUS parameters, cell culture medium and scaffold system to sufficiently overcome hypertrophic changes of MSCs, (2) understanding the detailed mechanism underlying the diverse activities of LIUS and (3) development of efficient and convenient LIUS stimulators fit for specific research or practical purposes, for example, an integrated system of bioreactor and LIUS stimulator for cartilage tissue engineering using MSCs.
Abbreviations
| 3-D | = | three-dimensional |
| BMP-2 | = | bone morphogenic protein-2 |
| ECM | = | extracellular matrix |
| ELISA | = | enzyme-linked immunoabsorbent assay |
| HIF-1α | = | hypoxia inducible factor-1α |
| LIUS | = | low-intensity ultrasound |
| MMP-3 | = | metalloproteinase-3 |
| MSCs | = | Mesenchymal stem cells |
| PCNA | = | proliferating cell nuclear antigen |
| PECAM-1 | = | pallet/endothelial cell adhesion molecule-1 |
| PGA | = | polyglycolic acid |
| RT-PCR | = | reverse transcriptase-polymerase chain reaction |
| TGFβ | = | transforming growth factor-β |
| TIMP-2 | = | tissue inhibitor of metalloproteinases-2 |
| VEGF | = | vascular endothelial growth factor |
Figures and Tables
Figure 1 Effects of LIUS on the chondrogenic differentiation of human MSCs in alginate layer culture. The expression of chondrogenic markers such as Sox-9, aggrecan and type II collagen was measured at one and two weeks by RT-PCR analysis. Relative band intensities normalized against those of GAPDH were presented from five independent experiments. *p < 0.05, **p < 0.01 and ***p < 0.001.
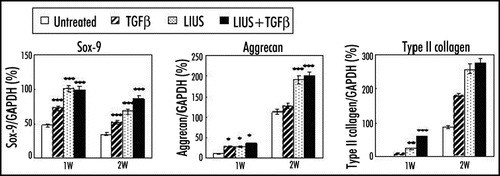
Figure 2 Effect of LIUS preconditioning on the chondrogenic differentiation and hypertrophic changes of rabbit MSCs in PGA scaffold in nude mice. (A) Safranin O/Fast green staining for the expression of proteoglycans in the implanted constructs at 1, 2, 4 and 6 weeks (x10). (B) von Kossa staining for the calcification of the implanted constructs (x10).
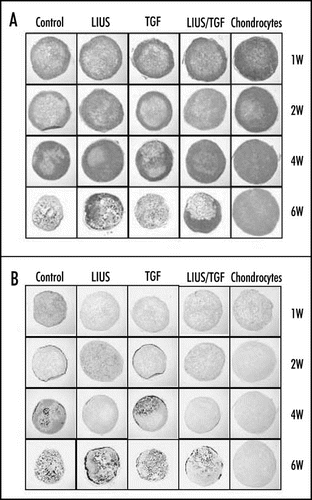
Figure 3 Effects of LIUS on the apoptosis of human MSCs during chondrogenic differentiation in alginate layer culture. (A) Thin sections of the constructs were stained with FragEL DNA Fragmentation Detection Kit (Calbiochem, Germany) at one and two weeks. The number of apoptotic cells was determined from five independent experiments in the histogram. The data were presented as a mean ± standard deviation (SD). *p < 0.05, **p < 0.01, and ***p < 0.001 (B) The expression of apoptosis and cell viability related genes (p53, bax, bcl-2 and PCNA) was examined by Western blot analysis. The level of action was measured as an internal control.
Acknowledgements
This study was supported by the grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (0405B001-0204-0006).
References
- Kassem M. Mesenchymal stem cells: Biological characteristics and potential clinical applications. Cloning Stem Cells 2004; 6:369 - 374
- Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering: The application of stem cell technology. Curr Opin Biotechnol 2005; 16:503 - 509
- Chen FH, Rousche KT, Tuan RS. Technology insight: Adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol 2006; 2:373 - 382
- Mastrogiacomo M, Cancedda R, Quatro R. Effect of different growth factors on the chondrogenic potential of human bone marrow stromal cells. Osteoarthritis Cartilage 2001; 9A:36 - 40
- Indrawattana N, Chen G, Tadoloro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun 2004; 320:914 - 919
- Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids. Arthritis Rheum 2003; 48:418 - 429
- De Bari C, Dell'Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum 2004; 50:142 - 150
- Pelttari K, Winter A, Steck E, Goetzke K, Henning T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 2006; 54:3254 - 3266
- Schumann D, Kujat R, Zellner J, Angele MK, Nerlich M, Mayr E, Angele P. Treatment of human mesenchymal stem cells with pulsed low intensity ultrasound enhances the chondrogenic phenotype in vitro. Biorheology 2006; 43:431 - 443
- Schumann D, Kujat R, Nerlich M, Angele P. Mechanobiological conditioning of stem cells for cartilage tissue engineering. Bio-med Mater Eng 2006; 16:S37 - S52
- Angele P, Yoo JU, Smith C, Mansour J, Jepsen KJ, Nerlich M, Johnstone B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J Orthop Res 2003; 21:451 - 457
- Ebisawa K, Hata K, Okada K, Kimata K, Ueda M, Torii S, Watanabe H. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng 2004; 10:921 - 929
- Cui JH, Park K, Park SR, Min BH. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: An in vivo study. Tissue Eng 2006; 12:75 - 82
- Lee HJ, Choi BH, Min BH, Son YS, Park SR. Low intensity ultrasound stimulation enhances the chondrogenic differentiation in alginate culture of mesenchymal stem cells. Artif Organs 2006; 30:707 - 716
- Park SH, Sim WY, Park SW, Yang SS, Park SR, Park K, Choi BH, Min BH. An electromagnetic compressive force cell exciter stimulates chondrogenic differentiation of bone marrow-derived mesenchymal stem cells (MSCs). Tissue Eng 2006; 12:3107 - 3117
- Cui JH, Park SR, Park K, Choi BH, Min BH. Preconditioning of mesenchymal stem cells with low intensity ultrasound for cartilage formation in vivo. Tissue Eng 2007; 13:351 - 406
- Parvizi J, Parpura V, Kinnick RR, Greenleaf JF, Bolander ME. Low intensity ultrasound stimulates proteoglycan synthesis in rat chondrocytes by increasing aggrecan gene expression. J Orthop Res 1999; 17:488 - 494
- Nishikori T, Ochi M, Uchio Y, Maniwa S, Kataoka H, Kawasaki K, Katsube K, Kuriwaka M. Effects of low-intensity pulsed ultrasound on proliferation and chondroitin sulfate synthesis of cultured chondrocytes embedded in Atelocollagen gel. J Biomed Mater Res 2002; 59:201 - 206
- Zhang ZJ, Huckle J, Francomano CA, Spencer RGS. The influence of pulsed low-intensity ultrasound on matrix production of chondrocytes at different stages of differentiation: An explant study. Ultrasound Med Biol 2002; 28:1547 - 1553
- Min BH, Woo JI, Cho HS, Choi BH, Park SJ, Choi MJ, Park SR. Effects of low-intensity ultrasound (LIUS) stimulation on human cartilage explants. Scand J Rheumatol 2006; 35:305 - 311
- Choi BH, Woo JI, Min BH, Park SR. Low-intensity ultrasound (LIUS) stimulates the viability and matrix gene expression of human articular chondrocytes in alginate bead culture. J Biomed Mater Res 2006; 79:858 - 864
- Min BH, Choi BH, Park SR. Low intensity ultrasound as a supporter of cartilage regeneration and its engineering. Biotechnol Bioproc Eng 2007; 12:22 - 31
- Cook SD, Salkeld SL, Popich-Patron LS, Ryaby JP, Jones DG, Barrack RL. Improved cartilage repair after treatment with low-intensity pulsed ultrasound. Clin Orthop Relat Res 2001; 391:231 - 243
- Nieminen HJ, Saarakkala S, Laasanen MS, Hirvonen J, Jurvelin JS, Toyras J. Ultrasound attenuation in normal and spontaneously degenerated articular cartilage. Ultrasound Med Biol 2004; 30:493 - 500
- Lee HJ, Choi BH, Min BH, Park SR. Low-intensity ultrasound inhibits apoptosis and enhances viability of human mesenchymal stem cells in three-dimensional alginate culture during chondrogenic differentiation. Tissue Eng 2007; 13:1049 - 1057
- Park SR, Jang KW, Park SH, Cho HS, Jin CZ, Choi MJ, Chung SI, Min BH. Effect of sonication on simulated osteoarthritis. Part I: Effects of 1 MHz ultrasound on hyaluronan into the rabbit synovium. Ultrasound Med Biol 2005; 31:1551 - 1558
- Park SR, Park SH, Jang KW, Cho HS, Cui JH, An HJ, Choi MJ, Chung SI, Min BH. The effect of sonication on simulated osteoarthritis. Part II: Alleviation of osteoarthritis pathogenesis by 1 MHz ultrasound with simultaneous hyaluronate injection. Ultrasound Med Biol 2005; 31:1559 - 1566
- Hangody L, Feczko P, Bartha L, Bodo G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res 2001; 391:328 - 336
- Stetler-Stevenson WG, Seo DW. TIMP-2: An endogenous inhibitor of angiogenesis. Trends Mol Med 2005; 11:97 - 103
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 2004; 16:558 - 564
- De Bari C, Dell'Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum 2004; 50:142 - 150
- Noel D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, Millet V, Turgeman G, Perricaudet M, Sany J, Jorgensen C. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells 2004; 22:74 - 85
- Schmitt B, Ringe J, Haupl T, Notter M, Manz R, Burmester GR, Sittinger M, Kaps C. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation 2003; 71:567 - 577
- Bai X, Xiao Z, Pan Y, Hu J, Pohl J, Wen J, Li L. Cartilage-derived morphogenetic protein-1 promotes the differentiation of mesenchymal stem cells into chondrocytes. Biochem Biophys Res Commun 2004; 325:453 - 460
- Bosnakovski D, Mizuno M, Kim G, Ishiguro T, Okumura M, Iwanaga T, Kadosawa T, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Exp Hematol 2004; 32:502 - 509
- Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 2004; 22:313 - 323
- Attisano L, Wrana JL. Signal transduction by the TGFβ superfamily. Science 2002; 31:1646 - 1647
- Salter DM, Millward-Sadler SJ, Nuki G, Wright MO. Integrin-interleukin-4 mechanotransduction pathways in human chondrocytes. Clin Orthop 2001; 291:S49 - S60
- Zhou S, Schmelz A, Seufferlein T, Li Y, Zhao J, Bachem MG. Molecular mechanisms of low intensity pulsed ultrasound in human skin fibroblasts. J Biol Chem 2004; 279:54463 - 54469
- Choi BH, Choi MH, Kwak MG, Min BH, Woo ZH, Park SR. Mechanotransduction pathways of low-intensity ultrasound in C-28/I2 human chondrocytes cell line. Proc Instn Mech Engrs, Part H: J Eng Med 2007; 221:527 - 535
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 1999; 5:623 - 628
- Wiltink A, Nijweide PJ, Oosterbaan WA, Hekkenberg RT, Helders PJM. Effect of therapeutic ultrasound on endochondral ossification. Ultrasound Med Biol 1995; 21:121 - 127
- Rawool D, Goldberg B, Forsberg F, Winder A, Talish R, Hume E. Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound. Trans Radiol Soc North Am 1998; 83:1185
- Zhang ZJ, Huckle J, Francomano CA, Spencer RGS. The effects of pulsed low-intensity ultrasound on chondrocyte viability, proliferation, gene expression and matrix production. Ultrasound Med Biol 2003; 29:1645 - 1651
- Huang MH, Ding HJ, Chai CY, Huang YF, Yang RC. Effects of sonication on articular cartilage in experimental osteoarthritis. J Rheumatol 1984; 24:1978 - 1984
