Abstract
Reactive oxygen species (ROS) production and scavenging in plants under drought stress have been studied intensively in recent years. Here we report a global analysis of gene expression for the major ROS generating and scavenging proteins in alfalfa root and shoot under gradual drought stress followed by one-day recovery. Data from two alfalfa varieties, one drought tolerant and one drought sensitive, were compared and no qualitative differences in ROS gene regulation between the two were found. Conserved, tissue-specific patterns of gene expression in response to drought were observed for several ROS-scavenging gene families, including ascorbate peroxidase, monodehydroascorbate reductase, and peroxiredoxin. In addition, differential gene expression within families was observed. Genes for the ROS-generating enzyme, NADPH oxidase were generally induced under drought, while those for glycolate oxidase were repressed. Among the ROS-scavenging protein genes, Ferritin, Cu/Zn superoxide dismutase (SOD), and the majority of the glutathione peroxidase family members were induced under drought in both roots and shoots of both alfalfa varieties. In contrast, Fe-SOD, CC-type glutaredoxins, and thoiredoxins were downregulated.
Keywords: :
Reactive oxygen species (ROS) are produced unavoidably in plant cells as a consequence of oxygen metabolism. Major forms of ROS include the superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and singlet oxygen (1O2), which differ in the extent of reduction or activation of oxygen.
Under optimal growth conditions, ROS generation and scavenging in plant cells are maintained in a dynamic balance.Citation1,Citation2 However, when plants are stressed, ROS generation tends to exceed scavenging and accumulated ROS may oxidize lipids, proteins and nucleic acidsCitation1,Citation3,Citation4 and further cause programmed cell death.Citation5,Citation6 Aside from negative effects, the role of ROS as signaling molecules has gained increasing attention in recent years.Citation4,Citation7–Citation9
Overproduction of ROS in plants under drought stress is well documentedCitation10–Citation12 and in most cases a general increase in plant antioxidant enzyme activities was measured under drought.Citation10,Citation13,Citation14 However, the majority of these studies monitored one or few ROS scavenging enzymes in the above ground tissues and the data were collected at limited time points.
Here, we present a genome-wide analysis of the expression of the major ROS generating and scavenging genes in alfalfa root and shoot under gradual 11-d soil drought stress followed by one-day recovery (Table S1). Indirect ROS scavengers including alternative oxidase and ferritin, and three protein redox regulating proteins were also included in the analysis. Genes involved in the metabolism of ROS-scavenging chemicals were not included.
Results of two alfalfa varieties, Wisfal (M. sativa ssp. falcata var Wisfal), a drought tolerant variety, and Chilean (M. sativa ssp. sativa var Chilean), a drought sensitive variety, were compared. The data were extracted from Kang et al.Citation15 and gene expression heat maps were constructed with Multi Array Viewer (www.tm4.org/mev/). Medicago truncatula ROS generating and scavenging genes were identified by homology to ArabidopsisCitation2,Citation16,Citation17 and updated according to annotations in UniProt (www.uniprot.org) and the M. truncatula Gene Expression Atlas (mtgea.noble.org/v2/). The corresponding M. truncatula Affymetrix probeset IDs were identified through Legoo (www.legoo.com).
Plasma membrane NADPH oxidase, also called respiratory-burst oxidase (RBO), catalyzes the production of O2-. NADPH oxidase is involved in various plant biotic and abiotic stress responses.Citation9,Citation18–Citation20 The expression of most NADPH oxidases increased in both roots and shoots of alfalfa under drought, especially under severe drought stress (D11; ). NADPH oxidase expression appeared to be more sensitive to drought in the drought-sensitive variety, Chilean, than in drought-tolerant Wisfal. On the other hand, the expression of the important ROS generator in the peroxisome, glycolate oxidase (GLO), declined rapidly upon drought stress in the root. In the shoot, reduction in GLO expression only became apparent at severe drought stress (). Previous studies on GLO regulation under drought stress found no consensus, with some finding reduction in GLO activityCitation11,Citation21 consistent with our results, and others finding an increase in GLO gene expressionCitation22 and protein activityCitation23–Citation25 under drought.
Figure 1. Expression profiles of selected ROS-generating genes in roots and shoots of Chilean and Wisfal subjected to drought stress and re-watering. Rboh, NADPH oxidase (respiratory burst oxidase homolog); GLO, glycolate oxidase. C, Chilean; W, Wisfal; R, root; S, shoot; CTL, control; D5, five-day drought; D8, eight-day drought; D11, 11-d drought; RW, one day after re-watering.
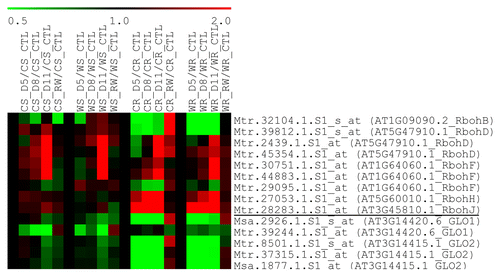
The expression of the major ROS scavenging genes was finely tuned at sub-family levels under drought stress (). SODs catalyze the dismutation of O2- to H2O2 and are located in various subcellular compartments in plants.Citation1,Citation26 The expression of FeSOD (FSD, chloroplast) was generally repressed under drought stress in alfalfa while Cu/ZnSOD (CSD, chloroplast and cytosol) was induced (). MnSOD (mitochondria) transcript level was too low to be detected (Table S1). Earlier studies reported increase in SOD activityCitation11,Citation27,Citation28 and abundanceCitation29 after drought stress, while MnSOD but not Cu/ZnSOD transcript was shown to be induced under drought in wheat.Citation30 However, both wheatCitation31 and common beanCitation32 SOD activity remained constant under moderate to severe drought.
Figure 2. Expression profiles of ROS-scavenging genes in roots and shoots of Chilean and Wisfal subjected to gradual drought stress and re-watering. AOX, Alternative oxidase; APX, ascorbate peroxidase; DHAR, dehydroascorbate reductase; MDHAR, monodehydroascorbate reductase; GPX, glutathione peroxidase; Grx, glutaredoxin; Prx, peroxiredoxin; Trx, thioredoxin; SOD, superoxide dismutase. C, Chilean; W, Wisfal; R, root; S, shoot; CTL, control; D5, 5-d drought; D8, 8-d drought; D11, 11-d drought; RW, one day after re-watering.

Ascorbate peroxidase (APX) is an important H2O2 scavenger at the expense of oxidizing ascorbate and glutathione in the ascorbate-glutathione cycle (Gill and Tuteja 2010).Citation33–Citation35 Ascorbate and glutathione regeneration requires the activity of dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), and glutathione reductase (GR). Transcript levels of APX were very high in the shoot under well-watered conditions (Table S1) but decreased gradually under drought, along with ascorbate levels,Citation15 which implies that oxidized ascorbate was not fully recycled. In contrast, APX regulation in the root was weak and not consistent (). Interestingly, neither MDHAR nor DHAR had similar expression patterns to APX; MDHAR was induced in the shoot but repressed in the root while DHAR was induced in both root and shoot during drought. The transcript level of GRs was below the detection limit (Table S1). In earlier studies with similar experimental design as this study, the activity of APX, DHAR and MDHAR in pea were found to decrease significantly under severe drought stress,Citation11 while in wheat, their activity changes were correlated with both the drought sensitivity of the cultivar and severity of the stress.Citation36
Glutathione peroxidase (GPX) and Peroxiredoxin (Prx) are H2O2 scavengers which use glutathione as reductant. In drought-stressed alfalfa, Prxs were repressed in the shoot but induced in the root while most GPXs were induced in both root and shoot except GPX2 and GPX3 (). Interestingly, AtGPX2 and AtGPX3 are neighbors in the phylogenetic tree.Citation37 In agreement with our results, increase in GPX transcript level or activity under drought have been reported in several plant species.Citation31,Citation32,Citation38
Alternative oxidase (AOX) reduces ROS generation by the mitochondrial electron transport chain under stress.Citation39 AOX1 is induced in response to various biotic and abiotic stresses in plants.Citation2,Citation39,Citation40 However, under drought stress in alfalfa, AOX1A transcript level was below the detection limit (Table S1), while AOX2 and AOX4 were strongly regulated but in different ways (). Two of the AOX2 homologs were induced in the root of Wisfal but repressed in Chilean, which may contribute to greater drought tolerance in Wisfal.
Among all the gene families analyzed, ferritins stand out as the most strongly and uniformly upregulated family under drought stress (Table S1). Upon recovery from drought stress, ferritin expression decreased rapidly, revealing high sensitivity to drought. Ferritin is an effective Fe sequestrator and plays a crucial role in plant oxidative stress primarily by removal of free Fe, which catalyzes the Fenton reaction that produces highly reactive hydroxyl radicals.Citation41,Citation42 Ferritin also serves as an active ROS scavenger because the oxidation of Fe(II) to Fe(III) consumes oxygen and H2O2 before it is stored in ferritin.Citation43 In plants, ferritin level is very low in mature leaves under normal growth conditions but it is induced by excess Fe application, ABA treatment and other oxidative stresses.Citation42,Citation44,Citation45 Ferritin mRNA accumulated in soybean nodules under drought stressCitation46 and in maize shoots under dehydration stress.Citation47 Ferritin protein abundance increased more than 2-fold in the root apical region of soybean under water stress.Citation48 Our data support the notion that removing free iron may be crucial for plant cells to survive drought induced oxidative stress.
Under drought stress, plant cells generate excessive ROS that oxidizes cellular proteins.Citation11,Citation49,Citation50 Oxidized proteins can be reduced by several families of “redoxins,” including thioredoxin (Trx), glutaredoxin (Grx), and Prx. However, almost all redoxin transcripts were repressed under drought stress in Arabidopsis.Citation2 In alfalfa, leaf 2-cys Prx protein abundance increased upon drought stress.Citation51 In our experiments, most of the redoxins were downregulated under drought except the classical-type Grx and Prx in the root (). Note that Prx is a H2O2 scavenger in addition to being a protein redox regulator as mentioned above. The Trx gene family is large in alfalfa but almost all of these genes did not change activity or were downregulated in response to drought (). One exception, corresponding to Mtr.40458.1.S1, was induced by drought in alfalfa (), as was a homolog, CDSP32/AT1G76080.1, in potato.Citation52,Citation53
In summary, ROS scavenging genes in alfalfa were highly responsive to drought and regulated in a conserved manner in a drought tolerant and a drought sensitive variety. It was intriguing to find many ROS-scavenging genes repressed under drought. However, the importance of a ROS-scavenging gene cannot be judged solely upon whether it is induced under stress. Some ROS-scavenging genes such as APXs are expressed at very high levels under optimal growth conditions (Table S1) and may still be the main ROS scavenger when slightly downregulated under drought stress. A systematic analysis of the changes in ROS-scavenging protein stability and activity under drought stress would be a useful complement to the results presented here. The promoter regions of alfalfa ROS-scavenging genes should also be analyzed to identify potential regulatory domains and transcription factor families that might control their tissue-specific and stress-responsive expression patterns. It is clear from our work that one cannot assume that it is always beneficial to increase the expression of ROS scavenging genes during stress, as many earlier studies have.Citation1 In future, it will be interesting to determine which drought-induced ROS genes contribute significantly to drought tolerance in alfalfa.
| Abbreviations: | ||
| AOX | = | Alternative oxidase |
| APX | = | ascorbate peroxidase |
| DHAR | = | dehydroascorbate reductase |
| MDHAR | = | monodehydroascorbate reductase |
| GLO | = | glycolate oxidase |
| GPX | = | glutathione peroxidase |
| GR | = | glutathione reductase |
| Grx | = | glutaredoxin |
| Prx | = | peroxiredoxin |
| Trx | = | thioredoxin |
| Rboh | = | NADPH oxidase (respiratory burst oxidase homolog) |
| ROS | = | reactive oxygen species |
| SOD | = | superoxide dismutase |
Additional material
Download Zip (169.7 KB)Acknowledgments
This work was supported by the Oklahoma Bioenergy Center (OBC) and The Samuel Roberts Noble Foundation. The authors would like to thank Dr. Mingyi Wang for assistance with data analysis and Eric Worley for valuable suggestions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 2010; 48:909 - 30; http://dx.doi.org/10.1016/j.plaphy.2010.08.016; PMID: 20870416
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci 2004; 9:490 - 8; http://dx.doi.org/10.1016/j.tplants.2004.08.009; PMID: 15465684
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 2010; 30:161 - 75; http://dx.doi.org/10.3109/07388550903524243; PMID: 20214435
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 2010; 33:453 - 67; http://dx.doi.org/10.1111/j.1365-3040.2009.02041.x; PMID: 19712065
- Dangl JL, Dietrich RA, Richberg MH. Death don't have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996; 8:1793 - 807; http://dx.doi.org/10.1105/tpc.8.10.1793; PMID: 12239362
- Gadjev I, Stone JM, Gechev TS. Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. In: Jeon KW, ed. Int Rev of Cell and Mol Biol, Vol 270, 2008:87-144.
- Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiol Plant 2010; 138:405 - 13; http://dx.doi.org/10.1111/j.1399-3054.2009.01321.x; PMID: 20028478
- Møller IM, Sweetlove LJ. ROS signalling–specificity is required. Trends Plant Sci 2010; 15:370 - 4; http://dx.doi.org/10.1016/j.tplants.2010.04.008; PMID: 20605736
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 2011; 14:691 - 9; http://dx.doi.org/10.1016/j.pbi.2011.07.014; PMID: 21862390
- Jubany-Marí T, Munné-Bosch S, Alegre L. Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiol Biochem 2010; 48:351 - 8; http://dx.doi.org/10.1016/j.plaphy.2010.01.021; PMID: 20199867
- Moran JF, Becana M, Iturbe-Ormaetxe I, Frechilla S, Klucas RV, Aparicio-Tejo P. Drought induces oxidative stress in pea plants. Planta (Heidelberg) 1994; 194:346 - 52
- Price AH, Hendry GAF. Stress and the role of activated oxygen scavengers and protective enzymes in plants subjected to drought. Biochem Soc Trans 1989; 17:493 - 4
- Srivalli B, Sharma G, Khanna-Chopra R. Antioxidative defense system in an upland rice cultivar subjected to increasing intensity of water stress followed by recovery. Physiol Plant 2003; 119:503 - 12; http://dx.doi.org/10.1046/j.1399-3054.2003.00125.x
- Tan M, Lu J, Zhang A, Hu B, Zhu X, Li W. The distribution and cooperation of antioxidant (iso)enzymes and antioxidants in different subcellular compartments in maize leaves during water stress. J Plant Growth Regul 2011; 30:255 - 71; http://dx.doi.org/10.1007/s00344-010-9189-1
- Kang Y, Han Y, Torres-Jerez I, Wang M, Tang Y, Monteros M, et al. System responses to long-term drought and re-watering of two contrasting alfalfa varieties. Plant J 2011; 68:871 - 89; http://dx.doi.org/10.1111/j.1365-313X.2011.04738.x; PMID: 21838776
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annu Rev Plant Biol 2005; 56:187 - 220; http://dx.doi.org/10.1146/annurev.arplant.56.032604.144246; PMID: 15862094
- Tripathi BN, Bhatt I, Dietz K-J. Peroxiredoxins: a less studied component of hydrogen peroxide detoxification in photosynthetic organisms. Protoplasma 2009; 235:3 - 15; http://dx.doi.org/10.1007/s00709-009-0032-0; PMID: 19219525
- Hao FS, Wang XC, Chen J. Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci 2006; 170:151 - 8; http://dx.doi.org/10.1016/j.plantsci.2005.08.014
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 2003; 22:2623 - 33; http://dx.doi.org/10.1093/emboj/cdg277; PMID: 12773379
- Sairam RK, Dharmar K, Chinnusamy V, Lekshmy S, Joshi R, Bhattacharya P. NADPH oxidase as the source of ROS produced under waterlogging in roots of mung bean. Biol Plant 2011; 55:741 - 6; http://dx.doi.org/10.1007/s10535-011-0179-3
- Gong C-M, Bai J, Deng J-M, Wang G-X, Liu X-P. Leaf anatomy and photosynthetic carbon metabolic characteristics in Phragmites communis in different soil water availability. Plant Ecol 2011; 212:675 - 87; http://dx.doi.org/10.1007/s11258-010-9854-2
- Rizhsky L, Liang HJ, Mittler R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 2002; 130:1143 - 51; http://dx.doi.org/10.1104/pp.006858; PMID: 12427981
- Chowdhury SR, Choudhuri MA. Hydrogen peroxide metabolism as an index of water stress tolerance in jute. Physiol Plant 1985; 65:476 - 80; http://dx.doi.org/10.1111/j.1399-3054.1985.tb08676.x
- Goyal A. Effects of water stress on glycolate metabolism in the leaves of rice seedlings (Oryza Sativa). Physiol Plant 1987; 69:289 - 94; http://dx.doi.org/10.1111/j.1399-3054.1987.tb04289.x
- Mukherjee SP, Choudhuri MA. Effect of some reducing agents on water stress induced oxidative and deteriorative processes of vigna-catjang cultivar pusa-barsati seedlings. Biologia Plantarum (Prague) 1983; 25:401 - 7; http://dx.doi.org/10.1007/BF02903135
- Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 2002; 53:1331 - 41; http://dx.doi.org/10.1093/jexbot/53.372.1331; PMID: 11997379
- Mittler R, Zilinskas BA. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J 1994; 5:397 - 405; http://dx.doi.org/10.1111/j.1365-313X.1994.00397.x; PMID: 8180623
- Zlatev ZS, Lidon FC, Ramalho JC, Yordanov IT. Comparison of resistance to drought of three bean cultivars. Biol Plant 2006; 50:389 - 94; http://dx.doi.org/10.1007/s10535-006-0054-9
- Aranjuelo I, Molero G, Erice G, Avice JC, Nogués S. Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.). J Exp Bot 2011; 62:111 - 23; http://dx.doi.org/10.1093/jxb/erq249; PMID: 20797998
- Wu G, Wilen RW, Robertson AJ, Gusta LV. Isolation, chromosomal localization, and differential expression of mitochondrial manganese superoxide dismutase and chloroplastic copper/zinc superoxide dismutase genes in wheat. Plant Physiol 1999; 120:513 - 20; http://dx.doi.org/10.1104/pp.120.2.513; PMID: 10364402
- Simova-Stoilova L, Demirevska K, Petrova T, Tsenov N, Feller U. Antioxidative protection in wheat varieties under severe recoverable drought at seedling stage. Plant Soil Environ 2008; 54:529 - 36
- Terzi R, Saglam A, Kutlu N, Nar H, Kadioglu A. Impact of soil drought stress on photochemical efficiency of photosystem II and antioxidant enzyme activities of Phaseolus vulgaris cultivars. Turk J Bot 2010; 34:1 - 10
- Asada K. Ascorbate peroxidase a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant 1992; 85:235 - 41; http://dx.doi.org/10.1111/j.1399-3054.1992.tb04728.x
- Ishikawa T, Shigeoka S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci Biotechnol Biochem 2008; 72:1143 - 54; http://dx.doi.org/10.1271/bbb.80062; PMID: 18460785
- Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, et al. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 2002; 53:1305 - 19; http://dx.doi.org/10.1093/jexbot/53.372.1305; PMID: 11997377
- Sgherri CLM, Maffei M, Navari-Izzo F. Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol 2000; 157:273 - 9; http://dx.doi.org/10.1016/S0176-1617(00)80048-6
- Dayer R, Fischer BB, Eggen RIL, Lemaire SD. The peroxiredoxin and glutathione peroxidase families in Chlamydomonas reinhardtii. Genetics 2008; 179:41 - 57; http://dx.doi.org/10.1534/genetics.107.086041; PMID: 18493039
- Anderson JV, Davis DG. Abiotic stress alters transcript profiles and activity of glutathione S-transferase, glutathione peroxidase, and glutathione reductase in Euphorbia esula. Physiol Plant 2004; 120:421 - 33; http://dx.doi.org/10.1111/j.0031-9317.2004.00249.x; PMID: 15032839
- Juszczuk IM, Rychter AM. Alternative oxidase in higher plants. Acta Biochim Pol 2003; 50:1257 - 71; PMID: 14740012
- Van Aken O, Giraud E, Clifton R, Whelan J. Alternative oxidase: a target and regulator of stress responses. Physiol Plant 2009; 137:354 - 61; http://dx.doi.org/10.1111/j.1399-3054.2009.01240.x; PMID: 19470093
- Becana M, Moran JF, Iturbe-Ormaetxe I. Iron-dependent oxygen free radical generation in plants subjected to environmental stress: Toxicity and antioxidant protection. Plant Soil 1998; 201:137 - 47; http://dx.doi.org/10.1023/A:1004375732137
- Briat J-F, Ravet K, Arnaud N, Duc C, Boucherez J, Touraine B, et al. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot 2010; 105:811 - 22; http://dx.doi.org/10.1093/aob/mcp128; PMID: 19482877
- Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 2009; 1790:589 - 99; http://dx.doi.org/10.1016/j.bbagen.2008.09.004; PMID: 18929623
- Briat JF, Lobréaux S, Grignon N, Vansuyt G. Regulation of plant ferritin synthesis: how and why. Cell Mol Life Sci 1999; 56:155 - 66; http://dx.doi.org/10.1007/s000180050014; PMID: 11213255
- Briat J-F, Fobis-Loisy I, Grignon N, Lobreaux S, Pascal N, Savino G, et al. Cellular and molecular aspects of iron metabolism in plants. Biol Cell (Paris) 1995; 84:69 - 81
- Clement M, Lambert A, Herouart D, Boncompagni E. Identification of new up-regulated genes under drought stress in soybean nodules. Gene 2008; 426:15 - 22; http://dx.doi.org/10.1016/j.gene.2008.08.016; PMID: 18817859
- Fobis-Loisy I, Loridon K, Lobréaux S, Lebrun M, Briat J-F. Structure and differential expression of two maize ferritin genes in response to iron and abscisic acid. Eur J Biochem 1995; 231:609 - 19; http://dx.doi.org/10.1111/j.1432-1033.1995.tb20739.x; PMID: 7649160
- Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, Rogers EE, et al. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ 2010; 33:223 - 43; http://dx.doi.org/10.1111/j.1365-3040.2009.02073.x; PMID: 19906149
- Gogorcena Y, Iturbe-Ormaetxe I, Escuredo PR, Becana M. Antioxidant defenses against activated oxygen in pea nodules subjected to water stress. Plant Physiol 1995; 108:753 - 9; PMID: 12228507
- Sade B, Soylu S, Yetim E. Drought and oxidative stress. Afr J Biotechnol 2011; 10:11102 - 9
- Hajheidari M, Eivazi A, Buchanan BB, Wong JH, Majidi I, Salekdeh GH. Proteomics uncovers a role for redox in drought tolerance in wheat. J Proteome Res 2007; 6:1451 - 60; http://dx.doi.org/10.1021/pr060570j; PMID: 17343403
- Pruvot G, Cuiné S, Peltier G, Rey P. Characterization of a novel drought-induced 34-kDa protein located in the thylakoids of Solanum tuberosum L. plants. Planta 1996; 198:471 - 9; http://dx.doi.org/10.1007/BF00620065; PMID: 8717138
- Rey P, Pruvot G, Becuwe N, Eymery F, Rumeau D, Peltier G. A novel thioredoxin-like protein located in the chloroplast is induced by water deficit in Solanum tuberosum L. plants. Plant J 1998; 13:97 - 107; http://dx.doi.org/10.1046/j.1365-313X.1998.00015.x; PMID: 9680968
