Abstract
The influence of anoxia and hypoxia on dynamic of intracellurar pH and ATP content in rice and wheat root tips was investigated with 31P-NMR spectroscopy. Both cereals responded to hypoxia similarly, by rapid cytoplasmic acidification (from pH 7.6-7.7 to 7.1), which was followed by slow partial recovery (0.3 units). Anoxia led to a dramatic pHcyt drop in tissues of both species (from pH 7.6-7.7 to less than 7.0) and partial recovery took place in rice only. In wheat, the acidification continued to pH 6.8 after 6 h of exposure. Anoxic wheat root tips were deficient in ADH induction, whereas increased activity of alcoholic fermentation enzymes took place in anoxic rice root tips, as well as in both species after hypoxic treatment. In both plants, NTP content followed the dynamics of pHcyt. There was a strong correlation between NTP content and cytoplasmic H+ activity ([H+]cyt = 10–pHcyt) for both hypoxic and anoxic conditions. In this addendum we want to focus the reader’s attention on the importance of adequate experimental design when hypoxia is under investigation and on some further perspectives of intracellular pH regulation in plants under anaerobic conditions.
Introduction
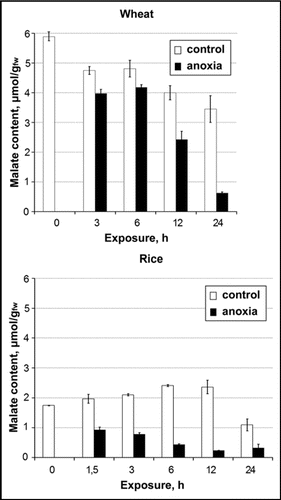
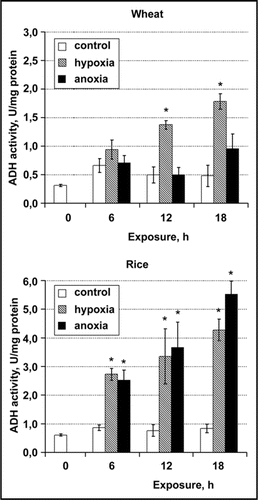
Intracellular pH regulation under oxygen deprivation stress is a complex interaction of biophysical (active H+ transport from the cytoplasm, accompanied with concurrent influxes of cations, or effluxes of anions to maintain the charge balance) and biochemical (H+ buffering and H+ producing/consuming reactions) pHcyt-stator mechanisms.Citation1,Citation2 Previously we have shown that there was a 6-fold decrease in malic acid content in rice roots after 6 h of anoxia, whereas in wheat roots malate decreased gradually during 24 h of exposure in oxygen-free conditions ().Citation3 Our recent NMR investigationsCitation4 brought to light the difference in pHcyt dynamics between rice and wheat root tips at the first hours of anoxic treatment: the initial cytoplasmic acidification in rice root tips was followed by partial recovery, which was absent in wheat root tips. The response to hypoxia was similar and recovery took place for both cereal species. There was a strong correlation between NTP content and cytoplasmic H+ activity ([H+]cyt=10−pHcyt) for both hypoxic and anoxic conditions.Citation4 Anoxic wheat root tips displayed the lowest pHcyt value and the least NTP content throughout the experiment. Further investigation showed that wheat root tips were deficient in ADH induction under anoxia, whereas in hypoxic wheat and in rice root tips in both anoxia and hypoxia the increase of ADH activity took place (, unpublished data).
The rapid decrease in endogenous malic acid content in anoxic rice roots reflects the operation of biochemical pH-stat, which seems not to be effective in wheat roots. Nevertheless, the response to hypoxia in both pHcyt dynamics and ADH induction was similar in rice and wheat root tips.
Low-oxygen state is of great interest because of hypoxia induced anoxia tolerance.Citation5 Previous investigations have concentrated predominantly on the differences between hypoxically pretreated and nonpretreated plant and tissues subjected to anoxia, whereas the investigations of hypoxic state itself are few in numbers. Hypoxia may be characterized as a condition, when oxygen concentration in the medium is lower than the critical O2 pressure for respiration (COP). This is a wide range of oxygen concentrations from zero oxygen (anoxia) to COP, where oxygen concentration is a determinant of the physiological state of cell/tissue/plant. The main O2-dependent physiological characteristic of plant cell under anaerobic condition is the energy state—ATP concentration and adenylate energy charge (AEC)—which determines the ability to maintain membrane transport and biosynthetic events. This is why in the experiments, where hypoxic conditions are applied, the degree of hypoxia should be assessed. Without such assessment it will be difficult to compare the data with any previous and future research results.
For this reason, we would like to focus the reader's attention in this addendum on the importance of adequate experimental design, especially when hypoxic state is under investigation. Also, we would like to discuss some further perspectives of investigations on intracellular pH regulation under anaerobic condition.
Hypoxic and Anoxic Experiments: The Importance of Experimental Design
The simplest approach to characterize hypoxia is to investigate the rate of O2 consumption of cell, tissue, or plant as a function pO2 followed by mathematical processing of experimental data to determine maximal rate of O2 consumption (Vmax) and COP. We have applied this approach in our investigation.Citation4 As a degree of hypoxia we used the ratio of oxygen consumption rate at exact O2 concentration to Vmax. The ratio is a dimensionless quantity and allows the comparison of plants, tissues, and conditions with different COP and Vmax.
Another characteristic of oxygen deprivation is the energy state - ATP concentration, ATP/ADP ratio or AEC. It has been shown long ago, that the value ATP/ADP ratio and AEC are controlled by pO2.Citation6 A decrease in oxygen concentration below COP lead to a decrease in the ATP content, ATP/ADP ratio and AEC value simultaneously with the rate of O2 consumption, but in tissues having a high glycolytic capacity the correlation between ATP/ADP ratio and the respiratory rate breaks down as O2 tension decreases, due to the contribution of fermentative processes. Replacement of ATP by inorganic pyrophosphate (PPi) on some steps of glycolytic cleavage under anaerobic condition may also lead to increased ATP yield of glycolysisCitation7 and affect the correlation. Hence, the measurement of O2 consumption in dependence on pO2 may not be enough to characterize experimental condition. In some cases, the measurements of ATP/ADP ratio and AEC should also to be done.
Anaerobic conditions may also be created by inhibiting the cytochrome c oxidase (COX) with azide or cyanide, rather than by removing O2 from the medium. This treatment is known as “chemical anoxia”. Despite the visual consequence of inhibitor application is similar with the real anaerobic treatment, the background could be different, as has been shown by earlier.Citation8 Sometimes inhibitor application leads to the same degree of ATP hydrolysis as anoxia, but without strong cytoplasmic acidification.Citation9 This fact reflects more site-specific effect of respiratory inhibitors compared with oxygen deprivation. Another difference between real and “chemical” anoxia is that in the latter oxygen is still present at relatively high concentrations in the medium and in the cell. When COX is inhibited, the key components of mitochondrial electron transport chain will be reduced leading to increased production of reactive oxygen species (ROS). There is evidence that ROS are signaling molecules and can regulate gene expression.Citation10 The ROS-signalling system may be expected to act in another way to the application of respiratory inhibitors compared with real anoxia and/or hypoxia.
pHcyt Regulation Under Oxygen Deprivation: Further Perspectives
To reveal the origin of cytoplasmic acidification and the mechanism of pHcyt regulation, an assessment of relative contribution from all H+-producing and consuming mechanisms should be made.Citation11 Our data show that the biochemical pH-stat plays an important role at the initial stages of low-oxygen conditions. A metabolomics approachCitation12 would allow the determination of the full set of anaerobic end-products and intermediates and to define their contribution to H+ balance in the cytoplasm. An application of metabolic control analysis (MCA)Citation13 to the processing of metabolomic data obtained, may help in the determination of enzyme reactions critical for biochemical pHcyt regulation under anaerobic condi tions. Metabolomic data combined with data of ion fluxes between the external medium, cytoplasm and vacuole, will create a full picture of pHcyt regulation in the plant tissue under investigation.
Abbreviations
| ADH | = | alcohol dehydrogenase |
| AEC | = | adenylate energy charge: [(ATP) + 0.5(ADP)] / [(ATP) + (ADP) + (AMP)] |
| ATP | = | adenosine triphosphate |
| ADP | = | adenosine diphosphate |
| COP | = | critical oxygen pressure for respiration |
| COX | = | cytochrome c oxidase |
| NMR | = | nuclear magnetic resonance spectroscopy |
| pHcyt | = | cytoplasmic pH |
| pO2 | = | partial oxygen pressure |
| Vmax | = | maximal rate of oxygen consumption |
Figures and Tables
Acknowledgements
This investigation has been supported by grants from Academy of Finland (no: 171987, 178918 and 1207898).
Addendum to:
References
- Greenway H, Gibbs J. Mechanisms of anoxia tolerance in plants. II. Energy requirement for maintenance and energy distribution to essential processes. Funct Plant Biol 2003; 30:999 - 1036
- Felle H. pH regulation in anoxic plants. Ann Bot 2005; 96:519 - 532
- Kulichikhin KYu, Kourcharova EV, Chirkova TV. The activity of malate dehydrogenases and endogenous malate content in roots of cereals under anoxic conditions. “Biologia” 2000; 3:Vestnik Sankt-Petersburgskogo Universiteta 70 - 76 Seria 3 [In Russian]
- Kulichikhin KYu, Aitio O, Chirkova TV, Fagerstedt KV. Effect of oxygen concentration on intracellular pH, glucose-6-phosphate and NTP content in rice (Oryza sativa L.) and wheat (Triticum aestivum L.) root tips: In vivo 31P-NMR study. Physiol Plantarum 2007; 129:507 - 518
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 2003; 30:1 - 47
- Raymond P, Pradet A. Stabilization of adenine nucleotide ratios at various values by an oxygen limitation of respiration in germinating lettuce (Lactuca sativa) seeds. Biochem J 1980; 190:39 - 44
- Plaxton WC. Organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 1996; 47:185 - 214
- Reid RJ, Loughman BC, Ratcliffe RG. 31P NMR measurement of cytoplasmic pH changes in maize root tips. J Exp Bot 1985; 36:889 - 897
- Gout E, Boisson A-M, Aubert S, Douce R, Bligny R. Origin of the cytoplasmic pH changes during anaerobic stress in higher plant cells. Carbon-13 and Phosphorus-31 nuclear magnetic resonance studies. Plant Physiol 2001; 125:912 - 925
- Møller IM. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 2001; 52:561 - 591
- Vartapetian BB, Jackson MB. Plant adaptation to anaerobic stress. Ann Bot 1997; 79:3 - 20
- Nicholson JK, Lindon JC, Holmes E. “Metabonomics”: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999; 29:1181 - 1189
- Plaxton WC. Storey KB. Principles of metabolic control. Functional Metabolism of Cells: Control, Regulation and Adaptation 2004; John Wiley and Sons Inc 1 - 24