Abstract
Three deep-water Mediterranean endemic species of Cystoseira spp. are found in the Port-Cros National Park (France, NW Mediterranean): C. zosteroides, C. spinosa var. compressa and C. funkii. They are abundant between 25 and 47m depth, but abundances are spatially heterogeneous, being greater on the seaward side of the park. Stands are usually dominated by one species and it is suggested that current regimes could be responsible for the observed partitioning patterns. Plant density varies depending on the species composition and location of the populations. Size structure patterns show a log-normal distribution indicating mature populations with low recruitment and/or high recruit mortality. The previously unnoticed existence of these deep-water, mature and vulnerable algal populations in one of the best studied Mediterranean areas, which was declared a National Park in 1963, indicates a need to increase basic research activities in coastal deep waters in order to increase our knowledge of Mediterranean biodiversity and underwater seascapes.
Introduction
The large literature dealing with shallow benthic algal assemblages provides considerable knowledge about their species composition, structure, dynamics and distribution. However, deep-water algal assemblages are less well known, especially in temperate waters (e.g. Spalding et al., Citation2003). Furthermore, the few existing studies of deep-water algal communities are focused on the species composition, zonation patterns (e.g. Littler et al., Citation1986; Vadas & Steneck, Citation1988; Spalding et al., Citation2003), or describe benthic algae associated with macrofauna (Sears & Cooper, Citation1978; Lewbel et al., Citation1981; Lissner & Dorsey, Citation1986; Vadas & Steneck, Citation1988; Foster & Schiel, Citation1992). Few studies are focused on deep algal assemblage structure (Ballesteros, Citation1990; Ballesteros et al., Citation1998, Citation2003; Spalding et al., Citation2003), dynamics (Ballesteros, Citation1991a; Garrabou et al., Citation2002) or biogeographic patterns (Hanisak & Blair, Citation1988).
Deep-water algal communities deserve more attention due to their floristic uniqueness (i.e. most of the species are restricted to deep water environments) and ecological and biogeographical interest (Sears & Cooper, Citation1978; Lewbel et al., Citation1981; Lissner & Dorsey, Citation1986; Vadas & Steneck, Citation1988; Foster & Schiel, Citation1992). Furthermore, as they are usually abundant close to the limits of their physiological light compensation point for growth (Markager & Sand-Jensen, Citation1992) deep-water algal species have low community dynamics, with low growth rates, long life-spans and low recruitment rates (Ballesteros, Citation1991b; Ballesteros et al., Citation1998; Garrabou & Ballesteros, Citation2000; Garrabou et al., Citation2002).
Because of their slow demographic changes, these communities may be easily affected by perturbations such as grazing or mechanical losses, changes in water turbidity, or competition with alien invasive species, and thus can be excellent indicators of anthropogenic disturbances (Markager & Sand-Jensen, Citation1992, Thibaut et al., Citation2005).
Species of the genus Cystoseira C. Agardh (Fucales, Phaeophyceae) dominate several Mediterranean rocky-bottom communities (Giaccone, Citation1973). These species are ecosystem engineers (sensu Lawton, Citation1994) as their canopy provides habitat for numerous other algae and invertebrates (Sauvageau, Citation1912; Funk, Citation1927; Rull & Gómez-Garreta, Citation1989; Ballesteros, Citation1990, Citation1992; Serio, Citation1994; Ballesteros et al., Citation1998; Bulleri et al., Citation2002). Although most species of algae inhabit shallow waters, some species only thrive in relatively deep waters where they constitute highly structured and diverse communities (Feldmann, Citation1937; Giaccone & Bruni, 1972; Ballesteros et al., Citation1998). Nevertheless, the composition, structure, dynamics and geographic distribution of these deep-water assemblages are poorly known because of the difficulties of sampling such inaccessible habitats.
Most endemic species of the genus Cystoseira in the Mediterranean and the communities they constitute are highly threatened (Boudouresque et al., Citation1990) and recent studies show that their abundance and distribution has been greatly reduced in the last 60 years (Cormaci & Furnari, Citation1999; Thibaut et al., Citation2005; Serio et al., Citation2006). Anthropogenic disturbances are generally considered to be factors in their decline, although the ultimate causes are seldom understood (Thibaut et al., Citation2005; Serio et al., Citation2006). The recent finding of a deep-water stand of Cystoseira spp. in the Port-Cros National Park (France) (Hereu et al., Citation2003) encouraged us to study the presence, composition and population structure of deep-water assemblages dominated by Cystoseira species in this Marine Protected Area. Our aims were: (i) to report on the abundance and distribution of deep-water Cystoseira species and communities throughout the Port-Cros National Park, (ii) to describe their population structure, and (iii) to incorporate our findings in order to update the known geographical distribution of these species.
Materials and methods
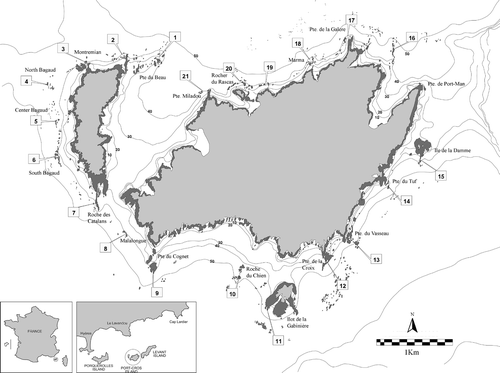
Port-Cros National Park encompasses two small islands of the Hyères archipelago (north-western Mediterranean, France), situated 5 miles offshore (). Twenty-one sites considered likely to be populated by deep water Cystoseira assemblages were a priori selected using recent topographic and bathymetric cartography (Belsher et al., Citation2005). The selected sites had rocky or pebble bottoms, and were preferably isolated rocks or seamounts highly exposed to predominating currents and situated at depths between 25 and 50m ().
Fig. 1. Map of the Port-Cros National Park with submerged rocky bottoms (shading areas). Labels indicate numbered sampling sites.
SCUBA dives were undertaken at all sites during April and May 2005, identifying all Cystoseira species in situ and collecting some plants as voucher specimens. Samples were kept in 4% formaldehyde:seawater and stored in personal herbaria. The density and size structure of Cystoseira populations were measured only when plants formed a canopy (i.e. densities higher than 1.25 ind 0.25m−2). Densities were measured by sampling haphazardly located 0.25m2 quadrats at each site (n>20, except Port-Man n=11). Size structure of the populations was evaluated by measuring the length of the main axis of all plants within the quadrats, a parameter that (i) is easy to measure underwater, (ii) is highly correlated with average yearly biomass and other morphological features of the plant, and (iii) has weak seasonal variation as it is independent of the phenological state of the plant (Ballesteros et al., Citation1998).
Data analysis
For each data series we calculated the mean size and standard deviation, kurtosis, and skewness on raw data according to Sokal & Rohlf (Citation1995). Size-frequency distributions were compared with each other (two-sample Kolmogorov–Smirnov test) and to log-normal distributions (single Kolmogorov–Smirnov test) using Lilleford probability adjustments (Legendre & Legendre, Citation1998). Similarity between size-frequency distributions was calculated with the Spearman rank-correlation coefficient by dividing colony numbers into 13 size classes based on a logarithmic scale (class borders were <1.35, 1.82, 2.46, 3.32, 4.48, 6.05, 8.16, 11.02, 14.88, 20.08, 27.11, 36.59, >49.4cm). To avoid deviations due to the low number of observations, we performed this analysis only with sites with more than 20 observations. Statistical analyses were performed using Systat 11.0 (SPSS Inc. 2004).
Results
We found three different species of Cystoseira: C. zosteroides, C. spinosa var. compressa and C. funkii and determined their distribution and abundance across sampling sites ().
Table 1. Distribution and abundance of species at the sampled sites.
Cystoseira zosteroides (Turner) C. Agardh
Cystoseira zosteroides was recorded at 15 of the 21 sites visited, distributed all around the islands (). In the north and west parts of the archipelago it was present only as isolated plants, except on Port-Man rocks (site 16), where it reached a density of 1.02 ind/0.25m2 (). At the Pointe du Vaisseau (site 13) and Ilot de la Gabinière (site 11), this species formed dense populations with 4.5 and 3.82 ind 0.25m−2, respectively, and in Ilot de la Gabinière it was mixed with isolated plants of C. funkii. In these sites, the C. zosteroides assemblage covered an extensive area (>100m2) on the flat basal part of the rocks surrounded by detritic sand. In the Pointe de la Croix (site 12) we also found a dense assemblage dominated by C. zosteroides, but within a narrow area of less than 10m2. Because of this limited distribution we did not measure population parameters ().
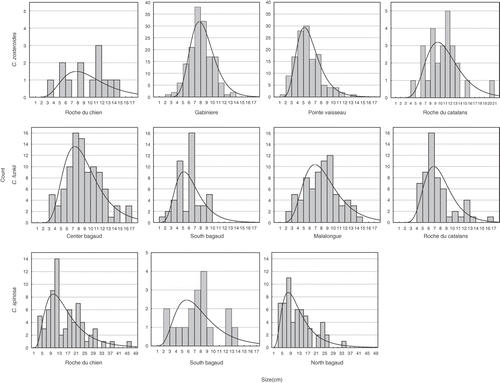
The population size structure in Pointe du Vaisseau (site 13) displayed a log-normal distribution (), with a peak within the size classes between 5 and 6cm, and a maximum length up to 14cm (). At this site we observed the introduced invasive alga Caulerpa racemosa var. cylindracea (Sonder) Verlaque, Huisman et Boudouresque (10–20% coverage) growing within the population of C. zosteroides. The size structure in Gabinière (site 11) was not log-normal (), but displayed a peak length around 7–8cm (). In the Roche des Catalans (site 7), an isolated seamount positioned between the islands of Port-Cros and Bagaud at 29–40m depth, C. zosteroides co-occurred with C. funkii and isolated individuals of C. spinosa var. compressa, as described previously by Hereu et al. (Citation2003). This mixed assemblage, with a total density of 3.89 ind 0.25m−2, covered a rocky platform of around 90m2 between 32 and 35m depth (). Cystoseira funkii was the most abundant species, with densities of 2.66 ind 0.25m−2, while C. zosteroides density was 1.19 ind 0.25m−2. Size structure of C. zosteroides showed a log-normal distribution (), but the peak was at 11–12cm, with a maximum size of 21cm (). In North Bagaud (site 4) and in Roche du Chien (site 10) Cystoseira zosteroides was present but at very low densities in assemblages dominated by C. spinosa var. compressa (). In the Port-Man rock (site 16), a flat isolated rock at 38–47m depth, an assemblage dominated by C. zosteroides with a density of 1.02 ind 0.25m−2 was found. This site was unique in the North part of the island, with a dense assemblage dominated by a Cystoseira species.
Cystoseira spinosa Sauvageau var. compressa (Ercegovic) Cormaci, Furnari, Giaccone, Scammacca et Serio
Cystoseira spinosa var. compressa was found at 13 sites at different abundances and associated with other Cystoseira species (). This species was mostly present as isolated individuals in the north and west sides of Port-Cros Island (sites 9, 12, 15, 17, 18 and 21), with dense populations found only on the south and west sides in North Bagaud (site 4) and Roche du Chien (site 10), growing on isolated rocks at depths of 35–38 and 37–42m, respectively.
Table 2. Distribution parameters data for Cystoseira species on sampled sites.
The population in North Bagaud had a density of 1.49 ind 0.25m−2 () and its size structure fits to a log-normal distribution (), with highest frequency at the 8–9cm interval and maximum height of 35cm (). In the Roche du Chien C. spinosa reached a density of 1.75 ind 0.25m−2 () displaying a log-normal size distribution (), with a peak in the 11–13cm interval and a maximum height of 47cm (). We also found isolated individuals of C. spinosa var. compressa in assemblages dominated by C. zosteroides and C. funkii on the west and the south side of the islands ().
Cystoseira funkii Schiffner ex Gerloff et Nizamuddin
Cystoseira funkii was recorded at 12 of the 21 sites. It was present all around the islands except on the east side. The abundance of this species was also variable, forming dense populations only on the west side of the islands (Centre Bagaud, site 5; South Bagaud, site 6; Catalans, site 7; Malalongue rock, site 8), growing together with C. spinosa var. compressa. These assemblages were distributed on isolated rocks at depths situated between 33 and 38m (). Densities ranged between 1.17 ind 0.25m−2 in South Bagaud to 2.74 and 2.28 ind 0.25m−2 in Centre Bagaud and Malalongue, respectively (). At the Roche des Catalans (site 7) a mixed assemblage with C. zosteroides was observed (, see results above), with C. funkii as the most abundant species reaching a density of 2.66 ind 0.25m−2. The size structure of these populations fit a log-normal distribution in all cases (), but the peak interval varied with maxima at 7–8cm, 6–7cm, 6–7cm and 9–10cm, and maximum sizes of 17, 10, 17 and 15cm, respectively ().
Comparison between communities
Size frequency distributions are given in . All distributions were more or less bell-shaped (), and all distributions except C. zosteroides from Gabinière were not statistically significantly different from a log-normal distribution (, p>0.05).
Differences in size-frequency distributions within species were relatively high (). The comparison between different species also showed a large number of significantly different distributions ().
Table 3. Probability that data between the different Cystoseira populations follow the same distribution (Kolmogorov-Smirnov test on log-transformed data).
We compared the general shape of the distributions by calculating the similarity between distributions (number of plants in 13 increasing size classes) using the Spearman rank-correlation coefficient. These comparisons showed that the similarity between distributions of the same species (from different sites) was very high in all cases (). The mean correlation coefficient of intraspecific comparisons (distributions of the same species from different sites) (mean=0.76; SD=0.19) was not different to the interspecific correlation (distribution of different species from different sites) (mean=0.61; SD=0.26) (p=0.112, Mann–Whitney U-test).
Table 4. Similarity between size-specific distribution of the different species and different sites (Spearman-rank correlation coefficient calculated on 13 size classes based on a logarithmic scale).
The relatively high degree of similarity between distributions from the same species from different sites, and between species also from different sites suggests that population structure has common characteristics for these species, independent of the site.
gives the geometric mean, minimum and maximum size, skewness, kurtosis and the probability that the sample comes from a log-normal distribution calculated on raw data and the sample size for specific populations at each site. Mean plant size was higher in C. spinosa when compared to C. zosteroides (Kruskal–Wallis 1-way ANOVA, t-statistic 63.96, p<0.001, n=447) and C. funkii (Kruskal–Wallis 1-way ANOVA, t-statistic 54.59, p<0.001, n=402), while there were no significant statistical differences between C. zosteroides and C. funkii (Kruskal–Wallis 1-way ANOVA, t-statistic 0.013, p<0.001, n=589).
Most Cystoseira size distributions were not symmetrical around the mean, but positively skewed (12 out of 13: ). This indicates plant-size distributions with relatively fewer individuals in the larger size classes and a preponderance of smaller individuals. Skewness did not vary significantly between species (F 2,10=0.196, p=0.824), reflecting similar rates of recruitment and mortality. Skewness and mean size were unrelated (r 2=0.002; p=0.87; n=10).
No significant between-species differences in Kurtosis were detected in the size-frequency distributions of Cystoseira populations (F 2,10=0.617, p=0.558).
General pattern of distribution
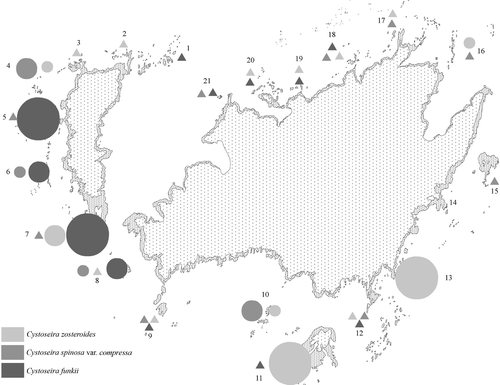
Plant densities and species composition were not homogeneous; densities were low in the north side of the island, where all species were present, while in the south and west sides of the islands high-density populations were common. The distribution of the different species also changed: C. zosteroides was dominant on the SE side of the island, while C. spinosa var. compressa and especially C. funkii dominated in the SW and W areas ().
Fig. 3. Map of the Port-Cros National Park with numbered study sites, where densities of the three Cystoseira species are represented. Diameters of the circles are proportional to the maximum densities measured: small circles: 0–1.25ind 0.25m−2; medium circles: 1.25–2.5ind 0.25m−2; big circles: >2.5ind 0.25m−2; triangles: presence. Specific values are shown in .
Discussion
The deep-water macroalgal assemblages in Port-Cros National Park are abundant and diverse. Although a large number of studies have been performed describing benthic assemblages of Port-Cros National Park (e.g. Augier & Boudouresque, Citation1967, Citation1974, Citation1975, Citation1976, Citation1978), deep-water Cystoseira communities have not previously been recorded.
Cystoseira zosteroides is relatively common in Port-Cros, especially on the east side of the island. It was previously recorded from Port-Cros on detritic bottoms around the island (Pérès & Picard, Citation1963) and observed rarely (Coppejans, Citation1977; Hereu et al., Citation2003). This species is distributed throughout the Mediterranean (Serio, Citation1995a) and can be the main species of communities occurring on rocky bottoms exposed to strong unidirectional currents and light ranging between 1% and 0.3% of surface irradiance (Giaccone & Bruni, Citation1973).
Cystoseira spinosa var. compressa was considered as a rare species in Port-Cros, being recorded as C. spinosa by Pérès & Picard (Citation1963) from detritic bottoms, and by Augier et al. (Citation1971), Belsher et al. (Citation1976) and Augier & Boudouresque (Citation1978) in several deep rocky bottoms between 36 and 45m depth. We found it mainly on the south and west sides of Port-Cros. This species is known to thrive on rocky reefs at depths ranging from 20 to 50m (e.g. Giaccone, Citation1973, Ballesteros et al., Citation1998). Cystoseira spinosa is distributed throughout the Mediterranean (Serio, Citation1995b) and can be the main taxa in communities that occur on Mediterranean rocky bottoms with light ranging between 2 and 10% of surface irradiance (Giaccone & Bruni, Citation1973).
Cystoseira funkii has been commonly misidentified as Cystoseira jabukae Ercegovic, but its taxonomic position has been validated by Verlaque et al. (Citation1999) who reported its distinctive features. The distribution of this species covers the Western Mediterranean and the westernmost part of the Eastern Mediterranean (Verlaque et al., Citation1999; Hereu et al., Citation2003; Thibaut et al., Citation2005). In this study we located several sites with dense stands of C. funkii, mainly distributed at the southwest side of the islands.
Our data indicated high spatial variability in the composition and structure of deep-water Cystoseira communities at relatively small geographic scales (hundreds to thousands of metres) in contrast to other studies that describe relatively homogeneous patterns in deep-water algal composition and distribution (Hanisak & Blair, Citation1988; Spalding et al., Citation2003). Unfortunately, at this time we do not know the causes of this heterogeneity. In this respect, depth (between 32 and 48m) and substratum (rocky bottoms) are similar at our sampling sites and anthropogenic disturbances (i.e. sewage, anchoring and small-scale, ‘artisanal’ fishing) and herbivory are low, or almost absent at all sites. The number of inhabitants of Port-Cros is around 40 people in winter, reaching a maximum of around 140 in summer and sewage treatment was implemented from 1999. Anchoring zones are outside the locations hosting Fucales, and although small-scale local ‘artisanal’ (net) fishing is permitted, its pressure is very low (a maximum of six fishermen in summer, one in winter) and is strictly regulated (PNPC, Citation2005). Diving is limited to few sites with organized mooring and is a significant distance from the majority of sampling sites. Densities of the herbivorous sea urchin Paracentrotus lividus Lamarck, 1916 are low in Port-Cros (Hereu et al., Citation2005) as its Mediterranean distribution is usually limited to shallower water environments (Chelazzi et al., Citation1997). In addition, many of the rocks were deep and isolated by surrounding sediment, thus having a low probability of being colonized by sea urchins. Sea urchins were absent at the study sites (authors' pers. obs.).
We hypothesize that differences in composition and structure of Cystoseira populations in Port-Cros National Park could be related to currents. The Port-Cros area lies within a branch of the liguro-provençal current, a general east to west geostrophic current that is confined between the Hyères archipelago (including Porquerolles, Bagaud, Port-Cros and Levant islands) and the continental coast. This current flows through the channels between the islands generating a small-scale pattern of high currents and counter-currents around the islands (Jeudy de Grissac, Citation1982). In fact, C. zosteroides, a species that thrives in strong currents (Giaccone et al., Citation1994), mainly occurs at the east side of Port-Cros where the current is funnelled by the presence of Levant Island to the East of Port-Cros. Cystoseira spinosa and C. funkii were mainly found in areas not directly exposed to the predominant east to west geostrophic current, in accordance with observations by Giaccone et al. (Citation1994). Other causes, such as patchy recruitment, or other local processes affecting these isolated populations, could also be important in determining composition and structure. However, these hypotheses need to be tested in the future by recording and describing accurately the current patterns at the different sites.
Apart from these differences, these assemblages had common characteristics in population structure that reflect low community dynamics. Plant sizes within stands of the three species were log-normal distributed, with a relatively high presence of old individuals. Ballesteros et al. (Citation1998, Citation2003) described a similar pattern for deep-water Cystoseira assemblages off Scandola (Corsica), hypothesizing that the log-normal distribution may result from intraspecific competition, where a canopy of older individuals inhibits juvenile recruitment. Recruitment would occur in pulses after a disturbance strong enough to remove part of the population (catastrophic storms, enhanced grazing). It has been reported from terrestrial studies that plant populations at equilibrium generally follow negative exponential distributions (Lorimer, Citation1985; Edmond et al., Citation1992; Berg & Harmig, Citation1994); however, in communities with strong herbivore pressure on small plants (Crisp & Lange, Citation1976; Frelich & Lorimer, Citation1985), or with strong inter-annual variations in recruitment rates (Tyrrell & Crow, Citation1994) the type of distribution can be normal or log-normal. An alternative explanation could be that recruitment is inhibited by filamentous algae such as the alien taxon Womersleyella setacea (Hollenberg) R.E. Norris (Ballesteros et al., Citation1998), an invasive species that has been found at our sampling sites (authors’ unpublished data). Nevertheless, density-dependent competition is the scenario that could better explain the log-normal size distribution. This distribution has also been found in other sites (Arrighi, Citation1995; Ballesteros et al., Citation1998, Citation2003) and seems to be a general pattern for these deep-water Cystoseira communities. Log-normal size structure distribution is also related to low population dynamics, since recruitment is low and there is an accumulation of medium and high size class individuals, with long-life spans. This pattern is supported by Ballesteros et al. (Citation2003) who describe very low annual growth rate of the main axis both for C. spinosa var. compressa and C. zosteroides (0.70cm year−1 and 0.46cm year−1 respectively), indicating that the longest specimens can reach ages of more than 50 years old. This finding agrees with some studies that describe a decrease of turnover and growth rates with depth (Bak & Luckhurst, Citation1980; Hughes & Jackson, Citation1985; Huston, Citation1985; Ballesteros, Citation1991b; Garrabou et al., Citation2002). These low dynamics reinforce the idea of the fragility of these communities since algae living in very deep waters are usually at their physiological light compensation point for growth, with no surplus energy to balance grazing, or mechanical losses (Markager & Sand-Jensen, Citation1992).
In conclusion, we have shown that deep-water Cystoseira species in Port-Cros have characteristics that are species and site specific. All species tend to have log-normal distributions in size structure and most species distributions vary at different sites. Because of the logistic problems of obtaining data due to the depth of the sampling sites, size-frequency distributions provide the only easily available indicators to assist in understanding the underlying dynamics of growth, survival and recruitment, since long-term data are lacking for most algal assemblages. Similar approaches and analyses have been used for other organisms, such as corals (Meesters et al., Citation2001), where they can detect differences in populations structure over gradients of environmental and anthropogenic disturbances.
Recent studies comparing present and historical data show a decline of Fucales in different areas of the Mediterranean (Thibaut et al., Citation2005; Serio et al., Citation2006). Furthermore, deep-water Cystoseira communities have disappeared over the last 30–40 years from several localities where they were previously known to exist, both on the continental coast of France and Spain (Thibaut et al., Citation2005; authors’ pers. obs.) and on isolated islands (Cormaci & Furnari, Citation1999; Serio et al., Citation2006). These assemblages still exist in the National Park of Port-Cros, suggesting that this area has not been affected by the causes of decline in other localities. Nevertheless, because the distribution of these deep-water communities is still largely unknown and Port-Cros has benefited from protected status for more than 40 years, further research is needed to determine if Port-Cros National Park is an exceptionally isolated site, or if these communities are also common in other places. Moreover, the previously unnoticed existence of these deep-water, mature and vulnerable algal populations in one of the best studied Mediterranean areas emphasizes the necessity of increasing basic research activities in coastal deep waters in order to increase knowledge of Mediterranean biodiversity and underwater seascapes, as a necessary step to guarantee conservation. Monitoring endemic species must be implemented in the localities where they are known to exist, even in protected, highly restricted areas, since non-controlled environmental changes such as the introduction of invasive species, or climate change will almost certainly have large effects on these assemblages in the future.
Acknowledgements
Financial support was provided by the Port-Cros National Park. We would like to thank H. Bergère, Chief de Secteur of the Port-Cros National Park, and the rangers who gave us free access to the reserve and provided all the technical, scientific and human support we requested. Special thanks also to all colleagues who assisted us in the field: J.M. Cottalorda, M.L. Susini, C. Linares, D. Diaz, and M. Zabala. We are very grateful to T. Belsher (IFREMER) for giving us access to the recent cartography of Port-Cros National Park, which allowed us to locate precisely the study sites. We are also grateful to M. Foster, A. Szoboszlai, A. Buschmann and M. Graham for their valuable comments and critical evaluation of previous versions of the manuscript. The manuscript has also benefited from a careful review by M. Verlaque and an anonymous referee. We also thank the support and help of A. Meinesz and all the researchers and technical staff of the Laboratoire Marin Littoral of the Université de Nice-Sophia Antipolis.
References
- Arrighi , F . 1995 . “ D.E.A. en Chimie de l'Environnement et Santé. ” . In Étude de la structure démographique des communautés de Cystoseira spinosa et d'un faciès de surpâturage dans la réserve naturelle de Scandola (Corse) , France : Université d'Aix Marseille .
- Augier , H and Boudouresque , CF . 1967 . Végétation marine de l’île de Port-Cros (Parc National). I. La baie de la Palu . Bull. Mus. Hist. Nat. Marseille , 27 : 93 – 124 .
- Augier , H and Boudouresque , CF . 1974 . Dix ans de recherches dans la zone marine du Parc National de Port-Cros (France). Deuxième partie . Ann. Soc. Sci. Nat. Archéol. Var. , 26 : 119 – 150 .
- Augier , H and Boudouresque , CF . 1975 . Dix ans de recherches dans la zone marine du Parc National de Port-Cros (France). Troisième partie . Ann. Soc. Sci. Nat. Archéol. Var. , 27 : 133 – 170 .
- Augier , H and Boudouresque , CF . 1976 . Végétation marine de l’île de Port-Cros (Parc National). XIII. Documents pour la carte des peuplements benthiques . Trav. Sci. Parc Nation. Port-Cros , 2 : 9 – 22 .
- Augier , H and Boudouresque , CF . 1978 . Végétation marine de Port–Cros (Parc national) XVI: contribution à l’étude de l’épiflore du détritique côtier . Trav. Sci. Parc Nation. Port–Cros , 4 : 101 – 125 .
- Augier , H , Boudouresque , C-F and Laborel , J . 1971 . Végétation marine de l’île de Port–Cros (Parc National). VII. Les peuplements sciaphiles profonds sur substrat dur . Bull. Mus. Hist. Nat. Marseille , 31 : 153 – 183 .
- Bak , RPM and Luckhurst , BE . 1980 . Constancy and change in coral reef habitats along depth gradients in Curação . Oecologia , 47 : 145 – 155 .
- Ballesteros , E . 1990 . Structure and dynamics of the community of Cystoseira zosteroides (Turner) C. Agardh (Fucales, Phaeophyceae) in the North-western Mediterranean . Sci. Mar. , 54 : 217 – 229 .
- Ballesteros , E . 1991a . Seasonality of growth and production of a deep-water population of Halimeda tuna (Chlorophyceae, Caulerpales) in the North-western Mediterranean . Botanica Marina , 34 : 291 – 301 .
- Ballesteros , E . 1991b . “ Structure and dynamics of north-western Mediterranean phytobenthos communities: a conceptual model. ” . In Homage to Ramon Margalef or, why there is such pleasure in studying nature? , Edited by: Ros , JD and Prat , N . 223 – 242 . Barcelona, , Spain : Universitat de Barcelona .
- Ballesteros , E . 1992 . Els vegetals i la zonació litoral: espècies, comunitats i factors que influeixen en la seva distribució , Barcelona, , Spain : Arxius Secció Ciències CI, Institut d’Estudis Catalans .
- Ballesteros , E , Sala , E , Garrabou , J and Zabala , M . 1998 . Community structure and frond size distribution of a deep water stand of Cystoseira spinosa (Phaeophyta) in the North-western Mediterranean . Eur. J. Phycol. , 33 : 121 – 128 .
- Ballesteros , E , Hereu , B , Zabala , M , Alcoverro , T , Garrabou , J and Sala , E . 2003 . Rapport mission Scandola. Cystoseira 2000 . Trav. Sci. Parc Nat. Rég. Corse , 60 : 95 – 115 .
- Belsher , T , Augier , H , Boudouresque , CF and Coppejans , E . 1976 . Inventaire des algues marines benthiques de la rade et des îles d’Hyères (Méditerranée, France) . Trav. Sci. Parc Nation. Port Cros , 2 : 39 – 89 .
- Belsher , T , Houlgatte , E and Boudouresque , CF . 2005 . Cartographie de la prairie à Posidonia oceanica et des principaux faciès sédimentaires marins du Parc national de Port-Cros (Var, France, Méditerranée) . Sci. Rep. Port-Cros Natl. Park , 21 : 19 – 28 .
- Berg , EE and Harmig , JL . 1994 . Spatial and genetic structure of two sandhill oaks: Quercus levis and Quercus margaretta (Fagaceae) . Am. J. Bot. , 81 : 7 – 14 .
- Boudouresque , CF , Ballesteros , E , Ben Maiz , N , Boisset , F , Bouladier , E , Cinelli , F , Cirik , S , Cormaci , M , Jeudy De Grissac , A , Laborel , J , Lanfranco , E , Lundberg , B , Mayhoub , H , Meinesz , A , Panayotidis , P , Semroud , R , Sinnassamy , JM , Span , A and Vuignier , G . 1990 . Livre Rouge «Gérard Vuignier» des Végétaux, Peuplements et Paysages Marins Menacés de Méditerranée , MAP Technical Reports Series (UNEP), no. 43. Available at: http://195.97.36.231/acrobatfiles/MTSAcrobatfiles/mts43.pdf, accessed 27 January 2008
- Bulleri , F , Benedetti-Cecchi , L , Acunto , S , Cinelli , F and Hawkins , SJ . 2002 . The influence of canopy algae on vertical patterns of distribution of low-shore assemblages on rocky coasts in the northwest Mediterranean . J. Exp. Mar. Biol. Ecol. , 267 : 89 – 106 .
- Chelazzi , G , Serra , G and Bucciarelli . 1997 . Zonal recovery after experimental displacement in two sea urchins co-occurring in the Mediterranean . J. Exp. Mar. Biol. Ecol. , 212 : 1 – 7 .
- Coppejans , E . 1977 . Bijdrage tot de studie van de wierpopulaties (Chlorophyceae, Phaeophyceae, Rhodophyceae) van het fotofiel infralittoraal in het noordwestelijk mediterraan bekken. Deel I: tekst , Thesis Belgium : University of Ghent .
- Cormaci , M and Furnari , G . 1999 . Changes of the benthic algal flora of the Tremiti Islands (Southern Adriatic) Italy . Hydrobiologia , 398–399 : 75 – 79 .
- Crisp , MD and Lange , RT . 1976 . Age structure, distribution and survival under grazing on the arid subzone shrub Acacia burkitii . Oikos , 27 : 86 – 96 .
- Edmond , RL , Thomas , TB and Maybury , KP . 1992 . Tree populations dynamics, growth and mortality in old growth forests in the Western Olympic Mountains, Washington . Can. J. Forest Res. , 23 : 512 – 519 .
- Feldmann , J . 1937 . Recherches sur la végétation marine de la Méditerranée. La côte des Albères , Rouen, , France : Imprimerie Wolf .
- Foster , MS and Schiel , DR . 1992 . “ Zonation, El Niño disturbance and the dynamic of subtidal vegetation along a 30m depth gradient in two giant kelp forests. ” . In Proceedings of the Second International Temperate Reef Symposium , Edited by: Battershill , CN , Schiel , DR , Jones , GP , Creese , RG and MacDiarmnid , AB . 151 – 162 . Wellington, Aukland, , New Zealand : NIWA Marine .
- Frelich , LE and Lorimer , CG . 1985 . Current and predicted long-term effects of deer browsing in hemlock forests in Michigan, USA . Biol. Cons. , 34 : 99 – 120 .
- Funk , G . 1927 . Die Algenvegetation des Golfs von Neapel . Pubbl. Staz. Zool. Napoli , 7 ( Suppl ) : 1 – 507 .
- Garrabou , J and Ballesteros , E . 2000 . Growth of Mesophyllum alternans and Lithophyllum frondosum (Corallinales, Rhodophyta) in the northwestern Mediterranean . Eur. J. Phycol. , 35 : 1 – 10 .
- Garrabou , J , Ballesteros , E and Zabala , M . 2002 . Structure and dynamics of north-western Mediterranean rocky benthic communities along a depth gradient . Estuar. Coast. Shelf Sci. , 55 : 493 – 508 .
- Giaccone , G . 1973 . Elementi di botanica marina. I. Bionomia bentonica e vegetazione sommersa del Mediterraneo , Trieste, , Italy : Pubblicazioni Istituto Botanico Università di Trieste, serie didattica .
- Giaccone , G and Bruni , A . 1973 . Le cistoseire e la vegetazione sommersa del Mediterraneo . Atti. Ist. Veneto di Sci. Lett. e Arti , 131 : 59 – 103 .
- Giaccone , G , Alongi , G , Pizzuto , F and Cossu , A . 1994 . La vegetazione marina bentonica fotofila del Mediterraneo: II. Infralitorale e circalitorale. Proposte di aggiornamento . Boll. Acc. Gioenia Sci. Nat. , 27 : 111 – 157 .
- Hanisak , MD and Blair , SM . 1988 . The deep-water macroalgal community of the East Florida continental shelf (USA) . Helgol. Meeresunt , 42 : 133 – 163 .
- Hereu , B , Zabala , M and Ballesteros , E . 2003 . On the occurrence of a population of Cystoseira zosteroides Turner and Cystoseira funkii Schiffner ex Gerloff et Nizamuddin (Cystoseiraceae, Fucophyceae) in the Port-Cros National Park (Northwestern Mediteranean, France) . Sci. Rep. Port-Cros Natl. Park , 19 : 93 – 99 .
- Hereu , B , Linares , C , Diaz , D , Dantart , L , Garrabou , J , Sala , E , Ballesteros , E , Harmelin , JG and Zabala , M . 2005 . “ Indicateurs de Biodiversité en milieu marin: les échinodermes. ” . In Fluctuations temporelles des peuplements d’Échinodermes a Port-Cros 1982–2003. , Technical report for the Port-Cros National Park (France)
- Hughes , TP and Jackson , JB . 1985 . Population dynamics and life histories of foliaceous corals . Ecol. Monogr. , 55 : 141 – 166 .
- Huston , MA . 1985 . Variation in coral growth rates with depth at Discovery Bay, Jamaica . Coral Reefs , 4 : 19 – 25 .
- Jeudy De Grissac , A . 1982 . Approche de la courantologie dans la baie de Port-cros et dans la passe entre Port-Cros et Bagaud . Trav. Sci. Parc Nation. Port–Cros , 8 : 93 – 106 .
- Lawton , JH . 1994 . What do species do in ecosystems? . Oikos , 71 : 367 – 374 .
- Legendre , P and Legendre , L . 1998 . Numerical Ecology , 2nd English Edition , Elsevier, Amsterdam, The Netherlands .
- Lewbel , GS , Wolfson , A , Gerrodette , T , Lippincott , WH , Wilson , JL and Littler , MM . 1981 . Shallow-water benthic communities on California's outer continental shelf . Mar. Ecol. Prog. Ser. , 4 : 159 – 168 .
- Lissner , AL and Dorsey , JH . 1986 . Deep-water biological assemblages of a hard-bottom bank-ridge complex of the southern California continental borderland . Bull. South. Calif. Acad. Sci. , 85 : 87 – 101 .
- Littler , MM , Littler , DS , Blair , SM and Norris , JN . 1986 . Deep water plant communities from an uncharted seamount off San Salvador Island, Bahamas: distribution, abundance, and primary productivity . Deep-Sea Res. , 33 : 81 – 92 .
- Lorimer , CG . 1985 . Methodological considerations in the analysis of forest disturbance history . Can. J. Forest Res. , 15 : 200 – 213 .
- Markager , S and Sand-Jensen , K . 1992 . Light requirements and depth zonation of marine macroalgae . Mar. Ecol. Prog. Ser. , 88 : 83 – 92 .
- Meesters , EH , Hilterman , M , Kardinaal , E , Keetman , M , De Vries , M and Bak , RPM . 2001 . Colony size-frequency distributions of scleractinian coral populations: spatial and interspecific variation . Mar. Ecol. Prog. Ser. , 209 : 43 – 54 .
- Pérès , JM and Picard , J . 1963 . Aperçu sommaire sur les peuplements marins entourant l’île de Port–Cros . Terre et Vie , 110 : 436 – 448 .
- PNPC . 2005 . Atlas du Parc National de Port-Cros .
- Rull , J and Gómez-Garreta , A . 1989 . Distribución de algas epífitas sobre los ejemplares de Cystoseira mediterranea Sauv . Anal. Jardín Bot. Madrid , 46 : 99 – 106 .
- Sauvageau , C . 1912 . À propos des Cystoseira de Banyuls et de Guéthary . Bull. Station Biol. d'Arcachon , 14 : 133 – 556 .
- Sears , JR and Cooper , RA . 1978 . Descriptive ecology of offshore deep-water, benthic algae in the temperate western North Atlantic Ocean . Mar. Biol. , 44 : 309 – 314 .
- Serio , D . 1994 . On the structure, typology and periodism of Cystoseira spinosa Sauvageau community and Cystoseira zosteroides C. Agardh community from eastern coast of Sicily (Mediterranean sea) . Giorn. Bot. Ital. , 128 : 941 – 973 .
- Serio , D . 1995a . Fenologia morfologica e riproduttiva di Cystoseira zosteroides C. Agardh (Fucales, Phaeophyceae) . Boll. Acc. Gioenia. Sci. Nat. , 28 : 45 – 58 .
- Serio , D . 1995b . Fenologia morfologica e riproduttiva di Cystoseira spinosa Sauvageau v. compressa (Ercegovic) Cormaci et al. (Fucales, Fucophyceae) . Boll. Acc. Gioenia. Sci. Nat. , 28 : 5 – 22 .
- Serio , D , Alongi , G , Catra , M , Cormaci , M and Furnari , G . 2006 . Changes in the benthic algal flora of Linosa Island (Strait of Sicily, Mediterranean Sea) . Bot. Mar. , 49 : 135 – 144 .
- Sokal , RR and Rohlf , FJ . 1995 . Biometry: the principles and practice of statistics in biological research , 3rd edition , New York, , USA : W. H. Freeman and Co. .
- Spalding , H , Foster , MS and Heine , JN . 2003 . Composition, distribution, and abundance of deep-water (>30m) macroalgae in Central California . J. Phycol. , 39 : 273 – 284 .
- Thibaut , T , Pinedo , S , Torras , X and Ballesteros , E . 2005 . Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean) . Mar. Poll. Bull. , 50 : 1472 – 1489 .
- Tyrrell , LE and Crow , TR . 1994 . Structural characteristics of old-growth hemlock-hardwood forests in relation to age . Ecology , 75 : 370 – 386 .
- Vadas , RL and Steneck , RS . 1988 . Zonation of deep-water benthic algae in the Gulf of Maine . J. Phycol. , 24 : 338 – 46 .
- Verlaque , M , Ballesteros , E , Sala , E and Garrabou , J . 1999 . Cystoseira jabukae (Cystoseiraceae, Fucophyceae) from Corsica (Mediterranean) with notes on the previously misunderstood species C. funkii . Phycologia , 38 : 77 – 86 .