Abstract
Polysaccharide A (PSA) derived from the human commensal Bacteroides fragilis is a symbiosis factor that stimulates immunologic development within mammalian hosts. PSA rebalances skewed systemic T helper responses and promotes T regulatory cells (Tregs). However, PSA-mediated induction of Foxp3 in humans has not been reported. In mice, PSA-generated Foxp3+ Tregs dampen Th17 activity thereby facilitating bacterial intestinal colonization while the increased presence and function of these regulatory cells may guard against pathological organ-specific inflammation in hosts. We herein demonstrate that PSA induces expression of Foxp3 along with CD39 among naïve CD4 T cells in vitro while promoting IL-10 secretion. PSA-activated dendritic cells are essential for the mediation of this regulatory response. When cultured with isolated Foxp3+ Tregs, PSA enriched Foxp3 expression, enhanced the frequency of CD39+HLA-DR+ cells, and increased suppressive function as measured by decreased TNFα expression by LPS-stimulated monocytes. Our findings are the first to demonstrate in vitro induction of human CD4+Foxp3+ T cells and enhanced suppressive function of circulating Foxp3+ Tregs by a human commensal bacterial symbiotic factor. Use of PSA for the treatment of human autoimmune diseases, in particular multiple sclerosis and inflammatory bowel disease, may represent a new paradigm in the approach to treating autoimmune disease.
Abbreviations
| B. fragilis | = | Bacteroides fragilis |
| DC | = | Dendritic cell |
| GF | = | Germ Free |
| MS | = | Multiple sclerosis |
| NCD4 | = | Naïve CD4 |
| PBMCs | = | Peripheral blood mononuclear cells |
| pDC | = | Plasmacytoid dendritic cell |
| PSA | = | Polysaccharide A |
| Sp1 | = | Streptococcus pneumoniae polysaccharide type 1 |
| SPF | = | Specific pathogen free |
| Treg | = | T regulatory cell |
| ZPS | = | Zwitterionic polysaccharide. |
Introduction
The intestinal microbiota profoundly shapes host immune responses.Citation1,2 Mice raised under germ free (GF) conditions lack commensal influence during development. As a result, GF animals exhibit disorganized lymphoid tissue and aberrant immune responses compared to specific pathogen free (SPF) mice, which undergo conventional microbial intestinal colonization.Citation3 Such deficits may be corrected by the introduction of commensal species to host mice suggesting a potent modulatory role for the microbiota.Citation4 Closer examination of several individual species clearly demonstrates their capacity to provoke divergent immune responses in mature mice. For example, Segmented filamentous bacteria promote inflammatory Th17 responsesCitation5 while Bacteroides fragilis (B. fragilis) colonization is associated with potentiating Th1 and Treg activity.Citation4
Of the 8 types of surface capsular polysaccharides expressed by B. fragilis, PSA, a zwitterionic polysaccharide (ZPS), is a known immunomodulator and symbiotic factor, directing host immune responses while promoting maintenance of the organism in vivo. This unique polysaccharide possesses the capacity to elicit T cell responses that are essential to the immune regulatory effects observed in hosts.Citation4,6 The otherwise Th2-skewed immune system in GF mice is rebalanced to reflect appropriate Th1 responses upon B. fragilis colonization. Furthermore, deficiency in Foxp3+ Tregs observed in GF mice is corrected upon exposure to PSA.Citation7,8 Later studies elaborated the biologically important role these Tregs play in B. fragilis survival in the host. Foxp3 Tregs were shown to be responsible for attenuating host Th17 cells in the gut, which would otherwise limit B. fragilis colonization.Citation9 The induction of Foxp3+ Tregs likewise is associated with PSA-mediated protection against murine autoimmune pathologies.Citation8,10 PSA significantly enhances the conversion of CD4+ T cells into IL-10-producing Foxp3+ Tregs. Furthermore, Foxp3+CD4+ Tregs in PSA-treated mice demonstrated enhanced functional suppression, increased Foxp3 and IL-10 compared to PBS controls.Citation8 Thus the induction of Foxp3+ Tregs directly represents a commensal mediated immune response that holds potential benefit for both bacteria and host alike.
To date, the association of PSA exposure and induction of Foxp3 in humans has not been reported. Whether induction of a Foxp3 population in humans is important for the maintenance of B. fragilis in human hosts has not been established. However, promotion of Foxp3 frequency and function by PSA would suggest the capacity of PSA to influence human disease in which Treg disparities have been observed such as multiple sclerosis (MS). We therefore investigated whether PSA induces Foxp3 in human T cells. In this report we demonstrate that this commensal-derived antigen promotes a CD39+Foxp3+ population among naïve CD4 T cells while enhancing IL-10 production. Induction of this population required cognate interactions with dendritic cells bearing HLA-DR, CD86, CD40 and PD-L1. PSA also increased the expression of Foxp3, CD39 and HLA-DR in Tregs, and enhanced their suppressive function in vitro.
Results
Dendritic cells are required for PSA-mediated human Foxp3+CD4+ T cell induction
Studies describing murine T cell responses to PSA in vivo demonstrate the induction of Tregs that protect against 2 distinct models of autoimmunity, experimental colitis (inflammatory bowel disease) and experimental autoimmune encephalomyelitis (multiple sclerosis). To determine whether PSA would promote Foxp3 expression in human T cells, DCs were isolated from whole peripheral blood and co-cultured with autologous naïve CD4+CD25− T cells (NCD4) in the presence or absence of PSA. As shown (), PSA promoted CD4+Foxp3+ T cells in a DC-dependent manner, as no enhancement of Foxp3 was detected in wells containing NCD4s alone. Other traditional antigen presenting cells derived from the peripheral circulation, including monocytes and B cells, were unable to induce this population (). Foxp3 induction was only observed in the DC-NCD4 context; by comparison, use of PSA in a mixed population of peripheral blood mononuclear cells (PBMCs), had no effect on Foxp3 expression (Fig. S1).
Figure 1. Dendritic Cells mediate PSA induction of Human CD39+Foxp3+ CD4+ T cells. PSA-mediated induction of CD39+Foxp3+ T cells was observed in the presence of DCs but not other APCs. 3 × 104 NCD4s were cultured in the presence or absence of 25 µg/ml PSA or Sp1 and 100 U/ml IL-2, alongside 5 × 103 of one of 3 primary autologous APC populations: CD19+ B cells, CD14+ monocytes, or total blood dendritic cells. (A) Fold induction of Foxp3+ frequency alone or in the presence of autologous DCs, n = 4. (B) Representative histograms showing frequency of Foxp3+ cells (red line) as an overlay against isotype control (shaded blue), panels were pre-gated on CD4+ cells. (C) Replicates of CD4+Foxp3+ frequency, n = 3. (D) Representative FACS plots showing frequency of CD25 and CD39 cells, pre-gated on Foxp3+ cells. Positive cells (red line) are overlaid against respective isotype controls (shaded blue). (E) PSA dose response of CD4+CD39+Foxp3+ T cells, n = 1 (Bars represent means of 4 culture wells per condition, error bars represent standard error of the mean). (F) Individual repeats of CD39+Foxp3+ T cells, n = 4–14 per condition. P values were calculated using 2-tailed Student's t-test (A, C) or one-way ANOVA using Dunnett's multiple comparison test (E, F).
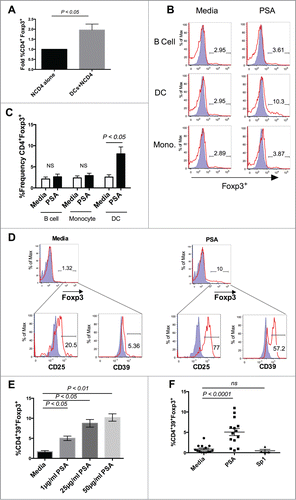
PSA promotes CD39 expression among human T cells
CD39 is an ectonuclease that cleaves extracellular ATP into ADP. In conjunction with the enzyme CD73, CD39 limits inflammation by converting inflammatory extracellular ATP into adenosine, which possesses anti-inflammatory properties. Human CD39 polymorphisms have been associated with inflammatory bowel disease.Citation11 Furthermore, in response to rapamycin-mediated acquisition of suppressive function, induced human Foxp3+ Tregs up-regulated CD39.Citation12 We recently showed that the absence of CD39 expression ablates PSA protection against murine CNS inflammation, supporting a regulatory role for this enzyme.Citation13 We evaluated PSA-induced Foxp3+ T cells for CD39 expression. In addition to dramatically up-regulating CD25, 60% of PSA-induced Foxp3+ T cells expressed CD39 (). PSA significantly enhanced this population compared to media controls in a dose dependent manner (). Induction of Foxp3+ T cells was not observed in the presence of control ZPS Streptococcus pneumoniae polysaccharide type 1 (Sp1) ().
PSA induction of CD39+Foxp3+ T cells requires engagement of HLA-DR and costimulatory molecules
Zwitterionic polysaccharides are unique in their capacity to generate αβCD4+ T cell responses via presentation on MHC-II.Citation14 Following endocytosis, PSA is processed prior to expression on the surface of APCs for presentation.Citation15 Both Sp1 and PSA were shown to interact with MHC-II as demonstrated by immunoprecipitation and confocal fluorescence.Citation16 Furthermore CD11c+ DCs from PSA-treated mice expressed enhanced levels of MHC-II as well as CD86 in a dose dependent manner.Citation4,10 We examined the capacity of PSA to modulate surface expression of several APC/Immunologic synapse proteins including HLA-DR, CD80, CD86, CD40, and PD-L1 by geometric mean fluorescence intensity (GMFI) approximately 16 hours post culture. PSA significantly upregulated HLA-DR (P = 0.009), CD86 (P = 0.02), and PD-L1 (P = 0.02) while also increasing surface expression of CD80 and CD40 ().
Figure 2. PSA induction of CD39+Foxp3+ T cells requires engagement of HLA-DR and costimulatory molecules. Blocking HLA-DR or costimulatory molecules limited PSA-mediated induction of CD39+Foxp3+ T cells and cytokine production. (A) 5 × 104 DCs were cultured with 25 µg/ml PSA for approximately 16 hours before being stained for surface expression of the above markers, n = 3–6. DCs were incubated with 10 µg/ml of blocking antibody specific to CD86, PD-L1, HLA-DR or CD40L for 30 min prior to co-culture with 3 × 104 autologous NCD4s in the presence or absence of 25 µg/ml PSA and 100 U/ml IL-2. Supernatants were assessed by ELISA for IL-10 or IFNγ. (B) CD4+CD39+Foxp3+ T cell frequency, n = 6–10 per condition (C) IL-10 and IFNγ production, n = 4–10 per condition. Error bars reflect standard error of the mean representing independent experiments, each using cells from different individuals. P values were calculated using 2-tailed Student's t-test (A) or one-way ANOVA using Dunnett's multiple comparison test (B, C).
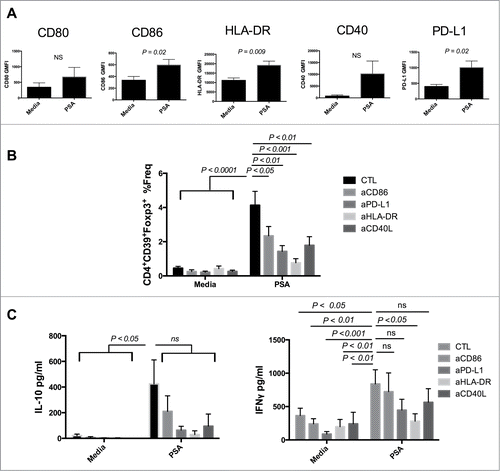
We investigated whether inhibition of the upregulated synaptic components in response to PSA impacted induction of human CD39+Foxp3+ T cells by pre-incubating DCs with blocking antibodies either to HLA-DR, CD86, CD40L or PD-L1. Blockade of any of these components significantly attenuated PSA-mediated induction of CD39+Foxp3+ T cell frequency. Substantial reduction followed blockade of CD86 (P < 0.05), PD-L1 (P < 0.01) or CD40L (P < 0.01) while near complete ablation occurred when HLA-DR (P < 0.001) was inhibited (). In vitro culture with PSA promotes T cell secretion of IFNγ and IL-10 in mice and humans.Citation4,17 We determined that compared to media controls PSA enhanced both cytokines in cultures containing PSA-induced CD4+CD39+Foxp3+ T cells (). No IL-17 was detected in supernatants (not shown). High inter-individual variation in IL-10 production was observed, whereas the levels of IFNγ detected were more consistent. We examined whether the same synaptic components assessed previously were central in mediating PSA-dependent cytokine production. Blockade of each component substantially reduced the average amount of IL-10, with anti-CD86 being least effective. There was significant attenuation of PSA-mediated IFNγ production following antibody blockade of HLA-DR (P < 0.05). In contrast, blocking CD86, or CD40L yielded little to no impact while inhibition of PD-L1 resulted in a further diminished, albeit non-significant, reduction of IFNγ ().
PSA enhances circulating CD39+ Treg frequency
Tregs expressing high levels of Foxp3 may be directly isolated from circulating human blood.Citation18 In addition to constitutive Foxp3, these circulating Tregs possess high levels of CD25 and may express low amounts of CD127 (). Circulating Foxp3+ Tregs represent a diverse array of subpopulations with varying suppressive potential. Numerical deficiencies or impaired ability to control pathological inflammation in particular subpopulations of these regulatory cells have been associated with autoimmune diseases, including human MS. Individuals with relapsing-remitting MS (RRMS) possess a reduced number of circulating CD39+Foxp3+ Tregs compared to healthy individuals.Citation19 In addition to being numerically deficient, these CD39+ Tregs are deficient in their capacity to suppress the production of IL-17 by inflammatory cells when compared to healthy individuals. We next evaluated whether CD39 expression was up-regulated on human circulating Tregs. Tregs cultured alongside DCs in the presence of PSA significantly increased expression of CD39 as measured by GMFI. PSA also enhanced the frequency of CD39+ circulating Tregs (). PSA did not significantly impact circulating Treg production of IL-10 or IFNγ (Fig. S2).
Figure 3. PSA increases the frequency of CD39-expressing Tregs and Treg function. PSA exposure significantly enhanced the frequency of CD39 and HLA-DR-expressing circulating Tregs and resulted in greater capacity to suppress monocyte secretion of TNFα. 1 × 104 Tregs were cultured in the presence or absence of PSA and 100 U/ml IL-2, alongside 5 × 103 autologous DCs. (A) Representative histograms of different Treg-associated markers. Positive cells (red line) are overlaid against respective isotype controls (shaded blue) (B) CD39 MFI, n = 11 (left), representative CD39+Foxp3+FACS panel pre-gated on CD4+ cells (center), replicates of CD39+Foxp3+Treg frequency, n = 12 (right). (C) HLA-DR MFI, n = 7 (left), Representative CD39+HLA-DR+ Treg FACS panel pre-gated on CD4+Foxp3+ cells (center), replicates of CD39+ HLA-DR+ Treg frequency, n = 7 (right). (D) Foxp3 MFI of CD4+ Tregs, n = 12 (E) Suppression of LPS-induced TNFα production, n = 3. Error bars reflect standard error of the mean representing independent experiments, each using cells from different individuals. P values were calculated using 2-tailed Student's t-test (B–D) or one-way ANOVA using Dunnett's multiple comparison test (E).
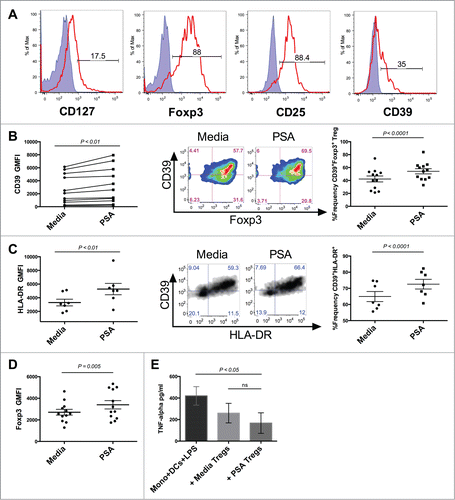
PSA promotes Treg suppressive function
HLA-DR+ Tregs are mature Tregs with acute suppressive functional capacity.Citation20 These Tregs express the highest levels of Foxp3, and are poor IL-10 producers, suppressing primarily through contact-dependent mechanisms. We examined PSA-exposed Treg cultures for HLA-DR+ cells finding that the majority of HLA-DR+ cells co-expressed CD39. Culturing with PSA significantly increased the frequency of this double positive population as well as HLA-DR GMFI () suggesting that PSA-exposed Tregs possess greater suppressive function. Foxp3 expression has been shown to correlate with Treg suppressive function.Citation21 An assessment of Foxp3 GMFI revealed that PSA significantly enhanced Foxp3 levels among Tregs, further supporting the notion that PSA promotes Treg suppressive function (). To evaluate enhanced suppression we measured the ability of PSA-exposed Tregs to suppress TNFα production by monocytes stimulated with LPS. PSA-exposed Tregs reduced the average amount of LPS-induced monocyte TNFα to a greater degree than Tregs cultured in media alone ().
Discussion
The capacity of gut derived commensal bacterial antigen to induce immune regulation is an important paradigm in understanding immune homeostasis. Homeostasis is achieved in the gut microbiome by the wide and diverse range of microorganisms that either effect inflammation or conversely regulate the host. This binary system of induction and regulation is essential to maintain immune balance. When the microbiome is distorted, the dysbiosis can lead to a disease state either by an excessive inflammatory response or poorly regulated immune control. In certain autoimmune conditions, including human MS, current evidence indicates that dysfunctional Tregs may be where homeostasis is lost leading to active disease. We have observed that a specific symbiotic factor and capsular polysaccharide antigen derived from the human commensal B. fragilis has the capacity to induce a population of phenotypically specific and biologically active regulatory T cells.
In this work, we demonstrate that in vitro culture of PSA with NCD4s in the presence of primary peripheral blood DCs leads to the generation of Foxp3+ T cells. These Foxp3+ cells bear high levels of CD25 and low amounts of CD127, matching fundamental surface phenotypic definitions for Tregs. The role of Foxp3 in Treg generation and function has been revised in recent years. Multiple studies reveal that expression of Foxp3 alone, such as that mediated by strong T cell receptor activation in combination with TGF-β, does not guarantee suppressive function.Citation22–26 Contemporary Foxp3+ Treg definitions describe a heterogeneous and plastic continuum of Foxp3+ CD4 T cells ranging in regulatory potential. A combination of appropriate transcriptional, epigenetic and environmental factors in vivo determines the acquisition, maintenance and enrichment of Foxp3 facilitating regulatory phenotype.Citation27 Although these studies are limited by the in vitro context of our experimental system we do show for the first time that an isolated commensal antigen is capable of inducing Foxp3 expression in human NCD4s in vitro without polyclonal anti-CD3 signaling or supplementation by exogenous agents such as TGF-β, and rapamycin.
CD39 along with CD73 are part of a sensitive purigenic signaling network by which extracellular nucleotides are detected and degraded in the pericellular environment thereby influencing immune responses.Citation28 Aside from direct contributions to suppression via generation of adenosine, CD39 may function to prolong Treg longevity by resistance to apoptosis and facilitate entry to inflamed tissue sites.Citation29 We show that CD39 is up-regulated by PSA on human Foxp3+ T cells. CD39 up-regulation was also observed after in vitro stimulation along with exposure to rapamyacin endowed suppressive function to human Foxp3+ T cells.Citation12 Interestingly, culture with Sp1 was unable to induce this population, stressing a unique functional role for PSA in mediating human T cell responses. Perhaps the endogenous presence of Bacteroides fragilis within the microbiota of donors used in this study and it's reported role in maturing host immune systems may contribute to the observed in vitro response to exogenous stimulation with PSA. Although CD39 possesses direct regulatory functions (in conjunction with CD73) and may be highly expressed on Foxp3+ Tregs, human lymphocytes of both regulatory and inflammatory disposition are capable of expressing CD39.Citation30,31 CD39+ Th17 cells have been reported in a number of studies, showing the enrichment of these cells within inflamed synovial and intestinal tissue of individuals with rheumatoid arthritis and inflammatory bowel disease.Citation32,33 IL-17 secretion by CD39+Foxp3− cells was consistently reported in these instances. It is therefore noteworthy that PSA cultures, despite containing CD39+Foxp3− CD4 T cells, did not produce detectable levels of IL-17.
The requirement of an APC for Foxp3 induction among human NCD4s is in line with well-defined mechanisms underlying processing and presentation of PSA to activate T cells. While other APCs may facilitate ZPS-mediated human T cell activation, DCs play a prominent role during PSA mediated protection from inflammatory disease in vivo as well as in vitro T cell responses to PSA. Fluorescently labeled PSA was shown to associate with DCs in the mesenteric lymph nodes of mice when orally delivered.Citation4 Further, DCs are essential mediators of PSA's downstream therapeutic effects. In response to oral treatment with PSA, CD103+ DCs were enriched in the cervical lymph nodes of EAE-afflicted mice mediating induction of Foxp3+ Tregs.Citation10 A recent study showed that plasmacytoid dendritic cells (pDCs) were the primary APCs responsible for promoting CD4+ T cell-produced IL-10 and protection from experimental colitis.Citation34 Our results are in agreement with this DC-centric paradigm, as PSA-mediated Foxp3 acquisition in human NCD4s required DCs whereas other APCs isolated from PBMCs were insufficient to promote this induction. Interestingly, we note that human primary pDCs isolated from the blood were unable to induce Foxp3 (not shown) suggesting that myeloid DCs may be primarily involved in PSA-mediated Foxp3 expression in humans consistent with our previously reported observations in mice. Finally, previous work that employed a culture system that utilized T-cell depleted PBMCs as APCs found that PSA did not induce Foxp3 among human CD4+ T cells.Citation17 We observed a similar readout when PSA was cultured with total PBMCs (data not shown). This lack of Treg activation potential may be due to the paucity of circulating DCs in the whole peripheral blood culture (less than 1% of total PBMCs).
Zwitterionic polysaccharides significantly up-regulate immunologic synapse components MHC-II, CD86, CD40, and PD-L1 on DCs as well as on other APCs. Further, HLA-DR, CD86, and CD40 are pivotal to downstream ZPS-mediated effects on murine and human T cells.Citation4,10,16,34,35 We utilized blocking antibodies to assess the role of CD86, PD-L1, HLA-DR and CD40L in the induction of human CD39+Foxp3+ T cells and cytokine production. Alongside CD39+Foxp3+ induction, both IL-10 and IFNγ were significantly enhanced in PSA-containing cultures. Disrupting PSA signal 1 by blocking HLA-DR consistently prevented both induction of Foxp3+ T cells and cytokine production.Citation4,16 By comparison, blockade of CD86 significantly reduced PSA-mediated induction by approximately 50%. This reduction of Foxp3+ T cell frequency by anti-CD86 parallels the central role of CD86 during human T cell proliferation mediated by the ZPS Sp1.Citation35 We observed that CD86 blockade also gave rise to a substantial (50%) reduction of the average amount of IL-10 produced. This was consistent with IL-10 inhibition observed when pDCs from CD86 knockout mice were used in vitro during a previous study.Citation34 However, given the lack of statistical significance, CD86, while necessary for activation and induction of Foxp3 by PSA, may play a less prominent role for PSA-mediated cytokine production by human cells in vitro.
PD-L1 is typically known as a negative regulator of T cell responses; however, this costimulatory molecule also plays an important role in generation, maintenance and function of murine inducible Foxp3+ Tregs.Citation36,37 We observed significant reductions in CD39+Foxp3+ T cell frequency, and marked decrease in IFNγ and IL-10 levels when DCs were incubated with anti-PD-L1. Likewise, the CD40-CD40L axis appears necessary for human in vitro responses to PSA as CD40L inhibition substantially disrupted both Foxp3 and cytokine induction to a similar degree.
Foxp3+ Treg dysfunction is a significant contributor to autoimmunity. Mutations in human Foxp3 gene yield lethal multi-organ systemic autoimmunity in humans with a similar phenotype replicated during Foxp3 deletion in mice.Citation38 Additionally, numeric and functional defects in Foxp3+ Treg subsets, including those expressing CD39, are also described in chronic autoimmune conditions such as MS.Citation19,29 PSA significantly increased the expression of CD39, on the surface of Foxp3+ Tregs in addition to enhancing the frequency of these CD39+Foxp3+ Tregs in vitro. Interestingly, 3 months of treatment with sphingosine-1-phosphate receptor antagonist FTY720 similarly enhanced CD39 GMFI as well as frequencies of CD39+Foxp3+ Tregs in patients, suggesting the therapeutic value of these cells.Citation39 Further support for the importance of these cells in MS stems from the positive correlation of increased CD39+ Treg frequency and disease remission.Citation40 Finally, these results parallel our findings in mice showing enhanced CD39+Foxp3+ Tregs in the CNS of PSA treated animals during EAE. In addition to reduced presence, attenuated suppressive function among Tregs has also been reported. Foxp3 protein levels correlate with the suppressive function of Foxp3+ Tregs.Citation41 That Foxp3 levels are reduced in MS patients corresponds with additional reports of diminished suppressive capacity among Foxp3+ Tregs in MS.Citation41–44 Exposing circulating Tregs to PSA-activated DCs increased Foxp3 protein expression among these cells. We also observed a significant increase in the frequency of HLA-DR+ Tregs, which co-expressed CD39. HLA-DR+ Tregs possess reduced suppressive activity in individuals with RRMS pointing to a potential role in regulating autoimmune disease.Citation45 Both low frequencies of HLA-DR+ Tregs, as well as low HLA-DR expression among these cells correlate with preterm labor and allograft rejection emphasizing the role of these cells in maintaining tolerance.Citation46 Lastly, we directly demonstrated that PSA promoted enhanced function by showing a greater reduction in the average amount of LPS-induced monocyte TNFα by cultures containing PSA-exposed Tregs compared to media Treg controls.
The intestinal microbiota can shape the development of murine host immune systems and impact a number of experimental autoimmune conditions. Indeed, preliminary studies profiling taxonomic shifts within the intestinal microbiota report differences between healthy individuals and those with relapsing MS or inflammatory bowel disease. Interestingly, Faecalibacterium prausnitzii, a noted producer of Foxp3+ Treg-inducing short-chain fatty acid butyrate,Citation47,48 was decreased in abundance for both autoimmune conditions.Citation49,50 This demonstration suggests that underlying shifts in the microbiota could relate to Foxp3+ Treg disparities implicated in human autoimmune disease. An increase in Foxp3+ Treg frequency and function by B. fragilis PSA in EAE raises the prospect of using microbiota-based interventions to understand and possibly treat those human conditions associated with autoimmune-related Foxp3+ Tregs deficiencies. Our pre-clinical observations in murine EAE and now human blood-derived cells demonstrate consistency in the capacity for PSA to promote Foxp3+ Treg frequency and function. Oral treatment with PSA derived from the human commensal B. fragilis may represent a novel shift in the paradigm for treating autoimmune disease such as human MS. To this end we have made preliminary observations that show that PSA can substantially enhance in vitro production of IL-10 by PBMCs from those with relapsing MS to a greater degree than healthy individuals. Further studies using human PBMCs focused on the capacity of PSA to induce both enhanced Treg functional activity as well as regulatory cytokine production in those with MS are currently underway.
Materials and Methods
Subjects
Fresh whole blood was obtained from healthy human subjects (25–60 years of age), volunteers for platelet pheresis enrolled under Institutional Review Board approval. PBMC were prepared from Terumo BCT leukoreduction system chamber content obtained from Dartmouth Hitchcock Medical Center by density gradient centrifugation over Ficoll-Histopaque.
Cell isolation and culture
Individual cell populations were isolated from whole peripheral blood using Miltenyi MACs magnetic isolation kits. Commercial standardized kits based on negative selection depleting non-target cells were used for the enrichment of naïve CD4+CD25− T cells (130–094–131), CD14+ monocytes (130–091–153), CD19+ B cells (130–091–151). Total blood dendritic cells were isolated via positive selection of CD1c+, CD141+ and CD304+ cells (130–091–379). CD4+CD25+Foxp3+ T regulatory cells were obtained by positive selection of cells highly expressing CD25, after depletion of non-target cells (130–094–775).
Reagents
For cell culture Aim V media (Life Technologies) was supplemented with 5% human serum (Valley Biomedical) and recombinant 100 U/ml IL-2 (Peprotech). Purified Bacteroides fragilis polysaccharide A and Streptococcus pneumoniae polysaccharide type 1 were provided by Dr. Dennis Kasper (Harvard Medical School). Polysaccharides were tested for LPS contamination (Less than 0.5%).
Flow cytometric analysis and ELISA
Surface staining was performed using the following human antibodies: anti-CD4 APC-H7 (clone RPA-T4, BD biosciences), anti-CD25 APC (clone BC96, Biolegend), anti-CD127 PE/Cy7 (clone A019D5, Biolegend), anti-CD39 FITC (clone A1, Biolegend), anti-CD86 APC (clone IT2.2, Biolegend), anti-PD-L1 PE (clone 29E.2A3, Biolegend), anti-CD40 PE (clone 5C3, Biolegend), anti-CD80 FITC (clone MAB104, Beckman Coulter), anti-HLA-DR PE/Cy7 (clone L243, Biolegend) antibodies. Foxp3 PE (clone 206D, Biolegend) was used to stain intracellular Foxp3 after cellular permeablization with a commercial kit (Ebioscience). Flow cytometric analysis was performed using Miltenyi MACs Quant 8-color cytometers. FACS quad gates were set using fluorescence minus one (FMO) controls. Histograms were set according to isotype control signal. Geometric mean fluorescence intensity values were set adjusting for isotype control background signal. For ELISA, cell cultures were washed in culture media following incubation and transferred to U-bottom 96 well plates coated with 1 µg/ml anti-CD3 (clone OKT3, BioXcell) in the presence of 0.5 µg/ml soluble anti-CD28 (clone CD28.2, Biolegend) for 24hrs. Cell culture supernatants were probed for IL-10, IFNγ, IL-17 or TNFα using commercial ELISA kits (Biolegend)
Foxp3 induction, antibody blockade, and suppression assays
Isolated naïve CD4+CD25− T cells or CD4+CD25+Foxp3+ Tregs were cultured in supplemented Aim V media in the presence of APCs, either B cells, monocytes, or DCs for 5 d before being assessed for relevant markers. Blockade of specific receptor/ligand interactions was accomplished by incubation of DCs with neutralizing antibodies against CD86 (polyclonal IgG, R&D systems), CD40L (clone 24–31, Biolegend), HLA-DR (G46–6, BD Biosciences), and PD-L1 (clone 29E.2A3, Biolegend).
For suppression of TNFα, DCs were cultured in the presence of absence of PSA for 24 hrs before being washed. Autologous 1 × 104 Tregs were then cultured with DCs for 72 hrs. Finally, 5 × 104 monocytes were added to cultures alongside 50ng/ml LPS (InvivoGen). Supernatants were analyzed for TNFα after 24 hrs.
Statistical analysis
Student's t-test (Paired, Two-tailed) or one-way ANOVA with Dunnett's multiple comparison test were used to show statistical differences between cell frequencies, and fluorescence intensity from FACS data and cytokine levels from ELISA. Bar graphs represent means of independent experiments, each using cells from different individuals unless otherwise stated. Error bars represent standard error of the mean. P-values were indicated in figures.
Disclosure of potential conflicts of interests
No potential conflicts of interest were disclosed.
Author Contributions
KMT conducted the research, performed the experiments and wrote the paper; YW, JOR, and SBH contributed to experiment design, data and provided critical review. AP, MC, and CK provided technical assistance. DLK graciously provided purified zwitterionic polysaccharides and comments on the research. LHK supervised the research and reviewed the manuscript.
Supplemental_Material.docx
Download MS Word (263.3 KB)Acknowledgments
We thank the members of Dartlab: Dr. Jacqueline Channon Smith, Dr. Daniel Mielcarz, John Delong, Alan Bergeron and Gary Ward for technical assistance on sample preparation and immunoassay technical support. We thank Dr. Isabelle Le Mercier, and Dr. Azizul Haque for critical scientific discussion.
Funding
Funding sources: The National Multiple Sclerosis Society (RG 4662A2/1) and National Institutes of Health (1R 41AI110170–01).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Wu H-J, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012; 3:4-14; PMID:22356853; http://dx.doi.org/10.4161/gmic.19320
- Ivanov II, Honda K. Intestinal commensal microbes as immune modulators. Cell Host Microbe 2012; 12:496-508; PMID:23084918; http://dx.doi.org/10.1016/j.chom.2012.09.009
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol 2004; 4:478-85; PMID:15173836; http://dx.doi.org/10.1038/nri1373
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122:107-18; PMID:16009137; http://dx.doi.org/10.1016/j.cell.2005.05.007
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485-98; PMID:19836068; http://dx.doi.org/10.1016/j.cell.2009.09.033
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal bacteroides fragilis depends on polysaccharide A expression. J Immunol 2010; 185:4101-8; PMID:20817872; http://dx.doi.org/10.4049/jimmunol.1001443
- Östman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol 2006; 36:2336-46; PMID:16897813; http://dx.doi.org/10.1002/eji.200535244
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 2010; 107:12204; PMID:20566854; http://dx.doi.org/10.1073/pnas.0909122107
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-Like Receptor 2 Pathway Establishes Colonization by a Commensal of the Human Microbiota. Science 2011; 332:974-7; PMID:21512004; http://dx.doi.org/10.1126/science.1206095
- Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology 2010:1-9; PMID:20531465
- Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 2009; 106:16788-93; PMID:19805374; http://dx.doi.org/10.1073/pnas.0902869106
- Lu Y, Wang J, Gu J, Lu H, Li X, Qian X, Liu X, Wang X, Zhang F, Lu L. Rapamycin regulates iTreg function through CD39 and Runx1 pathways. J Immunol Res 2014:1-8; PMID:24741640
- Wang Y, Telesford KM, raz JO-RA, Haque-Begum S, Christy M, Kasper EJ, Wang L, Wu Y, Robson SC, Kasper DL, et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat Commun 2014; 5:1-10; PMID:25043484
- Cobb BA, Kasper DL. Zwitterionic capsular polysaccharides: the new MHCII-dependent antigens. Cell Microbiol 2005; 7:1398-403; PMID:16153240; http://dx.doi.org/10.1111/j.1462-5822.2005.00591.x
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell 2004; 117:677-87; PMID:15163414; http://dx.doi.org/10.1016/j.cell.2004.05.001
- Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol 2002; 169:6149-53; PMID:12444118; http://dx.doi.org/10.4049/jimmunol.169.11.6149
- Kreisman LSC, Cobb BA. Glycoantigens Induce Human Peripheral Tr1 Cell Differentiation with Gut-homing Specialization. J Biol Chem 2011; 286:8810-8; PMID:21228275; http://dx.doi.org/10.1074/jbc.M110.206011
- Liu W. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203:1701-11; PMID:16818678; http://dx.doi.org/10.1084/jem.20060772
- Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, Tubridy N, Mills KHG. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol 2009; 183:7602-10; PMID:19917691; http://dx.doi.org/10.4049/jimmunol.0901881
- Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol 2006; 176:4622-31; PMID:16585553; http://dx.doi.org/10.4049/jimmunol.176.8.4622
- Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol 2008; 182:148-53; PMID:19109145; http://dx.doi.org/10.4049/jimmunol.182.1.148
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor- dependent but does not confer a regulatory phenotype. Blood 2007; 110:2983-90; PMID:17644734; http://dx.doi.org/10.1182/blood-2007-06-094656
- Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: A state attained by all activated human T-cells. Clin Immunol 2007; 123:18-29; PMID:17185041; http://dx.doi.org/10.1016/j.clim.2006.10.014
- Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol 2007; 19:345-54; PMID:17329235; http://dx.doi.org/10.1093/intimm/dxm014
- Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, Naldini L, Roncarolo MG, Soudeyns H, Levings MK. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 2007; 16:194-202; PMID:17984976; http://dx.doi.org/10.1038/sj.mt.6300341
- Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant 2011; 11:1148-57; PMID:21564534; http://dx.doi.org/10.1111/j.1600-6143.2011.03558.x
- Sawant DV, Vignali D. Once a Treg, always a Treg? Immunol Rev 2014; 259:173-91; PMID:24712466
- Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19:355-67; PMID:23601906; http://dx.doi.org/10.1016/j.molmed.2013.03.005
- Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110:1225-32; PMID:17449799; http://dx.doi.org/10.1182/blood-2006-12-064527
- Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, et al. Expression of CD39 by human peripheral blood CD4+CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant 2010; 10:2410-20; PMID:20977632; http://dx.doi.org/10.1111/j.1600-6143.2010.03291.x
- Schuler PJ, Schilling B, Harasymczuk M, Hoffmann TK, Johnson J, Lang S, Whiteside TL. Phenotypic and functional characteristics of CD4+CD39+ FOXP3+ and CD4+CD39+FOXP3neg T-cell subsets in cancer patients. Eur J Immunol 2012; 42:1876-85: n/a-n/a; PMID:22585562
- Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y, et al. CD39 and CD161 modulate Th17 responses in Crohn's disease. J Immunol 2014; 193:3366-77; PMID:25172498; http://dx.doi.org/10.4049/jimmunol.1400346
- Herrath J, Chemin K, Albrecht I, Catrina AI, Malmström V. Surface expression of CD39 identifies an enriched Treg-cell subset in the rheumatic joint, which does not suppress IL-17A secretion. Eur J Immunol 2014; 44:2979-89; PMID:24990235; http://dx.doi.org/10.1002/eji.201344140
- Dasgupta S, Erturk-Hasdemir D, Ochoa-Repáraz J, Reinecker H-C, Kasper DL. Plasmacytoid Dendritic Cells Mediate Anti-inflammatory Responses to a Gut Commensal Molecule via Both Innate and Adaptive Mechanisms. Cell Host Microbe 2014; 15:413-23; PMID:24721570; http://dx.doi.org/10.1016/j.chom.2014.03.006
- Stephen TL, Niemeyer M, Tzianabos AO, Kroenke M, Kasper DL, Kalka-Moll WM. Effect of B7-2 and CD40 signals from activated antigen-presenting cells on the ability of zwitterionic polysaccharides to induce T-Cell stimulation. Infect Immun 2005; 73:2184-9; PMID:15784561; http://dx.doi.org/10.1128/IAI.73.4.2184-2189.2005
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206:3015-29; PMID:20008522; http://dx.doi.org/10.1084/jem.20090847
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A 2008; 105:9331-6; PMID:18599457; http://dx.doi.org/10.1073/pnas.0710441105
- Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat Pub Group 2014; 14:343-9; PMID:24722479
- Muls N, Dang HA, Sindic CJM, van Pesch V. Fingolimod increases CD39-expressing regulatory T cells in multiple sclerosis patients. PLoS One 2014; 9:e113025; PMID:25411844; http://dx.doi.org/10.1371/journal.pone.0113025
- Peelen E, Damoiseaux J, Smolders J, Knippenberg S, Menheere P, Tervaert JWC, Hupperts R, Thewissen M. Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. J Neuroimmunol 2011; 240–241:97-103; PMID:22035960
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens J-L, Medaer R, Hupperts R, Stinissen P. Compromised CD4 + CD25 highregulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 2008; 123:79-89; PMID:17897326; http://dx.doi.org/10.1111/j.1365-2567.2007.02690.x
- Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, Bourdette D, Ziegler SF, Offner H, Vandenbark AA. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res 2005; 81:45-52; PMID:15952173; http://dx.doi.org/10.1002/jnr.20522
- Haas J, Fritzsching B, Trübswetter P, Korporal M, Milkova L, Fritz B, Vobis D, Krammer PH, Suri-Payer E, Wildemann B. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol 2007; 179:1322-30; PMID:17617625; http://dx.doi.org/10.4049/jimmunol.179.2.1322
- Pop SM. Single cell analysis shows decreasing FoxP3 and TGF 1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med 2005; 201:1333-46; PMID:15837817; http://dx.doi.org/10.1084/jem.20042398
- Baecher-Allan CM, Costantino CM, Cvetanovich GL, Ashley CW, Beriou G, Dominguez-Villar M, Hafler DA. CD2 costimulation reveals defective activity by human CD4+CD25hi regulatory cells in patients with multiple sclerosis. J Immunol 2011; 186:3317-26; PMID:21300823; http://dx.doi.org/10.4049/jimmunol.1002502
- Kisielewicz A, Schaier M, Schmitt E, Hug F, Haensch GM, Meuer S, Zeier M, Sohn C, Steinborn A. A distinct subset of HLA-DR. Clin Immunol 2010; 137:209-20; PMID:20822960; http://dx.doi.org/10.1016/j.clim.2010.07.008
- Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009; 294:1-8; PMID:19222573; http://dx.doi.org/10.1111/j.1574-6968.2009.01514.x
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504:451-5; PMID:24226773; http://dx.doi.org/10.1038/nature12726
- Bhargava P, Mowry EM. Gut Microbiome and Multiple Sclerosis. Curr Neurol Neurosci Rep 2014; 14:492; PMID:25204849; http://dx.doi.org/10.1007/s11910-014-0492-2
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014; 63:1275-83; PMID:24021287; http://dx.doi.org/10.1136/gutjnl-2013-304833

