Abstract
Huge groundwater reserves are under the foot of Mt. Fuji, the tallest volcanic mountain in Japan, and the residence time of the groundwater as estimated from the 36Cl/Cl isotope ratio is in the range of 20–35 years. All springwater samples contain nearly saturated concentrations of oxygen. The groundwater field was separated into four areas based on regional slopes of the groundwater table and estimated groundwater flow directions. Springwater samples showed that physicochemical characteristics of the groundwater in the region are relatively constant; Ion concentrations were characterized as the Ca-HCO3 type, irrespective of location at the foot of the mountain. The density of prokaryotes in the springwater, which was in the range of 102–104 cells mL−1, was low compared with any other groundwater so far reported. Although the density of prokaryotes was low, retrieved clones show that the prokaryotes belonged to eight bacterial and two archaeal phyla, and included both aerobic and obligate anaerobic prokaryotes. Some retrieved clones were related to thermophilic prokaryotes, which are optimally adapted to temperatures greater than 40°C. This finding suggests that at least some of the source of the groundwater was located at a depth of 600 m or greater, based on a temperature gradient of 4°C/100 m. This depth is far below the lava layer which was taken to be a substantial pool of groundwater. Microbiological information may provide insight into the transport route of groundwater until it emerges.
Introduction
The subsurface environment is highly variable constrained by the geological setting. The chemistry of the groundwater is a result of interactions between the geological environment and water supplied from rain or snow, and groundwater conditions support diverse distributions and activities of prokaryotes.
In contrast to subseafloor habitat, terrestrial subsurface habitats supporting prokaryotes assemblages are present in diverse geological settings, which include karst, lava, sedimentary rock, igneous rock and other environments. Studies of prokaryotes in terrestrial subsurface environments have been mainly restricted to shallow sedimentary aquifers that have been contaminated by surface waters or pollution (Harvey et al. Citation2010; Lin et al. Citation2012; Zhou et al. Citation2012) and deep igneous environments that have been considered as repositories for radioactive wastes (Pedersen Citation2000; Pedersen et al. Citation2008). Griebler and Lueders (Citation2009) summarized the density of prokaryotes in noncontaminated groundwater from granite and basalt systems ranged from 102 to 105 cells cm−3.
A pioneer work of Hazen et al. (Citation1991) in deep subsurface sedimentary geological setting of the Savannah River Site, South California, showed that the density of bacteria in groundwater ranged from 103 to 104 cells mL−1. And Kato et al. (Citation2009) reported that the density of prokaryotes ranged from 104 to 106 cells mL−1 in deep sedimentary geological setting. However, the number of studies on prokaryotes in springwater is still very limited.
A few recent preceding studies on prokaryotes in springwater were conducted in karst terrains (Farnleitner et al. Citation2005; Pronk et al. Citation2009; Wilhartitz et al. Citation2007), which have demonstrated that the abundance of prokaryotes in these environments amounted to 104 cells mL−1, and that the community consisted of approximately 10% Archaea. Pronk et al. (Citation2009) suggested that the residence time of groundwater in karst regions is less than 1 year, based on a study conducted in the Yverdon-les-Bains karst aquifer system in western Switzerland.
In contrast to the situation in karst terrains, the residence time of groundwater in the volcanic rocks of Mt. Fuji, which consist of a permeable layer containing abundant groundwater, have been reported to be in the range of 20–35 years (Tosaki et al. Citation2011). The relatively long interaction times of groundwater and rock in volcanic terrains may result in a more diversified community of prokaryotes in volcanic groundwater than in karst groundwater.
Prokaryotic communities in subseafloor and hydrothermal vent communities are known to be diverse (Inagaki et al. Citation2006; Jorgensen et al. Citation2012; Jungbluth et al. Citation2013). However, only a few preceding studies of prokaryotic diversity were conducted in springwater of karst (Farnleitner et al. Citation2005) and aquifer of igneous rock (Fukuda et al. Citation2010), which found clones belonging to the phylum of Proteobacteria and Bacteroidetes and clones of Proteobacteria and Firmicutes, respectively.
Here we first examine the distribution and diversity of prokaryotes in springwater from the volcanic environment at the foot of Mt. Fuji, using 16S rRNA gene sequencing analysis. Springwater that flushed out at the foot of Mt. Fuji is characterized by: (1) emergence at multiple springs distributed along the end or fissures in lava flows with a flushing rate ranging from 50,000 to 1 million m3 per day; (2) annually, constant water temperatures; and (3) saturated dissolved oxygen levels at almost all sites.
Based on those findings, this study aims to reveal how subsurface water-rock interactions affect the chemistry of groundwater and the influence of groundwater chemistry on prokaryotic communities. To this end, we employed the stable isotope signature of 87Sr/86Sr as an indicator of the history of water-rock interaction occurring in the subsurface environment, prior to emergence; we also measured groundwater chemistry, to know the influence of the geological setting on the aqueous environment. In addition, gene sequencing analyses were conducted to reveal the distribution and diversity of prokaryotes in the aquatic environment.
Materials and Methods
Site Description, Sample Collection, and Field Measurements
The study area is located at the foot of Mt. Fuji in the central part of the main island of Japan (). Two geological formations are present in the area of Mt. Fuji (Tsuya Citation1968); the upper formation (lava flow and tephra) is permeable and the lower formation (mudflow) is impermeable or low permeability.
Fig. 1. Geological map of the foot of Mt. Fuji showing sampling sites of springwater. The study area was divided into four slopes of E, SE, S and W (see text). The geological base map is from the National Institute of Advanced Industrial Science and Technology, AIST (http://riodb02.ibase.aist.go.jp/db084/). Black colors indicate lava flows. Gray and dark gray colors indicate tephra layers.
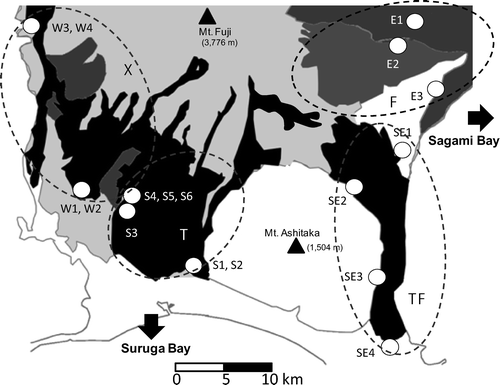
According to a water flow simulation (using the General Purpose Terrestrial Fluid-FLOW Simulator, GETFLOWS; Geosphere Environmental Technology Co., Ltd., personal communication), groundwater at the foot of Mt. Fuji flows in four general directions (); (1) toward Sagami Bay through eastern tephra layer (eastern slope, E), (2) toward Suruga Bay through Ohnohara (Mishima) Lava flow layer (southeastern, SE), (3) toward Suruga Bay through the western lava flows (western slope, W), and (4) toward Suruga Bay through southern lava flow (southern slope, S). Measurements of physical and chemical environmental parameters and water samplings for hydrological, chemical and prokaryotic analyses were conducted from 2009 to 2011 during the season from March to November.
shows the location of sampling sites for the study. Physical and chemical parameters of springwater were measured directly at the sampling site and samples were collected directly to the sterilized bottles or by using sterilized tools such as a jug, a dipper, and a bucket. The samples were then immediately subsampled into sterilized glassware for chemical and prokaryotic analyses, and were kept cool in the dark until analyzed.
The discharge of springwater was directly measured or estimated from velocity measurements using a velocity meter (Sencom, Inc., West Bloomfield, MI, USA) in case a stream was formed by springwater. In situ measurements of water temperature, pH, electric conductivity (EC), dissolved oxygen (DO) and oxidation reduction potential (ORP) were performed using a water quality checker either U-10 or U-52 (Horiba, Kyoto, Japan) and a Hach DO meter (Hach, Loveland, CO, USA). The ORP values were transformed to Eh values (Pt conversion) by the formula shown next (1).(1)
Chemical Analyses
Samples for ion analysis were filtered through a 0.22-μm Millex-GS filter (Millipore, Billerica, MA, USA) and stored at –20°C until analysis. Samples for the measurement of dissolved organic carbon (DOC) were kept in carbon free (burned at 550°C for 5 h) glass ampoules after filtration through glass fiber GF/F filters (Whatman, Maidstone, UK), which were burned at 450°C for 3 h and stored at –20°C until analysis.
Concentrations of dissolved ions were analyzed using either an ion analyzer (model IA-200; DKK-TOA, Tokyo, Japan) or an ion chromatograph (ICS-2100; Dionex, Sunnyvalley, CA, USA). DOC was measured using a TOC-5000A (Shimadzu, Kyoto, Japan). Analysis of 87Sr/86Sr was performed using a Multi-collector Mass Spectrometer (Finnigan, San Jose, CA, USA).
Microscopic Analyses
Total direct counting (TDC) was conducted after Porter and Feig (Citation1980) with some modifications and catalyzed reporter deposition - fluorescence in situ hybridization (CARD-FISH) was conducted after Pernthaler et al. (Citation2002) and Teira et al. (Citation2004). The samples for TDC were fixed in pH-neutral formaldehyde (Wako, Osaka, Japan). A 100-mL fixed sample was collected on Nucleopore filter (0.2 μm pore size; Whatman, Cambridge, UK).
Every treatment was conducted in a clean bench (Sanyo Electric, Osaka, Japan). The cells were stained with 4′6-diamidino-2-phenylindole (DAPI; final concentration, 0.01 μg mL−1); more than 500 prokaryotic cells were counted under epifluorescence microscopy (BX-51; Olympus, Tokyo, Japan). Samples for CARD-FISH were fixed in paraformaldehyde (final concentration 3%; Wako).
Hybridization was performed at 35°C for 2 h using horseradish-peroxidase labeled a Bacteria-specific EUB338 probe (5′-GCT GCC TCC CGT AGG AGT-3′; Amann et al. Citation1990) or 10 h using an Archaea-specific ARCH915 probe (5′-GTG CTC CCC CGC CAA TTC CT-3′; Stahl and Amann Citation1991). Numbers of Bacteria and Archaea were counted based on pictures taken under an epifluorescence microscopic system equipped with a digital camera (DP71, Olympus).
Sequencing of 16S rRNA Genes
Extraction, amplification, cloning and sequencing of DNA were conducted according to the method of Kimura et al. (Citation2007). A 10-L sample of springwater was filtered using a 0.22-μm Sterivex-GV filter (Millipore). Bulk DNA was extracted using the method described by Somerville et al. (Citation1989). The prokaryotic cells were lysed with a solution of lysozyme and proteinase K in Sterivex-GV. The bulk DNA was extracted with phenol.
Concentration and purity of extracted DNA were checked by Gene Quant 100 (GE Healthcare, Buckinghamshire, UK). The bacterial and archaeal 16S rRNA genes were PCR amplified using KOD DNA polymerase (Toyobo, Osaka, Japan) and the Bacteria or Archaea-specific primer set (Bac27F and Uni1492; Lane Citation1991, or Arch109F and Arch915R; Grosskopf et al. Citation1998; Stahl and Amann Citation1991). The PCR products were cloned using a Zero Blunt TOPO PCR cloning kit (Invitrogen, Carlsbad, CA, USA).
Clone libraries of the bacterial and archaeal 16S rRNA genes were constructed separately. The sequences of inserted PCR amplicons selected from recombinant colonies and used for choosing the Bacteria-specific primer (341F) and the Archaea-specific primer (109F) were determined by the Dragon genomics center.
All 16S rRNA gene sequences were checked for chimera formation using Bellerophon (Huber et al. Citation2004, http://foo.maths.vq.edu.au/∼huber/bellerophon.p1). Nonchimeric sequences were rapidly classified on the basis of the 16S rRNA gene sequences into high-order taxonomic units using Classifier from the Ribosomal Database Project II (RDP II, http://rdp.cme.msu.edu/index.jsp). Sequences were aligned using CLUSTAL W in the DNA Data Bank of Japan (DDBJ, http://www.ddbj.nig.ac.jp/; Thompson et al. Citation1994). Clones with homology values of >98.5% were grouped into one operational taxonomic unit (OTU). Well-known culturable and unculturable closest relatives were identified with the Basic Local Alignment Search Tool (BLAST; Altschul et al. Citation1997; Thompson et al. Citation1994). Phylogenetic trees were produced using the neighbor-joining algorithm of the NJ plot program (Saitou and Nei Citation1987). These fragments of the 16S rRNA gene sequence have been submitted to the DDBJ and assigned accession numbers AB794389–AB794587.
Results
Environmental Parameters of Springwater
Results of the physical and chemical analyses of the 17 springwaters are shown in . Daily discharge rates of each springwater ranged widely from 503 to 1,010,000 m3 day−1. Water temperature was in the range of 10.9–18.1°C for samples obtained in March–November 2009–2011. The values of pH were in the range of 5.69–8.47 and EC values were in the range of 54–229 μS cm−1. Value of DO ranged from 6.77 to 12.15 mg O2 L−1, suggesting that the springwater was nearly saturated or oversaturated with respect to DO concentration. Oxygen redox potential converted to Eh by platinum electrode ranged from 337 to 580 mV, reflecting a high concentration of DO. The DOC values were low (in the range of 7.8–63.0 μmol L−1).
Table 1. Environmental parameters of springwater at the foot of Mt. Fuji
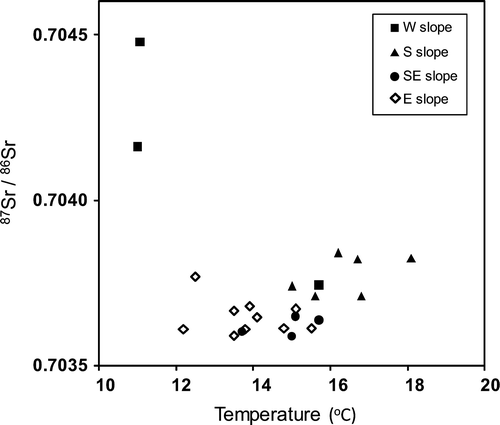
Among the measured ions, concentrations of dissolved Ca2+ and particularly HCO3− were high (Appendix Table 1). Data examined from the study site thus far can be categorized as Ca-HCO3 type. The concentrations of nitrate were low at all sites, based on observations obtained during August–October 2011. The 87Sr/86Sr isotope ratios of samples were mostly in the range of 0.703591–0.703841, and two exceptional large values over 0.7040 were found from high altitudes in western slope (). The data showed no correlation with either temperature or elevation of the site where the water was sampled.
To the above-mentioned general characteristics, the springwater from each of the four slopes showed unique features. On the eastern slope: shows (1) fluctuations in water temperature were small (within 1°C), and (2) pH and EC values were in relatively narrow ranges. Site SE4, located on the Kakita River at the lowest elevation of all the sites and at the end of a long and large lava flow on the southeastern slope, showed anomalously high flow rates of 1,010,000 m3 day−1. On the southern slope: (1) high discharge rates were observed in summer, and (2) differences in EC were larger than on other slopes. On the western slope, two exceptionally large 87Sr/86Sr ratios were obtained, with values of 0.704478 at Site W3, located at 691 m a.s.l., and of 0.704161 at Site W4, located at 726 m a.s.l. ().
Density of Prokaryotes and its Community Structure
The density of prokaryotes detected by TDC ranged from 7.75 × 102 to 4.30 × 104 cells mL−1. A sum of prokaryotes detected by CARD-FISH ranged from 7.7 to 20.2% of the total count of prokaryotic cells. Maximum densities of cells were observed at E1, where springwater emerged directly from a tephra layer, and minimum densities were observed at SE3, where springwater emerged directly from a lava flow. Samples from both E1 and E2 sites showed high abundances of prokaryotes; in both cases, the springwaters emerged from a tephra layer and showed high percentages of Bacteria.
The contribution of Archaea to the total prokaryote count was in the range of 0.26%–2.1%, with a maximum contribution observed at SE3 and a minimum observed at SE4.
Sequencing Analysis
To reveal the constituents of the bacterial and archaeal communities, we obtained 208 chimera-checked sequences of partial bacterial 16S rRNA genes and 51 chimera-checked sequences of partial archaeal 16S rRNA genes (average sequence length, 620 bp).
Bacteria
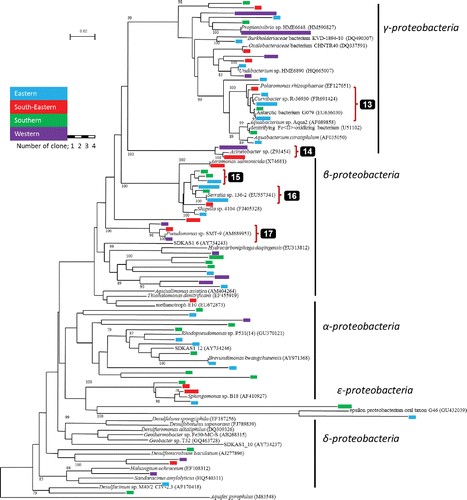
The retrieved clones were classified to the phyla and class levels using Classifier and a confidence threshold of >80% ( and ). Out of the 208 totally sequenced clones, 170 clones were classified as belonging to 8 phyla of Bacteria (a). Five phyla were retrieved from all slopes (106 clones of Proteobacteria, 33 clones of Bacteroidetes, 6 clones of Firmicutes, 6 clones of Planctomycetes, and 5 clones of Acidobacteria). The other three phyla were Cyanobacteria (retrieved from the eastern, southeastern, and western slopes), Actinobacteria (retrieved from the eastern, southern, and western slopes), and Nitrospirae (retrieved from the southern slope).
Table 2. Bacterial phylotypes (clone number) in springwater collected from the eastern (E), southeaster (SE), southern (S), and western (W) slopes of Mt. Fuji
Table 3. Archaeal phylotypes (clone number) in springwater collected from the foot of Mt. Fuji
Clones belonging to four classes such as Betaproteobacteria (39 clones), Gammaproteobacteria (39 clones), Deltaproteobacteria (6 clones) and Planctomycetecia (6 clones) were retrieved from all slopes from a total of 16 classes.
Archaea
Of the 51 totally sequenced clones, 35 were classified into two phyla of the Archaea (a): the Euryarchaeota (26 clones) and the Crenarchaeota (9 clones). Euryarchaeota clones were present in samples from all slopes; 4 of the clones belonged to Halobacteria (b) and 22 clones of unclassified Euryarchaeota were similar (by at most 79%) to clones of methane-producing prokaryotes belonging to Methanomicrobia. Crenarchaeota clones, including 9 clones of Thermoprotei (a hyperthermophilic archaea belonging to the Crenarchaeota), were collected from the southeastern, southern, and western slopes (b).
Phylogenetic Tree
A total of 208 bacterial clones and 51 archaeal clones were grouped into 141 and 43 OTUs, respectively, based on a matching criterion of 98.5% similarity. The phylogenetic tree summarizing the sequence data analyzed at OTU level for Bacteria as shown in and Appendix Figure 1, and for Archaea in .
Fig. 3. Phylogenetic tree of bacterial 16S gene rRNA sequences obtained from prokaryotes in springwater collected at the foot of Mt. Fuji (excluding the phylum Proteobacteria; see Appendix Figure 1). Aquifex pyrophilus was used as the outgroup. Blue, red, green, and purple indicate samples obtained from the eastern (E), southeastern (SE), southern (S), and western (W) slopes of the foot of the mountain, respectively. Length of color bar indicates the number of clones. Orange and blue arrows indicate thermophilic and obligate anaerobic bacteria, respectively. Bootstrap values above 50% for 1,000 replicates are shown.
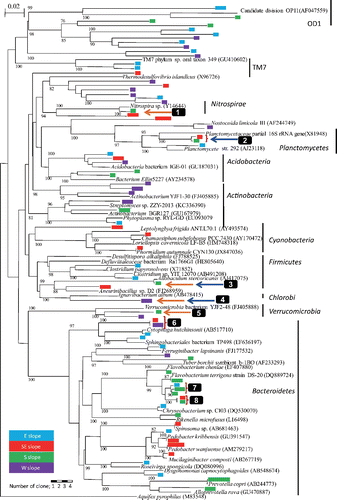
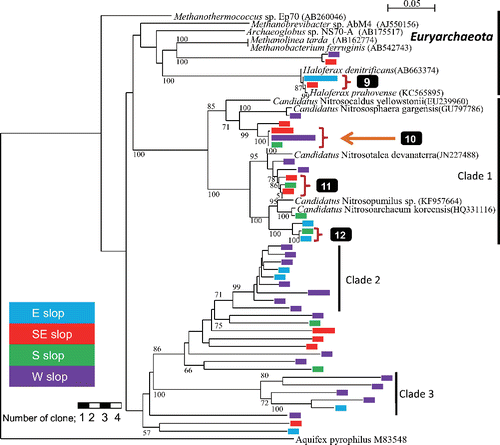
Fig. 4. Phylogenetic tree of archaeal 16S rRNA gene sequences obtained from prokaryotes in springwater collected at the foot of Mt. Fuji. Aquifex pyrophilus was used as the outgroup. Legends are the same as for .
Bacteria
A total of 102 retrieved clones belonging to Nitrospirae, Planctomycetes, Acidobacteria, Actinobacteria, Cyanobacteria, Firmicutes, Verrucomicrobia, Bacteroidetes, OD1 and TM7 group or unclassified are shown in a bacterial phylogenetic tree in (the tree for the phylum Proteobacteria is shown in the Appendix Figure 1). One group consisted of 2 OTUs (shown by 2 in ) belonging to Planctomycetes and three groups (shown by 6, 7 and 8 in ) belonging to Bacteroidetes were obtained from multiple slopes. Of the 106 clones belonging to the Proteobacteria (see Appendix Figure 1), two groups (shown by 13 and 14 in Appendix Figure 1) belonging to γ-Proteobacteria and three groups (shown by 15, 16 and 17 in Appendix Figure 1) belonging to β-Proteobacteria were obtained from multiple slopes of Mt. Fuji.
Archaea
A total of 51 retrieved clones belonging to Archaea are shown in the archaeal phylogenetic tree (). Only one group consisted of two OTUs (shown by 9 in ) belonging to Euryarchaeota was retrieved from multiple slopes.
Clade 1 consisted of 14 OTUs. Three OTUs among them (shown by 10 in ) were classified as Thermoprotei belonging to Creanarchaeota by Classifier. Three groups of clade 1 shown by 10, 11 and 12 in were retrieved from multiple slopes. Two unique clades, clade 2 and clade 3, retrieved from multiple slopes did not relate to any clones in the DDBJ database with >82%.
Characteristics of Prokaryotes
Sequence analysis shows that some OTUs may be thermophilic (orange arrows: 1, 3, 4, 5 and 10 in and ) and/or anaerobes (blue arrows: 2, 3 and 4 in ). The OTUs related with thermophilic prokaryotes were found in the phyla Nitrospirae, Firmicutes and Verrucomicrobia of Bacteria and Clade 1 of Archaea ( and , respectively), and obligate anaerobes were found in the phyla Planctomycetes, Firmicutes and unclassified group of Bacteria. One OTU (shown by 3 in ) similar to Allobaculum stercoricanis (AJ417075) with 98% in similarity was retrieved from the southern slope. Allobaculum stercoricanis has thermophilic and obligate anaerobic characteristics (Greetham et al. Citation2004).
Discussion
The densities of prokaryotes in springwater emerging from the volcanic subsurface environment at the foot of Mt. Fuji, which are in the range of 102–104 cells mL−1, are lower than densities previously reported from springwater in karst terrains, deep groundwater of granite setting (Fukuda et al. Citation2010; Pedersen Citation1997), and deep sedimentary groundwater (Kato et al. Citation2009), though somewhat similar densities were reported from other sedimentary groundwater (Hazen et al. Citation1991).
Irrespective of the low densities of prokaryotes in the volcanic environments of Mt. Fuji, the constituents of Mt. Fuji springwaters are more diverse than those of karst springwater; thus, the density of prokaryotes does not appear to be correlated with the diversity of the constituents. The number of phyla detected in this study was greater than that reported from both deep granitic and sedimentary groundwater samples (Fukuda et al. Citation2010; Kato et al. Citation2009). All of the phyla previously reported from both terrestrial subsurface aquifers (Farnleitner et al. Citation2005; Fukuda et al. Citation2010; Kato et al. Citation2009) were found in this study.
The features of prokaryotes identified in this study were found in the springwater characterized as simply that of the Ca-HCO3 type. In addition, the concentrations of DOC were significantly lower than those observed in karst springwater (which is recognized as an ultra-oligotrophic environment; Wilhartitz et al. Citation2009). Thus, prokaryotic diversity is not explained on the basis of water chemistry elucidated by the water flushed out alone.
Tosaki et al. (Citation2011) estimated the residence time of springwater emerging from the southern and western slopes to be 20–30 years old. As the ratios of 87Sr/86Sr were within the ranges reported for basaltic rock (Yokoo Citation2006), the examined springwater is assumed to have experienced prolonged water-rock interaction in a deep subsurface environment prior to emergence. Taking into account the flow rate of groundwater in lava, this suggests that the springwater source is at least partly from deep groundwater originating from below the 135 meters thick lava flow (Arai et al. Citation2003).
Regarding the characteristics of prokaryotes, The OTUs related with obligate anaerobic clones shown in (2, 3 and 4) were retrieved from multiple slopes; these clones may provide evidence that at least some of the springwater originated from an anaerobic environment, although the emerging springwater is almost completely saturated with respect to dissolved oxygen. Although unlikely, it is possible that anaerobes were embedded in the anaerobic environments in minute spaces in the soil or rock, or in biofilm that contains an anaerobic environment inside (Ziegler et al. Citation2013) and were then transported to the surface by groundwater flow.
On the other hand, the examined springwater contained thermophilic prokaryotes; these clones, which were retrieved from the southeastern, southern and western slopes, belonged to the Thermoprotei (b), which optimum growth temperature ranges from 65 to 100°C (Reysenbach Citation2001). Additionally, some of the clones retrieved from the southern slope were related to Allobaculum stercoricanis (3 in ), anaerobe that exhibits optimum growth at temperatures of 37–45°C (Greetham et al. Citation2004); members of the Thermoprotei are diverse, and include aerobic, facultative anaerobic and anaerobic characteristics (Reysenbach Citation2001). Although the temperatures of springwater were 10–17°C, the environment with temperature >40°C is estimated to be located at depth of 600 m or greater, based on a temperature depth gradient of 4°C per 100 m for a volcanic geological setting (Colwell Citation2001).
These findings suggest that the source of some portion of the springwater was at a depth of 600 m or greater, which is located below the lava flow layer, and that the water rose to the surface within a short period of time, enabling the examined bacterial DNA to remain intact. This finding changes the previous understanding that groundwaters have been reserved in lava flows with maximumly ca. 300 m (Arai et al. Citation2003) depth. The groundwater cannot simply have originated from the shallow layer of a geological setting.
Our data indicates that genetic analyses of prokaryotes in springwater may be a useful tool for estimating the depth of transport route of springwater; this information cannot be revealed by chemical analyses alone. The combination of molecular analyses of prokaryotes and chemical estimates of the turnover time of groundwater can provide precise information on the source and movements of groundwater in subsurface environments; this information can contribute to management strategies for groundwater resources.
Acknowledgments
We thank Numazu Office of River and National Highway, Chubu Regional Development Bureau, MLIT, Japan.
Funding
This study was partly supported by JSPS KAKENHI Grant Number 23241016 and 26257402 and by the Foundation for the Riverfront Research Center and River Found in Charge of River Foundation, Japan. This study was conducted with the partial support of Joint Research Grant for the Environmental Isotope Study of Research Institute for Humanity and Nature.
References
- Amann RI, Binder BJ, Olson RJ, Chrisholm SW, Devereux R, Stahl DA. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925.
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402.
- Arai K, Suzuki Y, Matsuda M, Chiba T, Futatsugi S, Koyama M, Miyaji N, Yoshimoto M, Tomita Y, Koizumi S, Nakashima K. 2003. Reevaluation of magma discharge volume of the 864-866 Jogan eruption of Fuji Volcano based on results of lake Senoumi drilling. Japan Geoscience Union Meeting abstract V055–P012 (in Japanese).
- Colwell FS. 2001. Constraints on the distribution of microorganisms in subsurface environments. In: Fredrickson JK, Fletcher M, editors. Subsurface Microbiology and Biogeochemistry, New York: John Wiley, p 71–95.
- Farnleitner AH, Wilhartitz I, Ryzinska G, Kirschner AKT, Stadler H, Burtscher MM, Hornek R, Szewzyk U, Herndl G, Mach RL. 2005. Bacterial dynamics in spring water of alpine karst aquifers indicates the presence of stable autochthonous microbial endokarst communities. Environ Microbiol 7:1248–1259.
- Fukuda A, Hagiwara H, Ishimura T, Kouduka M, Ioka S, Amano Y, Tsunogai U, Suzuki Y, Mizuno T. 2010. Geomicrobiological properties of ultra-deep granitic groundwater from the Mizunami Underground Research Laboratory (MIU), central Japan. Microb Ecol 60:214–225.
- Greetham HL, Gibson GR, Giffard C, Hippe H, Merkhoffer B, Steiner U, Falsen E, Collins MD. 2004. Allobaculum stercoricanis gen. nov., sp. nov., isolated from canine feces. Anaerobe 10:301–307.
- Griebler C, Lueders T. 2009. Microbial biodiversity in groundwater ecosystems. Freshwater Biol 54:649–677.
- Grosskopf R, Janssen PH, Liesack W. 1998. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol 64:960–969.
- Harvey RW, Metge DW, Barber LB, Aiken GR. 2010. Effects of altered groundwater chemistry upon the pH-dependency and magnitude of bacterial attachment during transport within an organically contaminated sandy aquifer. Water Res 44:1062–1071.
- Hazen TC, Jimrnez L, Lopez de Victoria G, Fliermans CB. 1991. Comparison of bacteria from deep subsurface sediment and adjacent groundwater. Microb Ecol 22:293–304.
- Huber T, Faulkner G, Hugenholtz P. 2004. Bellerophon; a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319.
- Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, Lauer A, Suzuki M, Takai K, Delwiche M, Colwell FS, Nealson KH, Horikoshi K, D’Hondt S, Jørgensen BB. 2006. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc Natl Acad Sci USA 103:2815–2820.
- Jorgensen SL, Hannisdal B, Lanzén A, Baumberger T, Flesland K, Fonseca R, Øvreås L, Steen IH, Thorseth IH, Pedersen RB, Schleper C. 2012. Correlating microbial community profiles with geochemical data in highly stratified sediments from the Arctic Mid-Ocean Ridge. Proc Natl Acad Sci USA 109:E2846–E2855.
- Jungbluth SP, Grote J, Lin HT, Cowen JP, Rappé MS. 2013. Microbial diversity within basement fluids of the sediment-buried Juan de Fuca Ridge flank. ISME J 7:161–172.
- Kato K, Nagaosa K, Kimura H, Katsuyama C, Hama K, Kunimaru T, Tsunogai U, Aoki K. 2009. Unique distribution of deep groundwater bacteria constrained by geological setting. Environ Microbiol Repts 1:569–574.
- Kimura H, Ishbashi J, Masuda H, Kato K, Hanada S. 2007. Selective phylogenetic analysis targeting 16S rRNA genes of hyperthermophilic archaea in the deep-subsurface hot biosphere. Appl Environ Microbiol 73:2110–2117.
- Lane DJ. 1991. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York: John Wiley and Sons, p115–175.
- Lin X, McKinley J, Resch CT, Kaluzny R, Lauber CL, Fredrickson J, Knight R, Konopka A. 2012. Spatial and temporal dynamics of the microbial community in the Hanford unconfined aquifer. The ISME J 6:1665–1676.
- Pedersen K. 1997. Microbial life in deep granitic rock. FEMS Microbiol Rev 20:399–414.
- Pedersen K. 2000. Exploration of deep intraterrestrial microbial life: current perspectives. FEMS Microbiol Lett 185:9–16.
- Pedersen K, Arlinger J, Eriksson S, Hallbeck A, Hallbeck L, Johansson J. 2008. Numbers, biomass and cultivable diversity of microbial populations relate to depth and borehole-specific conditions in groundwater from depths of 4–450 m in Olkiluoto, Finland. ISME J 2:760–775.
- Pernthaler A, Pernthaler J, Amann R. 2002. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68:3094–3101.
- Porter KG, Feig YS. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948.
- Pronk M, Goldscheider N, Zopfi J. 2009. Microbial communities in karst groundwater and their potential use for biomonitoring. Hydrogeol J 17:37–48.
- Reysenbach A-L. 2001. Class I. Thermoprotei. In: Boone DR, Castenholz RW, editors. Bergey's Manual of Systematic Bacteriology. 2nd ed., Volume 1. New York: Springer-Verlag, p169–210.
- Saitou N, Nei M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425.
- Somerville CC, Knight IT, Straube WL, Colwell RR, 1989. Simple rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol 55:548–554.
- Stahl DA, Amann RI. 1991. Development and application of nucleic acid probes. In: Stackebrandt E, Goofellow M, editors. Nucleoc Acid Techniques in Bacterial Systematics. New York: Wiley, p205–248.
- Teira E, Reinthaler T, Pernthaler A, Pernthaler J, Herndl JG. 2004. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl Environ Microbiol 70:4411–4414.
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res 22:4673–4680.
- Tosaki Y, Tase N, Sasa K, Takahashi T, Nagashima Y. 2011. Estimation of groundwater residence time using the 36Cl bomb pulse. Groundwater 49:891–902.
- Tsuya H. 1968. 1: 50,000 Geologic Map of Mt. Fuji. Kawasaki, Japan: Geological Survey of Japan 23 p (in Japanese).
- Wilhartitz IC, Kirschner AKT, Stadler H, Herndl GJ, Dietzel M, Latal C, Mach RL, Farnleitner AH. 2009. Heterotrophic prokaryotic production in ultraoligotrophic alpine karst aquifers and ecological implications. FEMS Microbiol Ecol 68:287–299.
- Wilhartitz IC, Mach RL, Teira E, Reinthaler T, Herndl GJ, Farnleitner AH. 2007. Prokaryotic community analysis with CARD-FISH in comparison with FISH in ultra-oligotrophic ground- and drinking water. J Appl Microbiol 103:871–881.
- Yokoo Y. 2006. Sr-Nd Isotopic Study on Geo-environmental Science. In: Yamanaka T, editor. Isotopic Tracer Techniques for Diagnosing Environmental Circulatory System, Electronic Monograph No. 2, Terrestrial Environment Research Center. Tsukuba, Japan: University of Tsukuba, p 54–59 (in Japanese).
- Zhou Y, Kellermann C, Griebler C. 2012. Spatio-temporal patterns of microbial communities in a hydrologically dynamic pristine aquifer. FEMS Microbiol Ecol 81:230–242.
- Ziegler S, Dolch K, Geiger K, Krause S, Asskamp M, Eusterhues K, Kriews M, Wilhelms-Dick D, Goettlicher J, Majzlan J, Gescher J. 2013. Oxygen-dependent niche formation of a pyrite-dependent acidophilic consortium built by archaea and bacteria. ISME J 7:1725–1737.
Appendix
Table A1. The range of ion values (ppm) in springwater at the foot of Mt. Fuji
Figure A1. Phylogenetic tree of proteobacterial 16S rRNA gene sequences at the foot of Mt. Fuji. Aquifex pyrophilus was employed as an outgroup. Blue, red, green, and purple indicate eastern (E), southeastern (SE), southern (S), and estern (W) slope of the foot, respectively. Length of color bar indicates the number of clones. Bootstrape values above 50% for 1,000 replicates are shown.