Abstract
Cervical cancer is the leading cause of cancer deaths among women in Latin America and the Caribbean, and disproportionately affects poorer women. Mortality rates in the region are seven times greater than in North America. In light of the significant public health burden, the Pan American Health Organization has drafted a Regional Strategy for Cervical Cancer Prevention and Control. The Strategy calls for increased action to strengthen programmes through an integrated package of services: health information and education; screening and pre-cancer treatment; invasive cervical cancer treatment and palliative care; and evidence-based policy decisions on whether and how to introduce human papillomavirus (HPV) vaccines. It calls for a seven-point plan of action: conduct a situation analysis; intensify information, education and counselling; scale up screening and link to pre-cancer treatment; strengthen information systems and cancer registries; improve access to and quality of cancer treatment and palliative care; generate evidence to facilitate decision-making regarding HPV vaccine introduction; and advocate for equitable access and affordable HPV vaccines. This proposed strategy, approved by the PAHO Directing Council on 1 October 2008, has the possibility of stimulating and accelerating the introduction of new screening technology and HPV vaccines into programmes throughout Latin America and the Caribbean.
Résumé
Le cancer du col de l’utérus est la principale cause de mortalité par cancer chez les femmes en Amérique latine et dans les Caraïbes, et il touche de manière disproportionnée les femmes pauvres. Les taux de mortalité dans la région sont sept fois plus élevés qu’en Amérique du Nord. C’est pourquoi l’Organisation panaméricaine de santé (OPS) a formulé une Stratégie régionale pour la prévention et le contrôle du cancer du col de l’utérus. La Stratégie demande une action accrue pour renforcer les programmes par un ensemble intégré de services : éducation en santé ; dépistage et traitement des lésions précancéreuses ; traitement et soins palliatifs des formes invasives ; et décisions de politique à base factuelle sur l’opportunité et les modalités de l’introduction des vaccins contre le papillomavirus humain (PVH). Elle prône un plan d’action en sept points : mener une évaluation de la situation ; intensifier l’éducation, l’information et le conseil ; affermir les programmes de dépistage et de traitement précancéreux ; renforcer les systèmes d’information et les registres du cancer ; améliorer l’accès et la qualité du traitement du cancer et des soins palliatifs ; créer des données probantes pour faciliter la prise de décision concernant l’introduction du vaccin anti-PVH ; et préconiser un accès équitable et abordable aux vaccins anti-PVH. Si elle est approuvée par le Conseil de directeur de l’OPS le 1er octobre 2008, cette stratégie pourrait stimuler et accélérer l’introduction de nouvelles technologies de dépistage et des vaccins anti-PVH en Amérique latine et aux Caraïbes.
Resumen
El cáncer cervical es la principal causa de muertes por cáncer entre las mujeres de Latinoamérica y el Caribe, y afecta desproporcionadamente a las mujeres más pobres. Las tasas de mortalidad en la región son siete veces mayor que en Norteamérica. En vista de la considerable carga de salud pública, la Organización Panamericana de la Salud elaboró la versión preliminar de una Estrategia Regional para la Prevención y el Control del Cáncer Cervical. La estrategia hace un llamado para aumentar las medidas destinadas a fortalecer los programas mediante una serie integrada de servicios: información y educación en salud; tamizaje y tratamiento pre-cáncer; tratamiento invasivo del cáncer cervical y atención paliativa; y decisiones de políticas basadas en evidencia respecto a si se deben introducir vacunas contra el virus del papiloma humano (VPH) y cómo. Hace un llamado para ejecutar un plan de acción de siete puntos: realizar un análisis de situación; intensificar la información, educación y consejería; ampliar el tamizaje y vincular al tratamiento pre-cáncer; fortalecer los sistemas de información y los registros de cáncer; mejorar el acceso al tratamiento del cáncer y la atención paliativa y la calidad de estos; generar evidencia para facilitar la toma de decisiones respecto al lanzamiento de vacunas contra el VPH; y abogar por igualdad de acceso y vacunas de VPH a precios asequibles. Si es aprobada por el Consejo Directivo de la OPS el 1 de octubre de 2008, esta estrategia tiene la posibilidad de estimular y acelerar el lanzamiento de una nueva tecnología de tamizaje y vacunas contra el VPH en los programas de Latinoamérica y el Caribe.
Cervical cancer is the leading cause of cancer deaths among women in Latin America and the Caribbean.Citation1 In light of the significant public health burden it represents, and in recognition of the limited impact of prevention programmes to date in low-resource settings, the Pan American Health Organization (PAHO) has drafted a Regional Strategy for Cervical Cancer Prevention and Control. Its aim is to illustrate to governments and stakeholders that cost-effective approaches are available for comprehensive cervical cancer prevention and control, which require a complete package of services: health education, screening, diagnosis and treatment; and depending on affordability, sustainability and country preparedness, HPV vaccination. The priority is to fortify programmes and evaluate whether and how new technologies, such as new screening techniques and HPV vaccines, can be used to improve the effectiveness of current programmes.
To enhance the development of the Regional Strategy, we conducted a literature review on PubMed of articles published in 1991-2008, using the search terms: cervical cancer in Latin America, cervical cancer incidence, cervical cancer mortality, cervical cancer screening programmes in Latin America and the Caribbean, cervical cancer screening technologies, HPV in Latin America and the Caribbean, HPV vaccines and cancer treatment. In addition, we analysed data on cervical cancer from the PAHO mortality database and the cancer epidemiology database of the International Agency for Research on Cancer.
Representatives from Ministry of Health cancer programmes from 25 countries in Latin America and the Caribbean were consulted on the proposed strategy. A standardised questionnaire with open-ended questions was sent in January 2008 to the PAHO/WHO country office contact and Ministry of Health cancer programme manager in each country, and written responses to the questionnaires were submitted to the PAHO central office by May 2008. Broader inputs to this Regional Strategy were also obtained during a Latin American stakeholders meeting on comprehensive cervical cancer prevention and control, convened by PAHO, WHO, the Sabin Institute and the US Centres for Disease Control and Prevention on 12-14 May 2008 in Mexico City. This meeting was attended by over 160 participants from 21 countries, representing country programmes in the areas of immunisation, adolescent health, sexual and reproductive health and cancer control.Citation1 Verbal comments on the Regional Strategy were provided during plenary discussion and working group sessions.
The Regional Strategy was then presented to the PAHO Directing Council on 1 October 2008, a governing body represented by Ministers of Health from all countries in the Americas. A resolution was endorsed that commits countries to scaling up comprehensive cervical cancer programmes, with increased technical cooperation from PAHO.
Preventable deaths
An estimated 72,000 new cases of cervical cancer and 33,000 deaths occur annually in Latin America and the Caribbean, representing an economic loss in productivity of approximately US$ 3.3 billion per year.Citation2Citation3
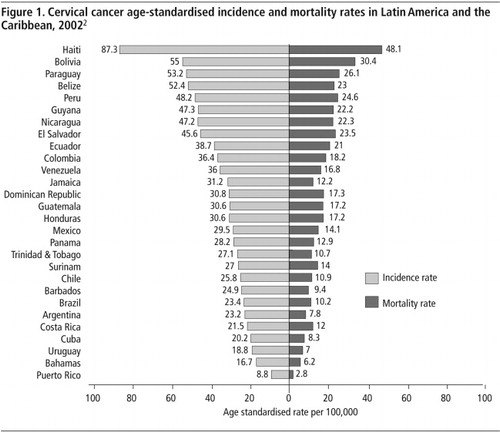
Cervical cancer disproportionately affects poorer countries, and within countries, poorer communities due to their lack of effective prevention programmes. Cervical cancer mortality rates are seven times greater in Latin America and the Caribbean than in North America. The highest disease burden is borne by Caribbean and Central American women, with mortality rates estimated at 16 per 100,000 and 15 per 100,000, respectively.Citation2 As illustrated in , Haiti, Bolivia, Paraguay, Peru and Nicaragua are among the countries with the highest cervical cancer mortality rates in the region.Citation2
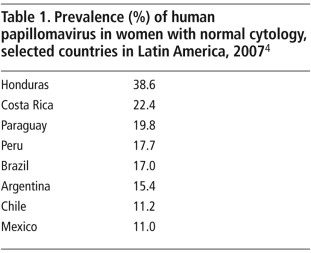
In the Americas, the prevalence of human papillomavirus (HPV), the main cause of cervical cancer, is estimated to be 15.6% among the general population of women aged 15 years and older. Countries such as Honduras, Costa Rica and Paraguay have HPV prevalence much higher than the regional average (Table 1).Citation4 As elsewhere in the world, HPV types 16 and 18 are most frequent in the Americas, and approximately 70% of invasive cervical cancer cases are attributed to HPV 16 and 18.Citation4
Limited programmes, coverage and treatment
To date, a very effective way to prevent cervical cancer is to screen women for pre-cancerous cervical lesions, followed by treatment of the lesions to prevent progression to invasive cancer. Cervical cytology screening (using Pap smears) has been in place in the Americas for approximately 30 years, yet countries have not experienced the same declines in mortality rates as have been observed in North America. Only Costa Rica, Chile and Cuba have observed significant reductions in cervical cancer rates, attributed to effective screening and treatment programmes.
The main reason for this situation is that it is very difficult to mount and sustain high quality screening programmes, particularly in low-resource settings, due to: a) challenges in achieving high screening coverage of women in the at-risk age group (>30 years), b) poor quality of Pap testing, and c) incomplete diagnosis and treatment of women found to be positive. For example, studies in Peru demonstrated that in one province, the sensitivity of the Pap test was only 27%; and of women with abnormal Pap test results, 75% did not receive appropriate follow-up diagnosis or treatment.Citation5Citation6
The failures of cervical cancer prevention programmes in the region can be characterised not only by factors related to the screening technology, but also health systems (access, organisation and quality of services, including training of health providers) and community perspectives. Women’s socio-cultural, economic and educational status, and ethnicity influence their access to information and the level of demand for and utilisation of cervical cancer prevention services.Citation7Citation8 Other key factors include:
| • | low awareness among women and men of the importance of screening; | ||||
| • | limited access to diagnostic services and treatment for pre-cancer; | ||||
| • | inadequate capacity for surgical and radiotherapy treatment for women diagnosed with invasive cancer.Citation8 | ||||
New technology
Alternative screening methods
Several cervical cancer screening techniques have been developed, partly as a response to the challenges of cytology screening. These include visual inspection with acetic acid (VIA), and HPV DNA tests, including a low-cost, fast HPV test. HPV DNA testing has in most cases proven to be more sensitive than Pap testing in accurately detecting pre-cancerous lesions; and VIA performs equal to or better than Pap testing in detecting pre-cancerous lesions.Citation9
An advantage of VIA screening is that women can be offered an immediate test result, and if eligible offered immediate pre-cancer treatment using cryotherapy (if health workers have been trained to provide it) in a single visit in primary care settings. VIA screening followed immediately by cryotherapy treatment for pre-cancerous lesions has demonstrated a 35% reduction in cervical cancer mortality in a seven-year period in a large randomised trial in India, for example.Citation10
Several countries in the region, including Bolivia, Colombia, Costa Rica, Guatemala, Mexico and Peru, are currently using alternative screening approaches. It is, therefore possible that an array of screening approaches can be adopted in countries, depending on health system access, availability of laboratory services, and human and financial resources. These new approaches and technologies offer opportunities for strengthening health services for screening and treatment.
HPV vaccines
The currently available HPV vaccines include a quadrivalent vaccine against genotypes 6,11,16 and 18, and a bivalent vaccine against genotypes 16 and 18. In clinical trials, both vaccines have demonstrated safety, high immunogenicity and almost 100% effectiveness in preventing infection and pre-cancerous lesions from HPV types 16 and 18, when given to adolescent girls prior to sexual debut.Citation11 Cost-effectiveness evaluations using data from Latin American countries demonstrate that at a price of US $5 per dose, HPV vaccination would be highly cost-effective, and actually result in cost-savings in most countries in the Latin American and Caribbean region. Vaccination alone, compared to the status quo, could lead to a 40% reduction in mortality over the lifetime of the vaccinated cohort. If vaccination is coupled with at least three screening tests over the woman’s lifetime, lifetime risk of cervical cancer could be reduced by 60% if vaccination and screening coverage reaches 70% of targeted women.Citation12Citation13
Twenty-six countries in Latin America and the Caribbean have licensed HPV vaccines; and Costa Rica, Mexico, and Peru are testing the HPV vaccine in demonstration projects or research trials. The affordability of HPV vaccines for public health programmes remains probably the greatest obstacle to their introduction. In addition, it will require adequate preparation, such as cold-chain requirements, delivery strategy, distribution method, and post-vaccination surveillance in order to address programmatic issues for sustainable introduction as part of a comprehensive cervical cancer programme.Citation14Citation15 The HPV vaccine is in the process of WHO pre-qualification for new vaccines, which would enable purchases in developing countries via United Nations agencies. In the meantime, PAHO has developed a framework for country-based policy decisions on new vaccine introduction, through its ProVac Initiative.Citation16 This initiative is to develop processes, tools and undertake cost-benefit analyses in support of evidence-based decision-making for the introduction of new vaccines, including the HPV vaccine.
PAHO regional strategy
The Regional Strategy for Comprehensive Cervical Cancer Prevention and Control proposes to improve country capacity for the sustained implementation of comprehensive cervical cancer prevention and control programmes, with the goal of reducing incidence and mortality. It promotes an integrated package of services for: health information and education; screening of asymptomatic women and pre-cancer treatment; invasive cervical cancer treatment and palliative care; and evidence-based policy decisions on whether and how to introduce the HPV vaccines.
A seven-point plan of action is recommended, with the immediate priority to strengthen current health services and consider the introduction of new approaches and technologies to improve coverage, quality and effectiveness. The plan includes the following points:
• Conduct a situation analysis
In the absence of current strategic information, collect information on sexual health; assess current investments and coverage, follow-up and quality of the screening programme; assess the burden of HPV, pre-cancer and cancer in the country; and examine adolescent and community perspectives, beliefs and needs related to cervical cancer prevention and control. This information would help inform decisions on whether and how to modify cervical cancer policies and practices; and also serve as a baseline for monitoring programme impact.
• Intensify information, education and counselling
Increase awareness about cervical cancer and HPV infection prevention and promote healthy sexual behaviour among adolescent populations, women and men, and health professionals. Engage communities in prevention services, focusing on women from disadvantaged and vulnerable groups, including women living in rural areas and indigenous women.
• Fortify screening and pre-cancer treatment programmes
In settings with sufficient resources to sustain quality Pap test screening and guarantee timely and appropriate follow-up for women who screen positive, strengthen screening programmes by: (i) improving the quality of screening tests; ii) consider introducing HPV DNA testing to triage HPV-positive women for further testing; (iii) increase the screening coverage of women in the at-risk age group (>30 years); and (iv) increase the proportion of timely and appropriate follow-up care for women with abnormal screening test results.
In settings where resources are not sufficient to sustain quality Pap smears, and where there are high rates of women who do not have access to timely and appropriate follow-up care, strongly consider incorporating VIA or fast HPV testing, using a single visit screen-and-treat approach, and ensuring appropriate health provider training and supervision.
• Establish or strengthen information systems and cancer registries
Establishment of an information and surveillance system is essential for ongoing monitoring of cervical cancer programme performance, including coverage, screening test results and follow-up diagnosis and treatment, to assess the pre-vaccine burden of HPV, pre-cancer and cervical cancer, and to monitor the impact, safety and effectiveness of HPV vaccines.
• Improve access to and quality of cancer treatment and of palliative care
Surgery and radiation therapy are the recommended treatment modalities for invasive cervical cancer, resulting in cure rates of 85–90% in early stages.Citation17Citation18 Investments are needed to ensure that radiation therapy and surgery are available and accessible, and linked to screening programmes, so that women detected with cancer can be treated appropriately and cured.
• Generate evidence to facilitate decision-making regarding HPV vaccine introduction
As countries decide whether and how to introduce the vaccine into public health programmes, evidence to inform their decisions will need to be gathered and several issues will need to be taken into account. PAHO, through the ProVac Initiative, will work with countries to enhance national capacity to make evidence-based vaccine introduction decisions through a five-year programme of scaled-up work.
• Advocate for equitable access and affordable HPV vaccines
Widespread access to the HPV vaccine will depend on having an affordable price and ensuring the necessary preparation for introducing a vaccine as part of a comprehensive cervical cancer programme. Since poor women are at greatest risk, access and equity are critical issues that need to be addressed for vaccine introduction,Citation19 particularly affordability of HPV vaccines. Partnerships and collaboration across multi-disciplinary health professional groups are needed to strengthen primary care services and immunisation programmes, in preparation for HPV vaccine introduction and to ensure a comprehensive approach to cervical cancer.
Discussion
Cervical cancer prevention is changing rapidly, with the potential to dramatically reduce mortality from this preventable disease. As a technical cooperation agency, PAHO is positioned to assist countries in their decision-making regarding new policies for the introduction of the new technology and in strengthening their public health programmes to be able to deliver them. PAHO’s experience implementing regional strategies that prioritise country support should contribute to the acceleration of prevention and control of cervical cancer in countries of Latin America and the Caribbean.Citation20
The inherent challenges of maintaining effective screening programmes in low-resource settings are well-recognised. Limited access to screening services and poor screening coverage are critical issues in many countries in the region, particularly among indigenous women. For example, in Ecuador and Guatemala, 70% and 58% respectively of indigenous women reported never having had a Pap smear.Citation21Citation22 This illustrates the need to direct resources towards previously unscreened women, particularly those in vulnerable and disadvantaged groups; and the importance of partnering with women’s advocacy groups to provide information that empowers women and enhances their participation in screening programmes.
Teenager receiving HPV vaccination during Vaccination Week in the Americas, El Paso, Texas, USA, April 2008
HPV vaccination will prevent, at most, 70% of the disease burden. Therefore, it must form a part of a package of interventions. Diminished incidence from vaccination of pre-adolescent cohorts should allow countries to focus on improving coverage and quality of screening, with 2-3 screening visits per woman’s lifetime. Finally, the process of policy development for introduction of HPV vaccination will promote a comprehensive strategy that will be most cost-effective for country implementation.
In the recent regional meeting on comprehensive cervical cancer in the region, mentioned above, public health professionals from national programmes on sexual and reproductive health, adolescent health, immunisation, and cancer control from all countries indicated that they would introduce the HPV vaccine into their programmes, if the price was affordable, if financing was available to assure its sustainability, and if criteria for introducing new vaccines were satisfied.Citation1 Additionally, almost all countries said that while they will continue to utilise Pap smear testing, they are willing to introduce alternative screening approaches such as VIA and HPV DNA testing, particularly in settings where the effectiveness of Pap smears has been limited.
Country representatives expressed concerns about the challenges and limitations of their current cervical cancer screening programmes and adopted a declaration to enhance comprehensive cervical cancer prevention and control. The declaration calls for increased resources and health system improvements to enhance the quality and coverage of screening programmes, as well as support for PAHO’s Revolving Fund to negotiate affordable vaccine prices and facilitate the introduction of the HPV vaccine into national immunisation programmes as soon as possible.Citation1
The acceptance of the Regional Strategy by the PAHO Directing Council in October 2008 commits countries to prioritise cervical cancer prevention and control, with technical assistance from PAHO, including introducing new cost-effective technology such as VIA and HPV DNA tests into screening programmes.
To implement this Regional Strategy, partnerships with community, national, and international organisations will be expanded, including across the UN system for resource mobilisation, advocacy and collaboration. Advocacy efforts will include raising awareness among policymakers and health professionals. Efforts to improve quality and coverage, by strengthening health services and training providers, will be focused on those countries with the highest mortality rates from cervical cancer and where screening programmes have not been successful, or where there are a high proportion of disadvantaged and vulnerable populations. In settings such as these, attention will be given to strengthening primary care services for the delivery of prevention, education and the possibility of a single visit screen-and-treat approach, using VIA or HPV DNA testing.
The information, knowledge and tools are all available to prevent cervical cancer. Our challenge now is to ensure that the policy environment, health care capacity and community support are in place to effectively deliver the necessary services to the women who need them most.
Acknowledgements
We wish to thank all of our colleagues who provided feedback and input into the development of PAHO’s Regional Strategy for Cervical Cancer Prevention and Control: Merle Lewis, Lucimar Coser-Cannon, Pablo Jimenez, Cuauhtemoc Ruiz Matus, Matilde Maddaleno, Ricardo Fescina, Roberto del Aguila, Marijke Velzeboer-Salcedo, James Hospedales, Meghan Blake, Marta Prieto, Gina Tambini, and Jarbas Barbosa.
References
- Pan American Health Organization. Towards comprehensive cervical cancer prevention and control: declaration from the regional meeting. Immunization Newsletter. June. 2008; PAHO: Washington DC.
- J Ferlay, F Bray, P Pisani. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. IARC CancerBase No. 5. Version 2.0. 2004; IARC Press: Lyon.
- Jara L, Suarez R. Analysis prepared on the economic impact of cervical cancer in Latin America and the Caribbean, 2007. (Unpublished)
- X Castellsagué, S de Sanjosé, T Aguado. HPV and cervical cancer in the world. 2007; WHO/ICO Information Centre on HPV and Cervical Cancer: Geneva.
- S Luciani, J Winkler. Cervical cancer prevention in Peru: lessons learned from the TATI demonstration project. 2006; PAHO: Washington.
- JC Gage, C Ferreccio, M Gonzales. Follow up care of women with abnormal cytology in a low-resource setting. Cancer Detection and Prevention. 27: 2003; 466–471.
- I Agurto, S Arrossi, S White. Involving the community in cervical cancer prevention programs. International Journal of Gynecology & Obstetrics. 89(Suppl 2): 2005; S38–S45.
- World Health Organization. Comprehensive Cervical Cancer Control: A guide to essential practice. 2006; WHO: Geneva.
- R Sankaranarayanan, L Gaffikin, M Jacob. A critical assessment of screening methods for cervical neoplasia. International Journal of Gynecology and Obstetrics. 89(2): 2005; S4–S12.
- R Sankaranarayanan, PO Esmy, R Rajkumar. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu: a cluster-randomized trial. Lancet. 370: 2007; 398–406.
- World Health Organization. Human papillomavirus and HPV vaccines: technical information for policy-makers and health professionals. 2006; WHO: Geneva.
- GP Garnett, JJ Kim, K French. Modeling the impact of HPV vaccines on cervical cancer and screening programs. Vaccine. S3: 2006; 178–186.
- S Goldie, M Diaz, D Constenla. Mathematical models of cervical cancer prevention in Latin America and the Caribbean. Vaccine. 26S: 2008; L59–L72.
- JK Andrus, J Sherris, JW Fitzsimmons. Introduction of HPV vaccine into developing countries: international strategies for funding and procurement. Vaccine. 26S: 2008; K87–K92.
- JK Andrus, M Lewis, SJ Goldie. Human papillomavirus vaccine policy and delivery in Latin America and the Caribbean. Vaccine. 26S: 2008; L80–L87.
- JK Andrus, C Toscano, M Lewis. A model for enhancing evidenced-based capacity to make informed policy decisions on the introduction of new vaccines in the Americas: PAHO’s ProVac Initiative. Public Health Reports. 122(6): 2007; 811–816.
- CA Perez, PW Grigsby, SM Nene. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 69(11): 1992; 2796–2806.
- PJ Eifel, TW Burke, L Delclos. Early stage I adenocarcinoma of the uterine cervix: treatment results in patients with tumors less than or equal to 4 cm in diameter. Gynecological Oncology. 41(3): 1991; 199–205.
- JK Andrus, JW Fitzsimmons. Introduction of new and under-utilized vaccines: sustaining access, disease control, and infrastructure development. PLoS Medicine. 2(10): 2005; e286.
- G Tambini, JK Andrus, JW Fitzsimmons. Regional programs for health: immunization as a model for strengthening inter-country cooperation and control of infectious diseases. Pan American Journal of Public Health. 20(1): 2006; 54–59.
- Ecuador National Institute of Statistics and Census. ENDEMAIN Survey. 2004; INEC: Ecuador.
- Guatemala National Institute of Statistics. Demographic and Health Survey. 2002; INE: Guatemala.