Abstract
Approximately 70% of cases of cervical cancer worldwide are caused by genotypes 16 and 18 of human papillomavirus (HPV), which is sexually transmitted. With the availability of an effective vaccine against these HPV types, there is real hope for reducing the global burden of cervical cancer in developing countries. Stakeholders faced with decisions about where to invest money to improve health must consider the burden of disease caused by cervical cancer relative to other priorities and the comparative benefits of different interventions. We conducted a series of analyses to obtain information for agencies drafting immunisation policy recommendations, financing coordination mechanisms, and country decision-makers on the benefits, cost requirements and cost-effectiveness of the HPV16,18 vaccine. We found that making an HPV16,18 vaccine accessible to 70% of young adolescent girls in 72 of the poorest countries, China, Thailand, and all of Latin America and the Caribbean, could prevent the future deaths of more than four million women vaccinated over the next decade. Provided the cost per vaccinated girl is less than $10–$25, adolescent HPV16,18 vaccination would be cost-effective even in relatively poor countries. Concerns about financial costs and affordability highlight the need for lowering vaccine prices, cost-efficient mechanisms for delivery of vaccinations to adolescents, and creative sources of financing.
Résumé
Environ 70% des cancers du col de l’utérus dans le monde sont causés par les génotypes 16 et 18 du papillomavirus humain (PVH) qui est transmis sexuellement. Depuis qu’on dispose d’un vaccin efficace contre ces souches du PVH, il est réaliste d’espérer réduire la charge mondiale de cette forme de cancer dans les pays en développement. Les responsables qui décident où investir les fonds pour améliorer la santé doivent tenir compte de la charge de morbidité du cancer du col de l’utérus au regard d’autres priorités et des avantages comparés des différentes interventions. Nous avons mené des analyses pour obtenir des informations d’institutions préparant des projets de recommandations politiques, de mécanismes de coordination du financement et de décideurs nationaux sur les avantages, les niveaux acceptables de prix et le rapport coût-utilité de ce vaccin. Nous avons constaté que si 70% des jeunes adolescentes dans les 72 pays les plus pauvres, la Chine, la Thaïlande et l’ensemble de l’Amérique latine et des Caraïbes avaient accès au vaccin anti-PVH16, 18, on éviterait les décès futurs de plus de quatre millions de femmes vaccinées au cours de la prochaine décennie. Pourvu que le coût par adolescente vaccinée soit inférieur à $US 10–25, cette intervention serait rentable même dans des pays relativement pauvres. Les inquiétudes sur les coûts financiers montrent qu’il faut baisser le prix des vaccins, créer des mécanismes efficaces de vaccination des adolescentes et trouver des sources novatrices de financement.
Resumen
Aproximadamente el 70% de los casos de cáncer cervical mundialmente son causados por los genotipos 16 y 18 del virus del papiloma humano (VPH), que es transmitido sexualmente. Con la disponibilidad de una vacuna eficaz contra estos tipos de VPH, existe una buena posibilidad de disminuir el impacto mundial del cáncer cervical en los países en desarrollo. Las partes interesadas que deben decidir dónde invertir el dinero para mejorar la salud, deben tomar en cuenta la carga de la enfermedad causada por el cáncer cervical con relación a otras prioridades y los beneficios comparativos de las diferentes intervenciones. Realizamos una serie de análisis para obtener información para las instituciones que están elaborando recomendaciones sobre las políticas de inmunización, los mecanismos de coordinación financiera y los tomadores de decisiones, respecto a los beneficios, los requisitos de costo y la costo-eficacia de la vacuna contra VPH16,18. Encontramos que si el 70% de las adolescentes en 72 de los países más pobres, China, Tailandia y toda Latinoamérica y el Caribe tienen acceso a la vacuna VPH16,18, se pueden evitar las futuras muertes de más de cuatro millones de mujeres vacunadas en la próxima década. Siempre y cuando el costo por adolescente vacunada sea inferior a $10–$25, la vacunación de las adolescentes contra el VPH16,18 sería rentable incluso en los países relativamente pobres. Las preocupaciones sobre los costos financieros y la asequibilidad resaltan la necesidad de reducir los precios de la vacuna y de contar con fuentes creativas de financiamiento y mecanismos costo-eficaces para la entrega de vacunas a las adolescentes.
Among the most tragic public health failures are the preventable deaths of women in developing countries from cervical cancer. Approximately half a million women develop cervical cancer each year, with the highest incidence rates in developing countries, where screening programmes have not been feasible. Nearly 80% of cervical cancer deaths currently occur in developing countries, and by 2020 this proportion is expected to increase to 90%.Citation1
Human papillomavirus (HPV), an extremely common sexually transmitted virus, is the known aetiologic agent responsible for cervical cancer. Although there is considerable epidemiological variation, HPV types 16 and 18 cause approximately 70% of cervical cancer,Citation2–4 and the eight most common genotypes (HPV 16, 18, 45, 31, 33, 52, 58 and 35) account for 90%. Persistent infection with high-risk HPV genotypes is also the cause of 90% of anal cancer, 40% of other anogenital cancers, and at least 12% of oropharyngeal cancers.Citation1 Regional variations in cervical cancer incidence are due to differences in underlying prevalence of high-risk HPV types as well as disparities in the availability of effective cervical prevention and treatment.
Unlike most cancers, cervical cancer is preventable through regular screening to detect and remove pre-cancerous lesions. A conventional screening programme can require up to three visits to collect a cervical cytology specimen, conduct a diagnostic evaluation, and provide any necessary treatment. In countries that have been able to achieve high coverage of adult women using cytology at frequent intervals, mortality has been reduced significantly. However, in countries with limited resources and inadequate health infrastructure, organised screening has proven difficult to implement.Citation5
New cervical cancer prevention opportunities
New opportunities to reduce cervical cancer deaths stem from new and more feasible screening alternatives for developing countries and, most recently, prophylactic vaccines against cancer-causing types of HPV. Promising screening approaches that have been found to be effective and cost-effective include HPV DNA testing and visual inspection methods, focusing efforts on screening women between the ages of 30 and 45 one to three times per lifetime, and minimising loss to follow-up by linking screening and treatment in as few visits as possible.Citation6–9
The two newly available vaccines prevent infection and disease associated with HPV high-risk types 16 and 18.Citation10Citation11 Results from ongoing clinical trials show high efficacy in preventing infection and cervical pre-cancerous disease in girls and women not previously infected with those types at the time of vaccination. With a much lower success rate among those already exposed to HPV, early emphasis has been placed on young adolescent girls as the priority target group. Among the most obvious barriers for developing countries is vaccine price. The three-dose series of the Merck vaccine is estimated to cost $360 in the United States, which even with tiered pricing still exceeds the reach of developing countries. Manufacturers have expressed their willingness to provide the vaccine at much lower costs for poor countries, but these prices are not yet known.
Challenging decisions about cervical cancer prevention
Adding a new vaccine to a national immunisation programme requires consideration of the avertable burden, relative value of the vaccine compared with alternative uses of resources, affordability, likelihood of public acceptability and political support for a vaccine against a sexually transmitted disease, and feasibility of achieving high coverage in young adolescent girls. The question of how to use screening in the context of vaccination adds additional layers of complexity. Because vaccination and screening are applicable to such different age groups, require monetary resources that may come from different sources, are subject to unique operational challenges and are dependent to different degrees on existing infrastructure, the feasibility of widespread coverage with each could very well vary between and within countries.
Predicting the population-level impact of a cervical cancer prevention programme is particularly complex, as the time course from infection to disease spans several decades, the best available data are based on intermediate endpoints, the primary and secondary prevention options target very different components of the disease spectrum, and randomised controlled trials are not feasible or ethical.Citation12–14 Computer-based mathematical models can be useful tools in overcoming these challenges because they provide a formal framework for synthesising data in an internally-consistent and epidemiologically plausible way.Citation8,12,14,15
Decision-makers need information on the relative value of investments in vaccination versus screening, on the synergies that might be realised with the use of both modalities, and on the “best bets” for a sustainable cervical cancer prevention programme. Furthermore, immunisation policy recommendations made by the World Health Organization, and financing coordination mechanisms such as the GAVI Alliance and Pan American Health Organization Revolving Fund, require information on the financial cost requirements and cost-effectiveness of adolescent HPV16,18 vaccination. In response to these needs, we conducted a series of analyses focused on the assessment of HPV16,18 adolescent vaccination for girls in low-and middle-income countries.Citation16–22 This paper summarises the most salient features from analyses focusing on 72 GAVI-eligible countries and 33 Latin American and Caribbean countries.
Methodological approach
Decision science and cost-effectiveness analysis
The disciplinary basis for our analytic approach, decision science, provides a structured, logical way of informing complex decisions with multiple choices or alternatives, inevitable trade-offs, and a number of possible perspectives. Inherent in a decision analytic approach is an explicit focus on identifying, measuring, and valuing the outcomes or consequences of decisions, as well as the uncertainty about these outcomes that exists at the time decisions are made. These elements are incorporated into a model to structure the decision problem over time. While different types of models may be chosen to accommodate the complexity of the decision problem, all rely on mathematical analysis to compare the performance of alternatives.Citation23
Cost-effectiveness analysis (CEA) is a specific type of decision analysis that formally compares the relationship between the incremental health and economic consequences associated with different interventions. A cost-effectiveness analysis addresses the following question: “How much health improvement can be gained, dollar for dollar, for any given health intervention compared to an alternative use of those resources?”Citation14Citation24 The underlying principle guiding the valuation of resources in cost-effectiveness analyses is opportunity cost, which reflects competing societal demands for limited resources. The performance of alternative interventions being compared in an analysis is described using incremental cost-effectiveness ratios, defined as the additional cost of a specific strategy, divided by its additional benefit, compared with the next best strategy.
In the analyses presented here, costs are expressed in constant International dollars (I$), a currency used to translate cost measures in a country’s currency to a common currency (the US dollar), reflecting differences in price levels between countries.Citation25Citation26 An international dollar has the same purchasing power as one US dollar has in the United States. In addition to reporting cancer deaths averted, to permit comparisons with other public health interventions, benefits are expressed as life-expectancy gains and disability-adjusted life years (DALYs) averted. DALY is a unit for measuring the health lost due to a particular disease, taking into account both premature death and years lived with disability and impaired quality.Citation27
There is no universal criterion that defines a threshold cost-effectiveness ratio, below which an intervention would be considered cost-effective. A commonly cited rule of thumb, based on a report by the Commission on Macroeconomics and Health, is that interventions are “very cost-effective” if they have cost-effectiveness ratios less than per capita Gross Domestic Product (GDP).Citation25,26,28 For an HPV16,18 vaccination strategy, there may indeed be a lower “threshold ratio” that would be required to compete for scarce resources if existing vaccines (e.g. childhood immunisation) have ratios that are much lower than the GDP rule of thumb cited above.Citation14
Analytic overview
Using population-based data from 72 countries eligible for assistance from the GAVI Alliance and 33 countries in Latin America and the Caribbean, together with country-and/or region-specific epidemiologic data, a model-based approach was used to estimate averted cases of invasive cancer, cancer deaths, life-expectancy gains, DALYs averted and incremental cost-effectiveness ratios (I$/DALY averted) for vaccination against HPV 16 and 18. Documentation of model development, data sources, data analysis and synthesis methods, and analytic assumptions may be found in recent publications and their respective appendices.Citation16–22,29–31 Analyses assumed the vaccine would be given to girls prior to age 12 (before sexual debut), was effective in preventing HPV 16 and 18 infection if used in girls with no prior infection, and provided life-long immunity. We adhered to standard guidelines for economic evaluation.Citation24,26–28 Sensitivity analyses assessed how uncertain parameters (e.g. vaccine efficacy) and assumptions (e.g. vaccination coverage levels) might influence results.
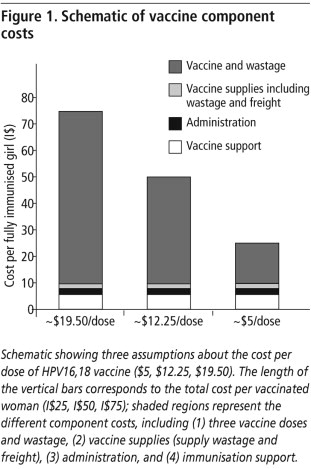
Since the vaccine price and delivery costs for low-and middle-income countries are currently uncertain, we defined a composite value as the “cost per vaccinated girl”. This composite cost was categorised into vaccine costs, wastage, freight and supplies, administration, immunisation support and programmatic costs.Citation17,32,33 For example, a cost of I$25 per vaccinated girl approximates three doses of vaccine at $5.00 each, wastage of $2.25, freight and supplies of $1.31, vaccine administration of $1.50, and immunisation support and programmatic costs of $4.94 ().Citation17 For countries in which screening strategies were analysed, direct medical costs (e.g. staff, supplies, equipment, and specimen transport), programmatic costs, and women’s time and transportation costs were included.Citation7,16,34
Results and insights
• To what degree could a reduction in cervical cancer be expected with adolescent HPV vaccination?
Assuming 70% coverage, the mean reduction in the lifetime risk of cancer was reduced by 40–50% in most countries, although was lower than 40% in some countries (e.g. Nigeria, Ghana, Chile) and higher than 50% in others (e.g. India, Uganda, Argentina). The absolute cervical cancer reduction in individual countries was influenced by cervical cancer incidence, population age structure, and vaccination coverage, while the relative reduction also depended on the fraction of cancer caused by HPV types 16 and 18.Citation17
• How much additional benefit would be realised from screening adult women who were previously vaccinated?
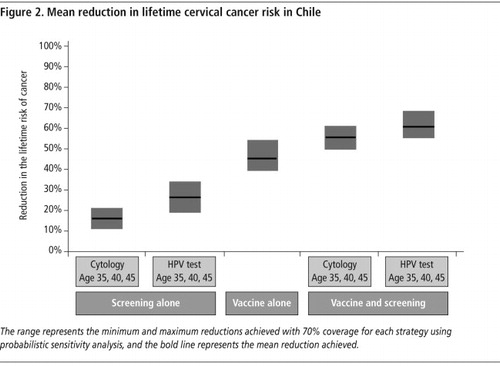
Screening was synergistic with vaccination as it reduced the risk of cervical cancer attributable to non-vaccine-targeted HPV types, as well as types 16 and 18. For example, assuming 70% coverage in Chile, there was a 25% additional cancer risk reduction when screening three times per lifetime (e.g. ages 35, 40 and 45) was conducted in women who were vaccinated as pre-adolescents ().Citation18 Analyses in other countries showed similar results, with the magnitude of the incremental benefit varying with screening test performance, frequency, and coverage.Citation8
• Would more women be saved by targeting those countries with the highest cancer rates?
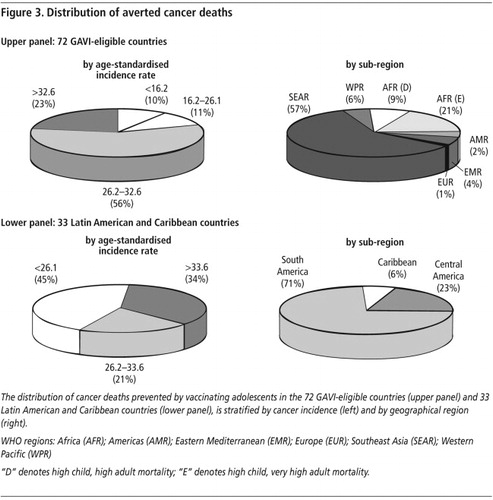
Countries with the highest rates of cervical cancer (age-standardised incidence rate (ASR) per 100,000 person-years at risk > 32.6) account for fewer absolute numbers of deaths than countries with moderate cervical cancer rates and large populations. In fact, among the 72 GAVI-eligible countries, those with the highest incidence rates represented less than 25% of averted deaths, and those with the lowest rates accounted for 10% (, upper panel). Approximately 57% of averted deaths would be in the WHO Southeast Asia region, with 41% in India alone. Countries in Africa would comprise an additional 30%.Citation17 Similarly, in the 33 Latin American and Caribbean countries, those with the lowest risk of cancer (ASR < 26.1) accounted for 45% of deaths averted (, lower panel). Approximately 71% of averted deaths would be in South America, 23% in Central America, and 6% in the Caribbean.Citation18
• How many women’s lives would be saved with a programme that vaccinated 70% of 12-year-old girls over ten consecutive years?
Approximately three million deaths would be prevented among women vaccinated as adolescents in the 72 GAVI-eligible countries.Citation17 A similar programme applied to 33 Latin American and Caribbean countries resulted in more than one million additional averted cases of cervical cancer.Citation18 We have reported elsewhere other scale-up scenarios that consider country-specific assumptions (per capita GNI, DPT3 coverage, percentage of girls enrolled in fifth grade) for the time of vaccine roll-out, rate of scale-up, and achievable coverage.Citation17
• Would adolescent HPV16,18 vaccination be cost-effective?
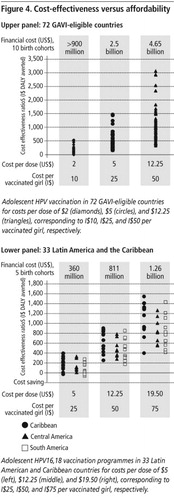
Provided the cost per vaccinated girl is much lower than the current price in developed countries, HPV16,18 vaccination could be very cost-effective even in the poorest countries, and offer comparable value to other new vaccines. In the GAVI-eligible countries, at I$10 per vaccinated girl (˜$2.00 per dose assuming three doses, plus wastage, administration, programme support), vaccination was very cost-effective in all 72 countries using the criterion of the cost-effectiveness ratio being less than the per capita GDP (, upper panel).Citation17 In the Latin America and Caribbean region, at I$25 per vaccinated girl ($5 per dose), for all 33 countries, the cost per DALY averted was less than I$400 (, lower panel).Citation18 While this ratio indicates good value for resources, in that it is less than each country’s per capita GDP, it also compares favourably to the ratios of other new vaccines.Citation25Citation28 For example, the cost-effectiveness of pneumococcal conjugate vaccination ranges from $110 to $2,150 across a range of countries, and rotavirus vaccination ranges from $290 to $12,300 in Latin American countries.Citation18
• Even if the vaccine is cost-effective, will it be affordable?
Despite favourable cost-effectiveness, assessment of financial costs required in the near term raised concerns about affordability (). For example, the financial requirements for vaccinating 12-year-old girls in the 72 GAVI-eligible countries at country-specific roll-out rates for just 10 calendar years varied from more than $900 million at $2 per dose (I$10 per vaccinated girl) to more than $4 billion at $12.25 per dose (I$50 per vaccinated girl).Citation17 In the 33 Latin American and Caribbean countries, vaccinating 70% of 12-year-olds for just five calendar years cost $360 million at $5 per dose but exceeded $1.25 billion at $19.50 per dose.Citation18
• Are there other benefits from prevention of cervical cancer deaths?
Using data from 72 countries on the average fertility rate, age-specific population size, and age-specific patterns of cervical cancer mortality, we estimated that a ten-year vaccination programme at 70% coverage would prevent the loss of a mother to cervical cancer for approximately 10 million children; between 1.5 and 2.9 million of these children would be under the age of 18.Citation17 Using wage rate data from the International Labour Organization,Citation35 we applied previously described methodsCitation36Citation37 to provide general insight into productivity costs associated with premature death from cervical cancer. Considering only one-third of countries, where data were easily available, when the stream of work (including household) conducted by women who will lose their lives to this disease was valued, lost future earnings (i.e. productivity costs) exceeded $2 billion.
• How do the benefits expected with the HPV16,18 vaccine compare to those of other new vaccines?
Considering the GAVI-eligible countries, 13 cervical cancer deaths were averted per 1,000 girls vaccinated, and among the poorest countries in Africa, 17 deaths were averted per 1,000 vaccinated.Citation17 In the Latin American and Caribbean countries, while the average averted cases per 1,000 girls vaccinated for the entire region was 27, in high-risk regions this number was higher (e.g. 41 averted cases per 1000 vaccinated in Haiti, and 32 averted cases per 1,000 vaccinated in Chile).Citation18 This compares favourably to reports estimating three averted deaths per 1,000 children vaccinated for rotavirusCitation38, as well as 6.8 deaths per 1,000 children vaccinated for pneumococcal conjugate vaccine in Brazil, 2.2 deaths per 1,000 children vaccinated in Chile, and 2.9 deaths per 1,000 children vaccinated in Uruguay.Citation39
• What uncertain assumptions had the greatest influence on the results?
For adolescent vaccination, the magnitude of the population benefits was most dependent on the age of vaccination, age-specific cervical cancer incidence, proportion of cancer attributable to HPV 16 and 18, and achievable coverage in young adolescent girls. Also influential were important vaccine uncertainties, such as the duration of immunity. Among the most influential uncertainties on cost-effectiveness were the vaccine price and the programmatic costs associated with an adolescent vaccination programme. Cost-effectiveness results were less favourable if the costs associated with invasive cervical cancer were reduced by 50% or if boosters were required for lifelong immunity.
The future cancer deaths that are prevented in HPV-vaccinated adolescent girls occur decades after vaccination costs are paid, while for other vaccines (e.g. rotavirus), health and economic outcomes are in close temporal proximity. Therefore, the impact of discounting future costs and benefits, recommended for standardising economic evaluations in public health,Citation14,24,40 is far more influential for HPV vaccination than for childhood vaccines. For example, without discounting benefits, cost-effectiveness ratios would be reduced (i.e. become more attractive) by 80%.Citation17Citation18
Sensitivity analyses that explore the influence of model input parameters, assumptions, and key uncertainties have been previously published for Brazil, Viet Nam and India,Citation16,21,22 as well as several other countries.Citation7,8,12,14,17–19,41
Comments
Our general findings indicate that making an HPV16,18 vaccine accessible to 70% of young adolescent girls in the 72 poorest GAVI-eligible countries could prevent the future deaths of about three million women vaccinated in the next decade. The addition of the non-GAVI eligible countries in the Latin American and Caribbean region, as well as China and Thailand,Citation19 would prevent almost one million additional future deaths. From a worldwide perspective, countries with the highest risk of cancer account for less than one-third of the projected avertable deaths, highlighting why a regional universal vaccination approach will be most effective in reducing the overall global burden. In fact, the greatest number of preventable deaths was expected in those countries with only moderate cervical cancer incidence but with large populations.
Both the annual financial costs to the payor (i.e. affordability) and the cost-effectiveness measurement (i.e. value for money) of a vaccine will need to be favourable as it will compete for dollars with existing immunisation programmes, initiatives for scale-up, and other new vaccines.Citation42 The price of the vaccine will be an obvious major determinant of both of these dimensions. Even at costs somewhat higher than traditional vaccines, provided the cost per vaccinated girl is less than $10–$25, adolescent HPV16,18 vaccination would be cost-effective even in relatively poor countries. For it to be affordable, current vaccine prices will need to be lowered, and effective cost-efficient mechanisms of achieving high coverage in adolescents will be needed. For instance, the World Bank has identified Latin America as one of the two most unequal world regions in terms of income distribution.Citation43 Assuming a cost near the current price of the vaccine in developed countries (US$120 per dose), the cost-effectiveness of vaccination in this region ranges from $2,400 to more than $10,000, well exceeding the ratios associated with childhood vaccines.Citation18 Furthermore, the financial implications for just five years of vaccination at this cost would approximate $6.38 billion in the 33 Latin American countries, compared with $811 million at $12.25 per dose.
The potential future introduction of HPV vaccines in conjunction with an adolescent immunisation programme could also create opportunities for strengthening health systems through the establishment of new mechanisms for vaccine delivery and surveillance of impact. If successful, the delivery of other adolescent health services might be facilitated, such as tetanus immunisation and sexual risk behaviour counselling.Citation44 Country-specific data using standardised costing instruments are needed to evaluate alternative programmatic and delivery options, costs associated with scale-up, and possible economies of scale with other programmes directed at an adolescent age group.
The most important evidence gaps bear emphasis. Model-based predictions of long-term vaccine benefits are dependent on several uncertain assumptions.Citation12,15,16,20,31,41,45 The long-term duration of vaccine-induced protection, necessity for a booster, and degree and duration of cross-strain protection against other oncogenic HPV types not included in the vaccine are not yet known. Other uncertainties are the efficacy of HPV vaccines in settings with high HIV prevalence, behaviour of non-targeted high-risk HPV types following vaccination, and magnitude of herd immunity. Future studies that address these issues are of high priority.
Given the range of uncertainties that exist, and the unavoidable limitations of model-based analyses, the insights from these analyses should be considered preliminary estimates of the health and economic outcomes of cervical cancer prevention based on the information available now. The policy decision facing low-and middle-income countries deciding on whether and how to implement HPV vaccination, given the reality that the individuals who benefit from vaccination will not realise those benefits for many years, is complex. Avertable burden, cost-effectiveness and affordability provide only three dimensions of the information necessary for decisions about vaccine adoption. Equally important to consider are criteria such as distributional effects and equity, capacity to deliver and sustain quality interventions, likelihood of cultural acceptability and political support.Citation14,27,42,46
Achieving broad coverage of young adolescent girls, negotiating affordable pricing and securing expedient vaccine financing will not be easy. During the typical multi-decade delay in making a new vaccine accessible to the poorest countries, several million more women will die of a preventable disease in their most productive years. A global commitment to meet the challenges required to make HPV16,18 vaccination available to young adolescent girls in the world’s poorest countries, coupled with provision of high quality and feasible secondary prevention services to adult women two to three times per lifetime, will save women’s lives. Although challenging to enumerate and monetise, these benefits will translate to an equal number of households and communities that will benefit, and an even greater number of children who will not lose their mothers to cervical cancer. Given enhanced efforts to leverage new resources for immunisxation through creative vaccine financing initiatives, the results of these analyses can both catalyse and inform early dialogue about investment decisions in HPV16,18 vaccine.
Acknowledgements
Funding support was from the Bill and Melinda Gates Foundation (30505), who had no role in the design or conduct of the study; collection, management, analysis or interpretation of data; or preparation, review or approval of the manuscript.
References
- DM Parkin, F Bray. The burden of HPV-related cancers. Vaccine. 24(S3): 2006; S11–S25.
- N Muñoz, FX Bosch, X Castellsagué. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. International Journal of Cancer. 111(2): 2004; 278–285.
- G Clifford, S Franceschi, M Diaz. HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 24(S3): 2006; S26–S34.
- JS Smith, L Lindsay, B Hoots. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. International Journal of Cancer. 121: 2007; 621–632.
- L Denny, M Quinn, R Sankaranarayanan. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 24(S3): 2006; S71–S77.
- SJ Goldie, LK Kuhn, L Denny. Policy analysis of cervical cancer screening strategies in low-resource settings: clinical benefits and cost-effectiveness. JAMA. 285: 2001; 3107–3115.
- SJ Goldie, L Gaffikin, JD Goldhaber-Fiebert. Cost-effectiveness of cervical cancer screening in five developing countries. New England Journal of Medicine. 353(20): 2005; 2158–2168.
- SJ Goldie, JJ Kim, E Myers. Chapter 19: Cost-effectiveness of cervical cancer screening. Vaccine. 24(S3): 2006; S164–S170.
- R Sankaranarayanan, L Gaffikin, M Jacob. A critical assessment of screening methods for cervical neoplasia. International Journal of Gynaecological Obstetrics. 89(S2): 2005; S4–S12.
- KA Ault, Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 369: 2007; 1861–1868.
- DM Harper, EL Franco, CM Wheeler. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 367(9518): 2006; 1247–1255.
- GP Garnett, JJ Kim, K French. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine. 24(S3): 2006; S178–S186.
- SJ Goldie. Chapter 15: Public health policy and cost-effectiveness analysis. Journal of the National Cancer Institute Monograph. 31: 2003; 102–110.
- SJ Goldie, JD Goldhaber-Fiebert, GP Garnett. Chapter 18: Public health policy for cervical cancer prevention: the role of decision science, economic evaluation, and mathematical modeling. Vaccine. 24(S3): 2006; S155–S163.
- JJ Kim, M Brisson, WJ Edmunds. Modeling cervical cancer prevention in developed countries. Vaccine. 26(11): 2008; K76–K86.
- SJ Goldie, JJ Kim, K Kobus. Cost-effectiveness of HPV16,18 vaccination in Brazil. Vaccine. 25(33): 2007; 6257–6270.
- SJ Goldie, M O’Shea, NG Campos. Health and economic outcomes of HPV16,18 vaccination in 72 GAVI-eligible countries. Vaccine. 26(32): 2008; 4080–4093.
- SJ Goldie, M Diaz, D Constenla. Mathematical models of cervical cancer prevention in Latin America and the Caribbean. Vaccine. 26(S11): 2008; L59–L72.
- Goldie SJ, Diaz M, Kim SY, et al. Mathematical models of cervical cancer prevention in the Asia Pacific region. Vaccine 2008;26(S12):M17-29.
- JJ Kim, B Andres-Beck, SJ Goldie. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. British Journal of Cancer. 97(9): 2007; 1322–1328.
- JJ Kim, KE Kobus, M Diaz. Exploring the cost-effectiveness of HPV vaccination in Vietnam: insights for evidence-based cervical cancer prevention policy. Vaccine. 26(32): 2008; 4015–4024.
- M Diaz, JJ Kim, G Albero. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. British Journal of Cancer. 99(2): 2008; 230–238.
- SY Kim, SJ Goldie. Cost-effectiveness analyses of vaccination programmes: a focused review of modelling approaches. Pharmacoeconomics. 26(3): 2008; 191–215.
- MR Gold, JE Siegel, LB Russell. Cost-effectiveness in Health and Medicine. 1996; Oxford University Press: New York.
- World Health Organization. Macroeconomics and Health: investing in health for economic development: report of the commission on macroeconomics and health. 2001; WHO: Geneva.
- World Health Organization Statistical Information System. CHOICE (Choosing Interventions that are Cost Effective). At <www.who.int/choice/en/. >. Accessed 29 January 2008.
- DB Evans, TT Edejer, T Adam. Methods to assess the costs and health effects of interventions for improving health in developing countries. BMJ. 331(7525): 2005; 1137–1140.
- Disease Control Priorities Project (DCPP). At <www.dcp2.org/main/Home.html. >. Accessed 28 January 2008.
- JD Goldhaber-Fiebert, NK Stout, J Ortendahl. Modeling human papillomavirus and cervical cancer for analyses of screening and vaccination. Population Health Metrics. 5(1): 2007; 11.
- JJ Kim, KM Kuntz, NK Stout. Multiparameter calibration of a natural history model of cervical cancer. American Journal of Epidemiology. 166(2): 2007; 137–150.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. New England Journal of Medicine 2008;359(8):821-32.
- U Kou. Guidelines for estimating costs of introducing new vaccines into the national immunization system. 2002; World Health Organization, Department of Vaccines and Biologicals: Geneva.
- World Health Organization. Adding a vaccine to a national immunization programme: decision and implementation. WHO/IVB/05.18. 2005. At: <www.who.int/vaccines-documents/DocsPDF05/777_screen.pdf. >. Accessed 1 August 2008.
- JD Goldhaber-Fiebert, SJ Goldie. Estimating the cost of cervical cancer screening in five developing countries. Cost Effectiveness and Resource Allocation. 4: 2006; 13.
- International Labour Organization. LABORSTA online database. At: <http://laborsta.ilo.org/. >. Accessed 21 September 2008.
- D Hu, SM Bertozzi, E Gakidou. The costs, benefits, and cost-effectiveness of interventions to reduce maternal morbidity and mortality in Mexico. PLoS ONE. 2(1): 2007; e750.
- W Max, DP Rice, HY Sung. Valuing human life: estimating the present value of lifetime earnings, 2000. (October 1, 2004). Center for Tobacco Control Research and Education. Economic Studies and Related Methods. Paper PVLE2000. At: <http://repositories.cdlib.org/ctcre/esarm/PVLE2000/. > Accessed 18 September 2008.
- IAVI/PATH. HPV vaccine adoption in developing countries: cost and financing issues. At: <http://www.iavi.org/viewfile.cfm?fid=47496. >. Accessed 29 February 2008.
- D Constenla, E Ortega-Barria, RD Rheingans. Economic impact of rotavirus vaccination in Panama. Anales de pediatría (Barcelona). 68(2): 2008; 128–135.
- MF Drummond, MJ Sculpher, GW Torrance. Methods for the Economic Evaluation of Health Care Programs. 3rd ed., 2005; Oxford University Press: New York.
- SJ Goldie, D Grima, M Kohli. A comprehensive natural history model of HPV infection and cervical cancer to estimate the clinical impact of a prophylactic HPV-16/18 vaccine. International Journal of Cancer. 106(6): 2003; 896–904.
- SY Kim, JA Salomon, SJ Goldie. Economic evaluation of hepatitis B vaccination in low-income countries: using cost-effectiveness affordability curves. Bulletin of World Health Organization. 85(11): 2007; 833–842.
- World Bank. Regional fact sheet. World Development Indicators 2008: Latin America and the Caribbean. At <http://siteresources.worldbank.org/DATASTATISTICS/Resources/lac_wdi.pdf. >. Accessed 11 September 2008.
- JM Agosti, SJ Goldie. Introducing HPV vaccine in developing countries–key challenges and issues. New England Journal of Medicine. 356(19): 2007; 1908–1910.
- CJ Haug. Human papillomavirus vaccination – reasons for caution. New England Journal of Medicine. 359(8): 2008; 861–862.
- P Musgrove, J Fox-Rushby. Cost-effectiveness analysis for priority setting. Disease Control Priorities in Developing Countries. 2006; 271–283.