Abstract
Background
The poor outlook of cervical carcinoma, a human papillomavirus (HPV)-related cancer mandates the search for new treatment modalities. Therapeutic targeting of tumor vasculature is a promising strategy. The aim was to study angiogenesis in cervical carcinoma in terms of VEGF expression and assessment of microvascular density (MVD) in relation to HPV antigen expression.
Methods
Thirty paraffin blocks of cervical carcinoma were studied for the immune expression of VEGF and MVD utilizing CD34 monoclonal antibody. Statistical analysis of these immunophenotypes in relation to tumor type, grade and HPV antigen expression was performed.
Results
This retrospective study comprised of 17 squamous cell carcinomas, 11 adenocarcinomas and two adenosquamous carcinomas. Eleven cases were low grade and 19 were high-grade cases. VEGF expression was detected in 100% of cases. The relation between carcinoma grade and VEGF expression and MVD was statistically significant. There was no relation between VEGF intensity and tumor type although more intense VEGF staining tended to occur in cervical adenocarcinomas. VEGF density was significantly lower in squamous cell carcinomas compared to adenocarcinomas. Mean MVD was 50.37 ± 20.0. The relation between MVD and VEGF expression was statistically significant. HPV immune expression was detected in 93.33% of cases. The relation between HPV antigen expression and each of tumor histotype and grade was not statistically significant. There was a statistically significant relation between HPV antigen expression and each of MVD and VEGF intensity. Multivariate statistical analysis showed MVD as an independent predictor of carcinoma grade.
Conclusion
VEGF was expressed in 100% of studied cervical carcinoma. There was a statistically significant relation between VEGF expression and MVD. Since HPV antigen expression was significantly correlated with MVD and VEGF staining intensity, we provide evidence that HPV infection may augment tumor angiogenesis in cervical carcinoma. MVD emerged as an independent predictor of cervical carcinoma grade and hence of progressive behavior.
Keywords:
1 Introduction
Cervical cancer is a worldwide public health problem being the second most common cancer in females after breast cancer.Citation1 In Egypt, the latest census by the National Cancer Institute in Cairo in 2003 showed predominance of cervical over endometrial carcinoma.Citation2
The latest WHO classification of cervical tumors Citation3 classifies epithelial malignancies mainly into four main groups: squamous carcinomas; adenocarcinomas; neuro-endocrine tumors; and others including adenosquamous carcinomas.Citation4
Carcinoma of the cervix is considered a sexually transmissible disease.Citation5 At the present time, viral agents particularly human papillomavirus are believed to be the most likely factors incriminated in the pathogenesis.Citation6
Approximately 200 types of human papillomavirus (HPV) have been identified, of which 85 have been genotypically characterized.Citation7 About 40 different types of HPV specifically infect the genital area.Citation8 According to their malignant potential, these viruses can be classified as high- or low-risk types. The high-risk types (predominantly HPV-16 and -18) are related to high-grade intraepithelial lesions and invasive cervical carcinoma.Citation9
However, most HPV infections either regress spontaneously or progress to cancer after a long period of latency.Citation10 Therefore, it was inferred that additional requirements other than HPV infection are likely to be needed for neoplastic transformation of cervical epithelial cells.Citation10
There is accumulating evidence that angiogenesis plays a pivotal role in cervical carcinogenesis.Citation11 However, little is known about the role of viral oncoproteins in regulating angiogenesis.Citation12
Angiogenesis is the development of new blood vessels from pre-existing vasculature.Citation13 When a tumor launches the growth of new vessels, it is said to have undergone an “angiogenic switch”. The principal trigger for this event appears to be hypoxia. Newly formed blood vessels will further promote tumor cell proliferation Citation14 and increase the possibility of metastasis.Citation15A group of angiogenic stimulators and inhibitors regulate the process of angiogenesis Citation16 Angiogenic stimulators include vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), acidic and basic fibroblast growth factors (FGF), transforming growth factors-α and β (TGF α and β) and others.Citation17 On the other hand, angiogenic inhibitors as angiostatin and endostatin exist to prevent excessive neoangiogenesis. Under normal circumstances, there is an equilibrium between pro and anti-angiogenic factors, this balance is disrupted in malignancy thereby favoring promotion of tumor angiogenesis.Citation18
A great deal of attention has been focused on therapy that can interrupt angiogenesis. Anti-angiogenic therapy may target endothelial cells directly, suppress the production and or action of pro-angiogenic peptides or augment the release of antiangiogenic factors within the tumor.Citation19
Microvascular density (MVD) is a commonly used marker for estimation of angiogenesis.Citation20 Measuring MVD necessitates labeling the vessels to be counted using antibodies against any of the antigens naturally expressed by endothelial cells like F-VIII, CD31, CD34 and CD105.Citation20 Different methods have been used for counting MVD, including manual counting of vascular hotspots and computerized image analysis.Citation21
VEGF represents the major inducer of angiogenesis.Citation16,Citation22 It is encoded by the human VEGF-A gene, on chromosome 6p21.3 Citation22 and belongs to the VEGF superfamily of growth factors which also includes VEGF-B, C, D, E and placental growth factor (PlGF).Citation23 VEGF exerts its biologic effect through interaction with transmembrane tyrosine kinase receptors (VEGFR). In tumors, VEGF expression is induced in response to hypoxia, genetic and cytokine stimulation.Citation24
Several functions have been described for VEGF including acting as a potent mitogen for vascular endothelial cells,Citation25 increasing microvascular permeability,Citation26 degradation of extracellular matrix,Citation23 acting as an antiapoptotic factor for endothelial cellsCitation24,Citation26 in addition to mobilizing bone marrow-derived endothelial cell precursors.Citation23
In the present work we aimed at studying the role of angiogenesis as measured by microvascular density and expression of vascular endothelial growth factor (VEGF) in relation to the status of HPV infection in some cases of cervical carcinoma.
2 Methods
This retrospective study was approved by the Ethics Committee of Alexandria Faculty of Medicine. It consisted of 30 consecutive cases of clinically and histologically proven cervical carcinomas. Patients were diagnosed and treated at the Alexandria University Hospital, Alexandria, Egypt, between May 2007 and April 2009. All tissue samples were formalin fixed and paraffin-embedded. Data about tumor stage were not always available so this parameter was not considered in analysis.
2.1 Histopathological examination
Five micron-thick sections cut from archival paraffin blocks were H&E stained and examined microscopically to determine tumor histologic type and grade. Squamous cell carcinomas were graded according to the modified Broders’ grading system (grades I–IV) that takes into account nuclear anaplasia in addition to the degree of differentiation.Citation27 Adenocarcinomas were graded according to the recommendations of the Association of Directors of Anatomic and Surgical PathologyCitation28 (grades I–III) taking into consideration architectural as well as nuclear criteria. For statistical purposes, cases of grades I and II were grouped together as low-grade tumors (11 cases, 36.7%), while grades III and IV cases were grouped together as high-grade tumors (19 cases, 63.3%).
2.2 Immunohistochemistry
Heat-induced epitope retrieval was performed by microwaving the sections in EDTA (1 mM, pH 8.0) for VEGF and in citrate buffer (10 mM, pH 6.0) (Thermo Fisher, Fremont, USA) for CD34 and HPV at 700W, 4 times, 2 min each. Endogenous peroxidase activity and nonspecific binding were blocked.
Three primary, mouse monoclonal antibodies were employed; VEGF antibody (Ab-7 Cat. #MS-1467-R7), human papillomavirus antibody (Ab-3, Clone K1H8, Cat. #MS-1826-R7) and CD34 antibody (Ab-1, Clone QBEnd/10, Cat. #MS-363-R7). All three primary antibodies were supplied in a pre-diluted format and were incubated with tissue sections at 4 °C overnight. (Thermo Fisher Scientific Inc., Fremont, CA, USA). The reaction product was visualized using the streptavidin–biotin peroxidase system with Diaminobenzidine as a chromogen (UltraVision Detection System Anti-Mouse, HRP/DAB, Cat #TM-015-HD, Thermo Fisher, Fremont, CA, USA) according to the manufacturer's protocol.
Sections without primary antibodies served as negative controls. Blood vessels in normal cervical tissue adjacent to the tumor served as internal positive controls for VEGF and CD34 immunostaining whereas commercially available positive control slides were used as positive control for HPV immunostaining (Cat. #MS-1826-CS7, Thermo Fisher, Fremont, CA, USA).Citation29,Citation30
Finally, sections were counterstained with hematoxylin, dehydrated and mounted.
2.3 Assessment of immunostaining
2.3.1 VEGF immunostaining
Positive VEGF immunostaining was defined as a cytoplasmic and or membranous staining of tumor cells.Citation31 VEGF-stained sections were studied at 100× magnification to assess VEGF staining density and intensity. VEGF density was defined as percentage of VEGF-stained tumor cells scored according to a 2-teired scoring systemCitation31,Citation32 whereby score 1 designated positive cytoplasmic staining in ⩽50% and score 2 in >50% of tumor cells. VEGF staining intensity was scored according to a 4-teired systemCitation32 into score 0: negative, 1: weak, 2: moderate and 3: strong staining. All sections were stained in a single run to avoid procedural variations affecting the credence of comparing VEGF intensity readings.
2.3.2 HPV-immunostaining
This was evaluated as either positive or negative, whereby any number of tumorous or non-tumorous cells showing brown nuclear staining qualified as a positive result.
2.3.3 CD34 immunostaining
A case was considered positive if any number of tumor vessel endothelial cells showed cytoplasmic brown staining.
2.4 Assessment of MVD
MVD was calculated according to the method described by Weidner et al.Citation21 CD34-stained sections were examined under low magnification (100×) to determine the area of highest vascularity (hot spot). Ten consecutive high power fields (HPFs) (400×) were assessed within a hot spot and vessel count was estimated in each HPF using the framework of an optical grid mounted onto the eye piece. Ten MVD readings were obtained for each tumor, the mean of which was recorded and expressed as the number of vessels HPF.Citation20,Citation21
Microvessels were identified as any CD34 positive endothelial cell or cluster of cells with or without visible lumen. In dense vascular networks, each distinct branch was interpreted as a single vessel. Large anastomosing sinusoidal vessels were counted as a single vessel.Citation33
3 Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for Social Sciences, version 13.0, Chicago, USA).
3.1 Bivariate analysis
Continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were expressed as numbers and percentages. Statistical relations between two categorical variables were tested using Chi-square (χ2) or Fisher exact tests. Relations between categorical and continuous variables were tested using t test and ANOVA. The level of significance was set at P ⩽ 0.05.
3.2 Multivariate analysis
In order to identify possible predictors of biological behavior of cervical carcinoma, all statistically significant variables with cervical carcinoma types or grade in the bivariate analysis were introduced into a logistic regression model. Odds ratio and 95% confidence intervals (CI) were calculated.
ROC curves were used to evaluate the specificity, sensitivity and overall accuracy of the predictive variables. The value that showed the highest overall accuracy was used as the cut-off points of the continuous variables.
4 Results
In the current study, 30 cases of cervical carcinoma were included. The mean age was 49.79 + 4.67 years ranging between 40 and 60 years. Histologically, 17 cases were squamous cell carcinomas (56.7%), 11 adenocarcinomas (36.7%) while two cases (6.7%) were adenosquamous carcinomas.
Cases of squamous cell carcinoma were classified into a case (5.8%) of grade I, three cases (17.6%) of grade II, eight cases (47.1%) of grade III and five cases (29.4%) of grade IV. Adenocarcinomas were classified into grade 1 in five cases (45.4%), grade II in two cases (18.1%) and grade III in four cases (36.8%).
Both cases of adenosquamous carcinoma were high grade (poorly differentiated glandular component).
Overall, 11 cases (36.7%) of low-grade carcinoma and 19 cases (63.3%) of high-grade carcinoma were included in the present study.
4.1 Immunohistochemistry
4.1.1 VEGF immunostaining
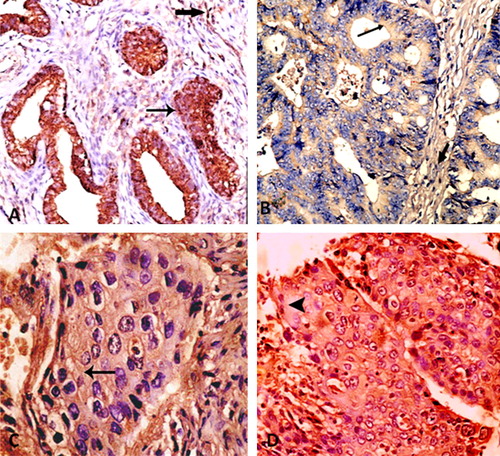
Heterogeneous VEGF positive cytoplasmic and membranous staining was seen both in neoplastic epithelial cells and in stromal cells (A). In addition, endothelial lining of intratumoral blood vessels showed positive cytoplasmic staining (internal control).
The difference in VEGF staining intensity among the three histologic types of cervical carcinoma was statistically insignificant (P = 0.315), while the difference in VEGF staining density was statistically significant (P = 0.002, Fisher exact test) with the highest VEGF density scores recorded in adenocarcinomas and lowest values recorded in squamous cell carcinomas ( and ).
Table 1 Relation between VEGF intensity and histologic subtypes of cervical carcinoma.
Table 2 Relation between VEGF staining density and histologic subtypes of cervical carcinoma.
The relation between cervical carcinoma grade and VEGF expression was statistically significant (P ⩽ 0.0001) (being direct with VEGF staining intensity and inverse with VEGF density) (A–D) ( and ).
Table 3 Relation between VEGF staining intensity and cervical carcinoma grade.
Table 4 Relation between VEGF staining density and cervical carcinoma grade.
4.1.2 CD34 immunostaining and assessment of MVD
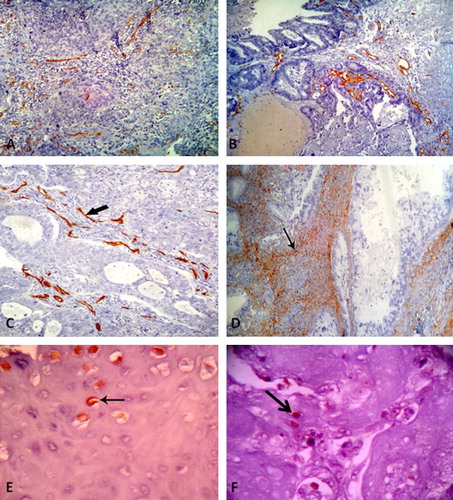
CD34 positive cytoplasmic staining was seen in endothelial cells lining intratumoral blood vessels and vessels in adjacent normal cervical tissue (internal positive control). MVD values ranged between 17.9 and 90.2 vessel/HPF with a mean of 50.37 ± 20.02.
The highest MVD values were recorded in adenosquamous carcinomas (65.70 ± 10.60) (C), while the least values were recorded in squamous cell carcinomas (47.32 ± 18.42) (A). However, the difference in MVD between the three histologic types was statistically insignificant (P = 0.450).
The difference in mean MVD values between low-grade (30.74 ± 8.25) and high-grade (61.74 ± 15.38) carcinomas was statistically significant (t test: 7.18, P ⩽ 0.0001).
4.1.3 HPV immunostaining
HPV positive nuclear staining was seen in infected neoplastic and non-neoplastic cells in 28 cases (93.3%).
Fifteen cases of squamous cell carcinoma (88.23%) (E), all adenocarcinomas (11 cases) (F) and both adenosquamous carcinomas (two cases) were positive. However, no statistical significance was found between HPV expression and histologic type (P = 0.304) or tumor grade (P = 0.13). ().
Table 5 Relation between HPV expression and cervical carcinoma grade.
4.2 Relationship between VEGF expression and MVD
The relation between VEGF staining intensity and MVD of cervical carcinoma was statistically significant (P = 0.0001) () and so was the inverse relation between VEGF staining density and MVD (P = 0.028). ().
Table 6 Relation between VEGF intensity and MVD.
Table 7 Relation between VEGF density and MVD.
4.3 Relationship between HPV expression and each of MVD and VEGF expression
The relation between HPV expression and MVD was statistically significant (t = 7.541, P ⩽ 0.0001). Furthermore, the relation between VEGF staining intensity and HPV expression was statistically significant (P = 0.042); however, the relation between VEGF staining density and HPV expression was not (P = 0.179). ( and ).
Table 8 Relation between HPV expression and VEGF intensity.
Table 9 Relation between HPV expression and VEGF density.
4.4 Results of multivariate statistical analysis
In a logistic regression analysis, including cervical carcinoma grade, MVD, VEGF intensity and density of expression; MVD emerged as an independent predictor of cervical carcinoma grade hence implying a poorer outcome. (P = 0.042) ().
Table 10 Logistic regression analysis.
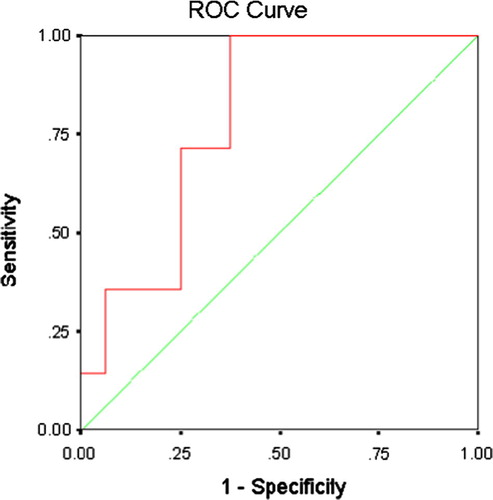
The sensitivity, specificity and overall accuracy of MVD were 92.9%, 75% and 85.5%, respectively and the value of MVD that recorded the highest overall accuracy (MVD = 40.01 vessel/HPF) was chosen as the cut-off point. According to this value, cervical carcinomas with MVD values ⩽40.01 vessel/HPF were of expected lower grade and carcinomas with MVD >40.01 vessel/HPF were of expected higher grade. ROC curve () showed that the area under the curve is 0.790 (confidence interval = 0.623–0.957, P = 0.007). Hence, MVD could be used as a predictive test for cervical carcinoma grade.
5 Discussion
Cancer of cervix is the second most common cancer among females worldwide with a ratio of mortality to incidence of 52%.Citation34 The annual number of new cervical cancer cases in Egypt in 2008 was 514 projected to increase by 93% to 846 in 2025.Citation35
The poor outlook of cervical carcinoma necessitates the search for new prognostic factors and therapeutic strategies. One such promising strategy is targeting tumor blood vessels. However, till the present time, the prognostic import of biological determinants of angiogenesis and their putative role in treatment of cervical carcinoma are not settled.Citation36
The poor prognostic implication of MVD in cervical cancer is well established,Citation37 however; so far, reports concerning the relation between VEGF and MVD in cervical cancer are profoundly contradictory.Citation38,Citation39
VEGF expression in cervical carcinoma has been studied at many levels including serum VEGF level,Citation40 VEGF mRNACitation36 and tissue VEGF protein.Citation36 The latter detected immunohistochemically was the method adopted in the present study being the most practically applicable.Citation41 Moreover, expression of VEGF mRNA was not always found to parallel VEGF protein expression, possibly due to epigenetic modification.Citation36
In the present study, VEGF expression was invariably detected in all cases which points to the crucial role played by VEGF in cervical carcinogenesis.Citation41 Cytoplasmic VEGF staining in tumor cells and adjacent stromal cells was previously reported.Citation42 Immunoreactive stromal cells are most probably considered to be macrophages that were attracted to the tumor to phagocytose VEGF positive-tumor cell debris.Citation42
In the present work, expression of VEGF by endothelial cells lining intratumoral microvessels was in keeping with previous observationsCitation43,Citation44 In these articles, such endothelial cells expressed mRNA of VEGF receptors implying that VEGF molecules released by tumor cells became bound to their receptors onto tumor vessel-endothelium. This receptor–ligand interaction may provide a mechanism of retaining and concentrating VEGF, thereby maximizing its activity within the tumor locale while preventing its diffusion elsewhere. This finding stands in contrast to the ubiquitous distribution of other pro-angiogenic peptides and thereby strongly favors the use of anti-VEGF therapy as specific anti-angiogenic agents with minimal side effects.Citation44
Most of the studied cases showed heterogeneous VEGF staining intensity within the same tumor. The same finding was previously reported by Tokumo et al.Citation45 who attributed such heterogeneity to regional ischemia stemming from regional differences in tumor cell growth. In addition, such staining heterogeneity may be ascribed to varying binding affinity and degradation of VEGF isoforms released within the same tumor.Citation46 In the current study, like in others,Citation47,Citation48 a statistically significant positive correlation was found between VEGF intensity and cervical carcinoma grade. Increasing tumor grade is coupled with augmented proliferation rate which calls for increased outgrowth of vascular supply. Such stresses generate a state of chronic hypoxia within the tumor. Hypoxia, known to be the most potent angiogenic stimulator, switches on tumor angiogenesis via up regulating a set of genes including the VEGF gene.Citation49
On the other hand, the statistically significant relation between VEGF density and cervical carcinoma grade was inverse. Other authors previously reporting the same findingCitation45 posed that once a certain microvascular density is obtained, growth reaches a plateau and the demand for VEGF declines, causing a decrease in its expression (negative feedback).Citation50 This explanation can be further extrapolated to decipher the inverse statistically significant relation between VEGF density and MVD (P = 0.028) which we reported as well as others.Citation51 MVD is assumed to reflect past angiogenic activity whereby the vascular network will remain long after the expression of VEGF has ceased.Citation52 This might explain why VEGF expression – unlike MVD – had no prognostic value in the work of Tjalma et al. Citation50
We were able to prove that VEGF density was higher in adenocarcinomas compared to squamous cell carcinomas and the relation was statistically significant. Such a finding is in line with results reported by Tjalma et al.Citation50 This can be ascribed to the fact that in the present study, most cases of squamous cell carcinoma were of high grade (and hence of low VEGF density) while most cases of adenocarcinoma were of low grade (and hence of higher VEGF density).
More intense VEGF staining was observed in cervical adenocarcinoma compared to other histotypes; however, no statistical significance was reached although previously reported.Citation45 This could possibly be ascribed to the small number of adenocarcinomas enrolled in the present study.
MVD is considered a measure of tumor angiogenesis and a significant prognostic factor that correlates with increased likelihood of metastasis and worse prognosis in many tumor types.Citation36
In our work, MVD showed significant correlation with tumor grade. This finding comes in accord with previous reportsCitation48 but still contradicts with others.Citation53 Such discrepancy may be attributed to variation in the studied histologic types of cervical carcinoma, different methodologies adopted in measuring MVD and the immunohistochemical markers used for highlighting endothelial cells.
The highest values of MVD that we recorded were in adenocarcinomas and the lowest values were in squamous cell carcinomas. Both findings were previously reported.Citation4,Citation37 We again ascribe our inability to reach statistical significance to the small number of adenocarcinomas included in the present study.
Highlighting blood vessels immunohistochemically is an essential prerequisite for measuring MVD. Several antibodies have been employed for that purpose including factor VIII, CD105, CD34 and CD31 antibodies.Citation54 Our selection of CD34 was based on its superior sensitivityCitation55 with detection of a greater number of microvessels in cervical tumors compared to other antibodies.Citation56 Furthermore, it stains neoplastic endothelium a deeper shade than normal endothelium.Citation55 However, compared to other antibodies as CD31 and factor VIII, it was found to be less specific in evaluating angiogenesis as it stains non-endothelial cells (e.g. hematopoietic progenitor cells) as well.Citation57 For these considerations, it is imperative for the evaluator to interpret the results of immunostaining in the proper morphological context to better assess MVD as an index of angiogenesis.
Since infection by high-risk types of HPV is strongly associated with cervical carcinoma,Citation7,Citation8 we investigated the possible association between virus infection and tumor angiogenesis. HPV expression in cervical carcinoma has been studied at many levels including estimation of serum HPV virus load,Citation58 HPV DNA by PCR, in situ hybridizationCitation59 and immunohistochemical detection of HPV antigens.Citation60
In the current work we were able to immunohistochemically detect HPV expression in 93.3% of the studied cervical carcinomas which is comparable to other data reported from Muslim and Middle East countries as Morocco, (70.5%)Citation61 and Iran (85.5%).Citation62 In a study covering 22 countries and coordinated by the International Agency for Research on Cancer (IARC), the overall detection rates of HPV DNA were found to vary little among different regions of the world (83–89%).Citation63 Furthermore, in an Egyptian study,Citation64 prevalence of HPV infection (detected by PCR) among Egyptian females 35 years and above was 2.6% while HPV infection was positive in 94.3% of cervical neoplastic lesions (pre-invasive and invasive). Actually, some authors allege that HPV causes practically 100%Citation65 of cervical carcinomas, and ascribe the underestimation of its prevalence in these tumors to “limitations of study methodologies”.Citation66
All cases of cervical adenocarcinoma examined were positive for HPV which further endorses the notion that positivity for HPV can be used to confirm the endocervical origin of an adenocarcinomaCitation67.
In the current work, HPV immune expression of the studied cases of cervical carcinoma showed positive statistically significant correlation with VEGF intensity. This is a novel finding on the immunohistochemical level. The same import, however, was reached by Song et al. who reported that VEGF expression is related to HPV load detected by PCR.Citation68 The concept of higher VEGF expression in HPV-16 E6 positive cells compared to HPV-16 E6-negative ones was previously reported.Citation69 The latter study group explained that HPV-16 E6 oncoprotein might contribute to tumor angiogenesis by direct stimulation of the VEGF gene promoter.Citation69 In the same context, other researchers added that HPV-16 E6 oncoprotein interfered with the ubiquitin mediated degradation of HIF-1α, a transcription factor involved in activating VEGF gene promoter in response to hypoxia.Citation49 Furthermore, it has been pointed out that the HPV-16 E7 oncoprotein enhanced the release of VEGFCitation70 while, HPV-16 E5 oncoprotein up-regulated VEGF expression through the activation of a diversity of signaling pathways.Citation71
Not only did HPV immune expression correlate significantly with VEGF intensity but also with MVD of studied tumors, a notion which lends credibility to the new concept claiming that HPV infection stimulates tumor angiogenesis in cervical carcinoma.
6 Conclusions
VEGF was expressed in all studied cases of cervical carcinoma. There was a statistically significant relation between VEGF expression and MVD. Since HPV immune expression was significantly correlated with MVD and VEGF staining intensity, we hereby provide further evidence that HPV infection may augment tumor angiogenesis in cervical carcinoma. In cervical carcinoma, MVD could be used as an independent predictor of cervical carcinoma grade hence implying a poorer outcome especially if anti-VEGF therapy is to be considered.
Conflict of interest
The authors have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 13 January 2012
References
- F.X.BoschA.LorinczN.MuñozC.J.MeijerK.V.ShahThe causal relation between human papillomavirus and cervical cancerJ Clin Pathol5542002244265
- Elattar IA. Cancer statistics at National Cancer Institute, Cairo; 2002–2003: Cancer statistics for specific site; cancers of female genital system. Available at: <http://www.nci.edu.eg/lectures/NCI/registry2002-2003.doc> [last accessed 10.10.2011].
- WHO histological classification of tumours of the uterine cervix. Available at: <http://www.screening.iarc.fr/atlasclassifwho.php>. [last accessed 16.10.2011].
- A.J.TiltmanThe pathology of cervical tumoursBest Pract Res Clin Obstet Gynaecol1942005485500
- I.I.KesslerHuman cervical cancer as a venereal diseaseCancer Res3619767891
- R.D.BurkZ.ChenK.Van DoorslaerHuman papillomaviruses: genetic basis of carcinogenicityPublic Health Genomics125-62009281290
- H.Zur HausenPapillomaviruses in human cancersProc Assoc Am Physicians1111999581587
- N.MunozF.X.BoschS.de Sanjose’Epidemiologic classification of human papillomavirus types associated with cervical cancerN Engl J Med3482003518528
- M.StanleyPathology and epidemiology of HPV infection in femalesGynecol Oncol1172l2010S5S10
- N.WentzensenS.J.KlugCervical cancer control in the era of HPV vaccination and novel biomarkersPathobiology76220098289
- J.H.NoH.JoS.H.KimI.A.ParkD.KangS.S.HanExpression of vascular endothelial growth factor and hypoxia inducible factor-1alpha in cervical neoplasiaAnn N Y Acad Sci11712009105110
- E.Toussaint-SmithD.B.DonnerA.RomanExpression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factorsOncogene23200429882995
- R.RibattiThe crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical reviewBr J Haematol12832005303309
- M.KirshM.A.DimopoulosAngiogenesis in human cancer: implications in cancer therapyEur J Int Med142003459469
- H.F.DvorakRous-Whipple Award Lecture. How tumors make bad blood vessels and stromaAm J Pathol162200317471757
- N.FerraraH.GerberJ.Le CouterThe biology of VEGF and its receptorsNat Med92003669676
- G.D.YancopoulosS.DavisN.W.GaleJ.S.RudgeS.J.WiegandJ.HolashVascular-specific growth factors and blood vessel formationNature45112000242248
- A.RiceC.M.QuinnAngiogenesis, thrombospondin, and ductal carcinoma in situ of the breastJ Clin Pathol552002569574
- M.RaicaA.M.CimpeanD.RibattiAngiogenesis in pre-malignant conditionsEur J Cancer4511200919241934
- A.DellasH.MochE.SchultheissG.FeichterA.C.AlmendralF.GudatAngiogenesis in cervical neoplasia: microvessels quantification in precancerous lesion and invasive carcinomas with clinicopathalogical correlationsGynecol Oncol6719972733
- N.WeidnerJ.P.SempleW.R.WelchJ.FolkmanTumour angiogenesis and metastasis-correlation in invasive breast carcinomaN Eng J Med324199118
- J.PatardN.Rioux-LeclercqP.FergelotUnderstanding the importance of smart drugs in renal cell carcinomaEur Urol492006633643
- N.FerraraVascular endothelial growth factor as a target for anticancer therapyOncologist92004210
- M.ToiT.MatsumotoH.BandoVascular endothelial growth factor: its prognostic, predictive, and therapeutic implicationsLancet Oncol22001667673
- H.ZengH.F.DvorakD.MukhopadhyayVascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) receptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathwaysJ Biol Chem27620012696926979
- N.FerraraRole of vascular endothelial growth factor in regulation of physiological angiogenesisAm J Physiol Cell Physiol280200113581366
- A.C.BrodersCarcinoma: grading and practical applicationArch Pathol21962376
- W.D.LawrenceF.W.Abdul-KarimC.CrumY.-S.FuRecommendations for the reporting of surgical specimens containing uterine cervical neoplasms. Grading adenocarcinomaMod Pathol134200010291033
- K.J.O’ByrneM.I.KoukourakisA.GiatromanolakiG.CoxH.TurleyW.P.StewardVascular endothelial growth factor, platelet-derived endothelial cell growth factor and angiogenesis in non-small-cell lung cancerBr J Cancer82200014271432
- J.L.CordellK.A.PulfordB.BigernaG.RoncardoA.BanhamE.ColomboDetection of normal and chimeric nucleophosmin in human cellsBlood931999632642
- Y.EralpP.SaipB.SakarS.KucucukA.AydinerM.DincerPrognostic factors and survival in patients with metastasis or recurrent carcinoma of the cervixInt J Gynecol Cancer132003497504
- D.ShweikiA.ItinD.SofferE.KeshetVascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesisNature3591992843845
- G.L.BremerA.T.TieboschH.M.Van der PuttenH.J.SchoutenJ.De HaanJ.W.ArendsTumor angiogenesis: an independent prognosis parameter in cervical cancerAm J Obstet Gynecol1741996126131
- F.T.CuttsS.FranceschiS.GoldieX.CastellsagueS.de SanjoseG.GarnettHuman papillomavirus and HPV vaccines: a reviewBull World Health Organ8592007719726
- WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Human papillomavirus and related cancers in Egypt. Summary Report 2010. Available at: <http://www.who.int/hpvcentre> [accessed 10.10.2011].
- V.M.NagyR.BuigaI.BrieN.TodorO.TudoranC.OrdeanuExpression of VEGF, VEGFR, EGFR, COX-2 and MVD in cervical carcinoma, in relation with the response to radio-chemotherapyRom J Morphol Embryol5220115359
- D.De LeónC.Lopez-GranielM.Frias MendivilG.Chanona VilchisC.GomezJ.De La Garza SalazarSignificance of microvascular density (MVD) in cervical cancer recurrenceInt J Gynecol Cancer132003856862
- D.K.GaffneyD.HaslamA.TsodikovE.HammondJ.SeamanJ.HoldenEpidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively affect overall survival in carcinoma of the cervix treated with radiotherapyInt J Radiat Oncol Biol Phys5642003922928
- J.S.LeeH.S.KimJ.T.ParkM.C.LeeC.S.ParkExpression of vascular endothelial growth factor in the progression of cervical neoplasia and its relation to angiogenesis and p53 statusAnal Quant Cytol Histol2562003303311
- L.Y.DirixP.B.VermeulenA.PawinskiA.ProvéI.BenoyC.De PooterElevated levels of the angiogenic cytokines basic fibroblast growth factor and vascular endothelial growth factor in sera of cancer patientsBr J Cancer761997238243
- J.FujimotoH.SakaguchiR.HiroseS.IchigoT.TamayaExpression of vascular endothelial growth factor (VEGF) and its mRNA in uterine cervical cancersBr J Cancer801999827833
- T.A.BrockH.F.DvorakD.R.SengerTumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cellsAm J Pathol1381991213221
- Y.DaiX.ZhangY.PengZ.WangThe expression of cyclooxygenase-2, VEGF and PGs in CIN and cervical carcinomaGynecol Oncol97200596103
- G.SouflaS.SifakisS.BaritakiA.ZafiropoulosE.KoumantakisD.A.SpandidosVEGF, FGF2, TGFB1 and TGFBR1 mRNA expression levels correlate with the malignant transformation of the uterine cervixCancer Lett2212005105118
- K.TokumoJ.KodamaN.SekiY.NakanishiY.MiyagiS.KamimuraDifferent angiogenic pathways in human cervical cancersGynecol Oncol6819983844
- S.KuemmelA.ThomasS.LandtA.FugerP.SchmidM.KrinerCirculating vascular endothelial growth factors and their soluble receptors in pre-invasive, invasive and recurrent cervical cancerAnticancer Res292009641646
- N.MuñozF.X.BoschS.de SanjoséR.HerreroX.CastellsaguéK.V.ShahEpidemiologic classification of human papillomavirus types associated with cervical cancerN Engl J Med3482003518527
- I.J.LeeK.R.ParkK.K.LeeJ.S.SongK.G.LeeJ.Y.LeePrognostic value of vascular endothelial growth factor in Stage IB carcinoma of the uterine cervixInt J Radiat Oncol Biol Phys542002768779
- H.K.HauglandV.VukovicM.PintilieA.W.FylesM.MilosevicR.P.HillExpression of hypoxia-inducible factor-1 α in cervical carcinomas: correlation with tumor oxygenationInt J Radiat Oncol Biol Phys532002854861
- W.TjalmaJ.WeylerB.WeynE.Van MarckA.Van DaeleP.Van DamThe association between vascular endothelial growth factor, microvessel density and clinicopathological features in invasive cervical cancerEur J Obstet Gynecol Reprod Biol9222000251257
- M.NeemanR.AbramovitchY.S.SchiffenbauerC.TempelRegulation of angiogenesis by hypoxic stress: from solid tumours to ovarian follicleInt J Exp Pathol7819975770
- L.F.BrownB.BerseR.W.JackmanK.TognazziA.J.GuidiH.F.DvorakExpression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancerHum Pathol2619958691
- H.F.DvorakL.F.BrownM.DetmarA.M.DvorakVascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability and angiogenesisAm J Pathol146199510291039
- M.MiettinenA.E.LindenmayerA.ChaubalEndothelial cell markers CD31, CD34 and BNH9 antibody to H- and Y-antigens – evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factorMod Pathol119948290
- I.KuzuR.BicknellA.L.HarrisM.JonesK.C.GatterD.Y.MasonHeterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumorsJ Clin Pathol451992143148
- S.Di LeoS.CaschettoG.GarozzoG.NuciforoN.CassaroM.T.MeliAngiogenesis as a prognostic factor in cervical carcinomaEur J Gynaecol Oncol191998158162
- W.F.ChengC.N.LeeJ.S.ChuC.A.ChenT.M.ChenW.Y.ShauVascularity index as a novel parameter for the in vivo assessment of angiogenesis in patients with cervical carcinomaCancer851999651657
- J.S.ParkE.J.KimH.J.KwonE.S.HwangS.E.NamkoongS.J.UmInactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesisJ Biol Chem275200067646769
- A.M.De Roda HusmanJ.M.WalboomersE.HopmanO.P.BlekerT.M.HelmerhorstL.RozendaalHPV prevalence in cytomorphologically normal cervical scrapes of pregnant women as determined by PCR: the age-related patternJ Med Virol46199597102
- C.UngureanuD.SocolovG.AntonM.S.MihailoviciS.TelemanImmunocytochemical expression of p16INK4a and HPV L1 capsid proteins as predictive markers of the cervical lesions progression riskRom J Morphol Embryol5132010497503
- K.LalaouiM.El MzibriM.AmraniM.A.BelabbasP.A.LazoHuman papillomavirus DNA in cervical lesions from Morocco and its implications for cancer controlClin Microbiol Infect922003144148
- S.MortazaviM.ZaliM.RaoufiM.NadjiP.KowsarianA.NowrooziThe prevalence of human papillomavirus in cervical cancer in IranAsian Pac J Cancer Prev3120026972
- G.M.CliffordJ.S.SmithM.PlummerN.Mun˜ozS.FranceschiHuman papillomavirus types in invasive cervical cancer worldwide: a meta-analysisBr J Cancer8820036373
- H.S.el-AllA.RefaatK.DandashPrevalence of cervical neoplastic lesions and human papilloma virus infection in Egypt: National Cervical Cancer Screening ProjectInfect Agent Cancer42007212
- J.M.WalboomersM.V.JacobsM.M.ManosF.X.BoschJ.A.KummerK.V.ShahHuman papillomavirus is a necessary cause of invasive cervical cancer worldwideJ Pathol189119991219
- G.CliffordS.FranceschiM.DiazN.MuñozL.L.VillaChapter 3: HPV type-distribution in women with and without cervical neoplastic diseasesVaccine24Suppl. 320062634
- A.YemelyanovaR.VangJ.D.SeidmanP.E.GravittB.M.RonnettEndocervical adenocarcinomas with prominent endometrial or endomyometrial involvement simulating primary endometrial carcinomas: utility of HPV DNA detection and immunohistochemical expression of p16 and hormone receptors to confirm the cervical origin of the corpus tumorAm J Surg Pathol332009914924
- S.H.SongJ.K.LeeJ.Y.HurI.KimH.S.SawY.K.ParkThe expression of epidermal growth factor receptor, vascular endothelial growth factor, matrix metalloproteinase-2, and cyclooxygenase-2 in relation to human papilloma viral load and persistence of human papillomavirus after conization with negative marginsInt J Gynecol Cancer166200620092017
- O.López-OcejoA.Viloria-PetitM.Bequet-RomeroD.MukhopadhyayJ.RakR.S.KerbelOncogenes and tumor angiogenesis: the HPV-16 E6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent mannerOncogene19200046114620
- M.Bequet-RomeroO.Lopez-OcejoAngiogenesis modulators expression in culture cell lines positives for HPV-16 oncoproteinsBiochem Biophys Res Commun27720005561
- S.H.KimY.S.JuhnnS.KangS.W.ParkM.W.SungY.J.BangHuman papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/AktCell Mol Life Sci632006930938