Abstract
Background
Leishmaniasis is one of the neglected diseases included in the World Health Organization's list of the top guns of antimicrobial resistance. Miltefosine (MIL) was the first successful oral agent used against visceral leishmaniasis in India. As regards cutaneous leishmaniasis (CL), multiple experimental and clinical studies have investigated its efficacy in treatment of New World CL, while only few trials have focused on Old World CL. Therefore, this work was designed to study the efficacy of MIL in experimental Old World CL caused by Leishmania major (MHOM/IL/81/FEBNI), one of the causative species of CL in the Middle East.
Results
Groups of infected mice were given MIL orally, at a dose of 20 mg/kg/day for 20 days. Results showed that untreated infected mice suffered from autoamputation of the inoculated footpads. While, those treated with MIL showed complete clinical cure, significant reduction of parasite burden and improvement of the histopathological changes of the cutaneous lesions. The drug causes evident ultrastructural morphological alterations of L. major amastigote form. One month post-treatment, no clinical sings of relapse were observed, and parasite density continued to decrease significantly.
Conclusion
The present study revealed activity of MIL against experimental Old World CL in the mouse model caused by L. major (MHOM/IL/81/FEBNI), one of the causative species of CL in the Middle East.
1 Introduction
Protozoan diseases of different types lie among the major worldwide health problems, especially in the poorest populations. Leishmaniasis, caused by several species of flagellated protozoa belonging to the genus Leishmania of the family Trypanosomatidae, represents one of these threats [Citation1,Citation2]. Clinical manifestations may include single self-healing or disseminated cutaneous lesions, mucocutaneous destruction and visceral commitment, all resulting from Leishmania replication within mononuclear phagocytes [Citation3].
In the last decade, regions that are endemic for leishmaniasis have expanded significantly. Leishmaniasis is now endemic in 88 countries, 72 of which are in developing countries, and 13 are among the least developed ones [Citation4]. It is estimated that worldwide 12 million people are affected by leishmaniasis; this figure includes cases with overt disease and those with no apparent symptoms [Citation2].
Parasites of the genus Leishmania have a dimorphic lifecycle, alternating between the motile flagellated promastigote and the intracellular amastigote. Once inside the host, the promastigotes are internalized by tissue macrophages, where they differentiate into non-motile amastigotes. These amastigotes are the major target of chemotherapy for leishmaniasis [Citation5]. Currently, the genomic sequence of Leishmania has provided a rich source of vaccine trials. Nevertheless, as yet no effective vaccines against leishmaniasis are available and control of the disease relies primarily on chemotherapy [Citation6,Citation7]. Anti-leishmanial chemotherapy is currently limited to a few compounds such as the pentavalent antimonials glucantime (N-methylglucamine antimoniate) and pentostam (sodium stibogluconate), amphotericin B, paramomycine, and pentamidine. Pentavalent antimonials have been the first-choice drugs for leishmaniasis treatment since the 1920s. Although they are generally effective against most leishmaniasis cases, severe side effects are often observed. These include hazards of parenteral administration, long courses of treatment, toxic side effects and high costs. Furthermore, the emergence of antimony-resistant strains has been reported [8–10]. The high toxicity of the antimonial formulation has been implicated in the death of several patients under treatment [Citation11].
Miltefosine (1-O-hexadecylphosphocholine, MIL), which is an alkylphosphocholine, registered as Impavido®, is a simple molecule, very stable, relatively safe, and was highly efficient in clinical trials [Citation12]. MIL was originally developed as an anti-cancer agent [Citation13]. Then came the evidence of MILs excellent anti-leishmanial activity both in vitro and in experimental animals [14–16]. Phosphocholines are slowly metabolized by phospholipases to form products such as choline and long-chain alcohols that are physiological metabolites and can be recycled into phospholipids [Citation12]. MIL is degraded rather slowly in vivo, with an extended half-life, this may allow treatment with a few doses. Being an oral drug with long half-life gave it the potential to be one component of anti-leishmanial combination chemotherapy. Combination chemotherapy makes the treatment of leishmaniasis more effective, attractive and available to all sections of the society. This also improves compliance, and prolongs the useful therapeutic life-span of the drugs [Citation17,Citation18].
Currently, MIL is the first oral drug licensed for the treatment of VL. As such, it represents a major advance in that it makes home treatment possible, with the advantage of large-scale use, by avoiding the expense and inconvenience of hospitalization, which makes it an attractive alternative [Citation19]. Given daily under medical supervision for four weeks, it cures 94–97% of patients (both children and adults), is reasonably safe, and is successfully used to treat cases resistant to antimonials [Citation20]. MIL has great potential for improving access to treatment and overall control of VL and will be critical in the VL elimination campaign in the Indian subcontinent, and can be recommended for treatment of VL in Ethiopia [Citation21].
Multiple experimental and clinical studies have investigated MILs efficacy in treatment of New World cutaneous leishmaniasis (CL) [22–24]. In vitro data showed that not all subspecies of the Leishmania parasite have the same sensitivity to MIL, this indicates that the response of leishmaniasis to treatment with MIL seems to be variable, depending on several factors such as, the causative species, different strains of each species and the affected geographical areas. Given the variable response between subspecies and geographical areas, thus it is evident that chemotherapy of CL needs to be evaluated in each endemic region, even if the “same” species of Leishmania causes disease in these locales [Citation25,Citation24].
For unusual forms of the disease that require long period of treatment such as diffuse cutaneous leishmaniasis, Zerpa et al., 2007, found that MIL produced a dramatic clinical and parasitological response in those patients and improvement continued during drug administration but they relapsed later [Citation26]. As regards efficacy of MIL against Old World CL (in Middle East and Indian subcontinent) where the parasites responsible are Leishmania tropica and Leishmania major subspecies, only little information has recently become available [Citation27,Citation28]. In view of its promising value against a number of Leishmania species, the present work was carried out to investigate its efficacy in a controlled study against experimental Old World CL caused by L. major (MHOM/IL/81/FEBNI), one of the causative species of CL in the Middle East.
2 Material and methods
2.1 Leishmania strain and its maintenance
The Leishmania strain used in this study was L. major (MHOM/IL/81/FEBNI). It was kindly provided by Prof. Dr. Tamas Laskay (Professor of Immunology, Innate Immunity Research Unit, Institute for Medical Microbiology and Hygiene, University of Lübeck, Germany). It was further maintained in the Laboratory of Medical Parasitology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt, both in vitro on N.N.N. medium and in vivo by serial passage in Swiss strain albino mice.
2.2 Culture media
N.N.N. medium was used for in vitro maintenance of the L. major strain and mass cultivation of leishmania required for animal inoculation [Citation29].
2.3 Drug
Miltefosine (Virbac): milteforan® 2% veterinary oral solution was used. It was kindly supplied by Dr. Paolo Bianciardi, Scientific Advisor, Virbac, Italy.
2.4 Experimental animals
All animal studies presented here have been approved by was approved by the ethics committee of Faculty of Medicine, Alexandria University, Egypt. All animal experiments comply with national regulations for animal experimentation. Eighty laboratories bred Swiss albino mice, 4–6 weeks old were used. They were kept in separate cages in air conditioned rooms. Animals were fed wheat, milk and bread on alternate days. Water was also supplied. For animal inoculation, promastigotes of L. major were harvested from N.N.N. medium on the 7th day of culture. The parasites were adjusted to the required concentration of 20 × 106, and inoculated into the right hind footpad. They were categorized into two main groups.
2.4.1 Group I: Control group
Forty mice were used as a control. This group was subdivided into two equal subgroups:
2.4.1.1 Group (Ia): Normal non-infected control group
Animals were injected intradermally with 0.1 ml of sterile saline in the right hind footpad.
2.4.1.2 Group (Ib): Infected non-treated control group
Animals of this group were infected by intradermal injection of 20 × 106 stationary-phase promastigotes into the right hind footpad [Citation30].
2.4.2 Group II: Experimental group: Infected treated group
Forty mice were infected by intradermal injection of 20 × 106 stationary-phase promastigotes into the right hind footpad [Citation30]. They were treated with MIL starting from the third week post-infection. MIL was given orally at a dose of 20 mg/kg/day for 20 days [Citation24]. Animals of this group were further subdivided equally into two subgroups according to the time of sacrifice.
2.4.2.1 Group (IIa)
This group was sacrificed at the end of treatment [Citation31].
2.4.2.2 Group (IIb)
This group was sacrificed one month after the end of treatment.
The control subgroups (Ia, Ib) were sacrificed simultaneously as well.
2.5 The efficacy of MIL was assessed by the following parameters in comparison to infected non-treated control
2.5.1 Skin lesions
Observation of the inoculated footpad for the progression of the cutaneous lesion development as regards redness, swelling, crust formation, ulceration, gangrene and autoamputation [Citation30].
2.5.2 Parasitological study
Through the following.
2.5.2.1 Quantitative grading of Leishmania in the cutaneous lesions
Impression smears were made from cutaneous lesions of infected and infected treated groups. Smears were fixed with methanol, stained with Giemsa stain for examination under oil immersion lens. The parasite density in the stained smear was examined under oil immersion, and was recorded on empirical scale as follows [Citation32]:
Grade 6+: >100 parasites/field.
Grade 5+: 10–100 parasites/field.
Grade 4+: 1–10 parasite/field.
Grade 3+: 1–10 parasite/10 fields.
Grade 2+: 1–10 parasite/100 fields.
Grade1+: 1–10 parasite/1000 fields.
Grade 0+: No parasite/1000 fields.
2.5.2.2 Culture
Culture was performed to detect the presence of viable parasites in the lesions. The footpads of infected and infected treated groups were sterilized with 70% ethyl alcohol. The footpads were sliced longitudinally into small parts under complete sterile technique. These parts were cultured aseptically on N.N.N. medium and kept at 23–25 °C. The culture was recorded as negative if no parasite was observed till the 10th day of culture.
2.5.3 Histopathological study
Portions of the cutaneous lesions from all groups were fixed in 10% formalin, embedded in paraffin and cut into sections of 5 μm each. They were stained with hematoxylin and eosin stain.
2.5.4 Transmission electron microscope (TEM) study
Parts of the cutaneous lesions were sliced into strips about 1 × 1 × 1 mm in cold 1.5% glutaraldehyde fixative, and processed for estimation by JOEL TEM [Citation33].
3 Results
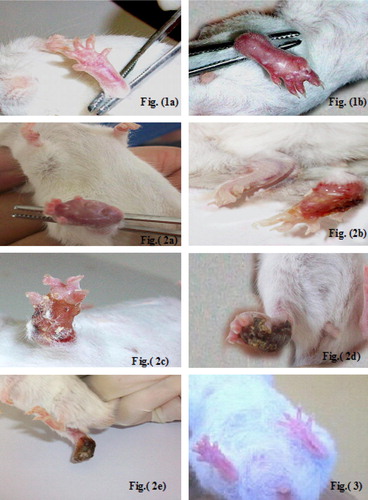
Clinically, cutaneous lesions in all infected subgroups (Ib, IIa and IIb) started with redness and swelling at the site of inoculation on the 7th day post-infection ( and ). Swelling increased progressively onwards, crust formation of the footpads occurred only in the infected non-treated control mice (subgroup Ib) at the 5th week post-infection (). Subsequently, cutaneous ulcers were developed and slowly increased in size ( and ), then gangrene started to develop by the 7th week post-infection. At first it was partial, involving one or few digits or parts of the footpad. Later on, it extended to involve the whole foot (d) and terminated by auto amputation of the inoculated feet by the 8th week post-infection in all survived mice (). No crust formation, ulceration, gangrene, or autoamputation were observed in the infected treated subgroups (IIa and IIb).
Plate 1 Gross appearance of inoculated footpads of infected (subgroup Ib) and infected treated mice (subgroup IIa and IIb). Fig. (1) Inoculated footpad of an infected mouse (subgroup Ib) at the 7th day post-infection showing redness and swelling. Fig. (2) Inoculated footpad of an infected untreated mouse (subgroup Ib) showing: (a) crust formation, (b and c) ulcer formation, (d) gangrene extending to involve the whole foot, (e) autoamputation of the inoculated footpad. Fig. (3) Infected mouse treated with miltefosine (subgroup IIb) showing grossly normal inoculated footpad.
Footpad thicknesses were measured weekly for the studied groups, and their mean was calculated (). The mean footpad thickness of the normal non-infected control mice (subgroup Ia) was increased gradually to16.6 ± 0.7 mm at the 10th week of the experiment. In subgroup Ib, it could not be measured after the 5th week post-infection due to ulceration, gangrene and autoamputation of the foot.
Table 1 Effect of miltefosine on the mean footpad thickness of infected treated subgroups compared to their corresponding controls.
Regarding mice of the infected treated subgroups (subgroups IIa and IIb), the swelling increased progressively till the 3rd week post-infection, miltefosine was given at that time. It was observed that footpad swelling started to decrease gradually after the onset of treatment regimen. There was statistically significant decrease in the mean footpad thickness of infected treated subgroups (IIa and IIb) in comparison to the infected non-treated controls. The swelling continued to decrease, and the mean values of footpad thickness significantly diminished to 16.8 ± 0.9 mm and, 17.4 ± 0.9 mm in subgroups IIa and IIb, respectively, at the 5th week post-infection (two weeks after the treatment onset). From the 6th till the 10th week post-infection, the mean values of footpad thickness for infected treated subgroups couldn't be compared to those of the infected non-treated controls (). At the end of treatment (6th week post-infection), subgroups’ IIa and IIb footpads became grossly normal with no clinical relapse one month post-treatment (subgroup IIb) (). There was no statistically significant difference between the mean footpad thickness of normal non-infected control subgroup and the infected treated subgroups; neither at the 6th week post-infection nor one month later.
Parasitologically, impression smears prepared from the infected non-treated control subgroup (Ib) (). There was statistically significant decrease in the mean grades of parasite density of the infected treated mice as compared to the infected non-treated controls (subgroup Ib) whether sacrificed immediately at the end of treatment regimen (subgroup IIa), or one month later (subgroup IIb) () (). Moreover, treatment with MIL didn't lead to sterile cure of lesions and Leishmania was shown to remain present and viable as demonstrated by their ability to grow in culture in both treated subgroups.
Plate 2 Impression smears from cutaneous lesions of infected and infected treated mice. Fig. (4) Impression smear from cutaneous lesion of infected untreated mouse showing parasite density grade 5+ (Giemsa ×1000). Fig. (5) Impression smear from cutaneous lesion of infected treated mouse showing parasite density grade 3+ (arrow) (Giemsa ×1000).
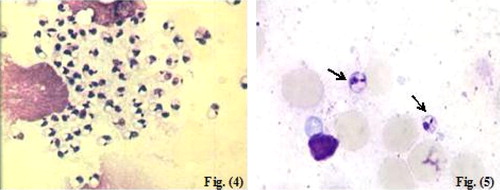
Table 2 Effect of miltefosine on the mean grade of parasite density of the infected treated subgroups compared to the infected non-treated controls.
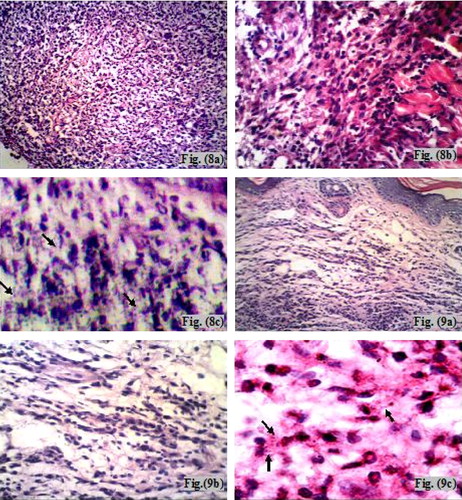
Examination of H&E stained sections of the infected non-treated control mice sacrificed at the 6th week post-infection (subgroup Ib) showed surface epithelial ulcerations and areas of intact epidermis. There was a moderately dense, localized dermal infiltrate composed of mixed acute and chronic non-specific inflammatory cellular infiltrates which were composed of macrophages admixed with lymphocytes and neutrophils. Congested dilated blood vessels were observed and numerous Leishmania amastigotes were seen either inside or outside the macrophages (–). At the 10th week post-infection (subgroup Ib), there was intense dermal infiltrate which was seen extending to the deeper layers of the footpad involving the muscle layer (–). The findings of the infected treated mice sacrificed immediately at the end of treatment (at the 6th week post-infection – subgroup IIa), showed an intact epidermis with a moderately dense infiltrate, and the intensity of the infection was less than that of the infected non-treated controls (–). Whereas, infected treated mice that were sacrificed at the 10th week post-infection (one month after stoppage of treatment – subgroup IIb) showed essentially a milder grade of affection in terms of both the inflammatory response as well as the numbers of visible amastigotes (–).
Plate 3 Light micrograph (LM) of cutaneous lesions of infected (subgroup Ib) and infected treated mice (subgroup IIa) at the 6th week post-infection. Fig. (6) LM of skin of infected untreated mouse (subgroup Ib) at the 6th week post-infection showing epidermal ulceration overlying dense acute and chronic non-specific inflammatory cellular infiltrate, congested dilated blood vessels and numerous Leishmania amastigotes (a) H&E ×200, (b) H&E ×400, (c) H&E ×1000. Fig. (7) LM of skin of infected treated mouse (subgroup IIa) at the 6th week post-infection showing an intact epidermis with a moderately dense infiltrate of chronic non-specific inflammatory cells and few visible Leishmania amastigotes (a) H&E ×200, (b) H&E ×400, (c) H&E ×1000.
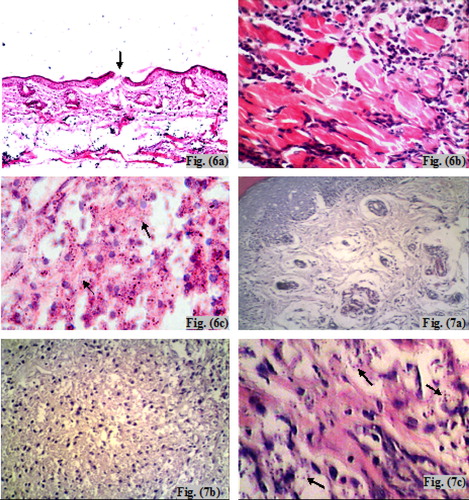
Plate 4 LM of cutaneous lesions of infected (subgroup Ib) and infected treated mice (subgroup IIb) at the 10th week post-infection. Fig. (8) LM of skin of infected untreated mouse (subgroup Ib) at the 10th week post-infection showing an intense non-specific inflammatory cellular infiltrate involving the muscle with numerous amastigotes (a) H&E ×200, (b) H&E ×400, (c) H&E ×1000. Fig. (9) LM of skin of infected treated mouse (subgroup IIb) at the 10th week post-infection showing mild inflammatory infiltrate with scarcity of the organisms (a) H&E ×200, (b) H&E ×400, (c) H&E ×1000.
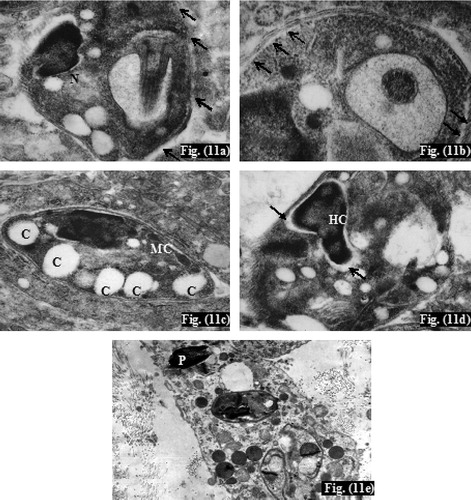
Ultrathin sections of infected non-treated controls (subgroup Ib) showed numerous Leishmania amastigotes. They were seen extracellularly near the macrophage cells and intracellularly inside parasitophorous vacuole ranging in number from 2 to 10 organisms per vacuole (). Amastigotes were generally spherical or slightly ovoid having spherical nucleus with heterochromatin on the inner surface of the nuclear membrane, and a visible nucleolus (). There was intact parasite electron dense plasma membrane with subpellicular microtubules (). Mitochondria and flagellar pocket of the amastigote were also observed (). In the infected treated mice (subgroup IIa), TEM showed mono-nucleated amastigotes with disintegrated plasma membrane (), disappearance of subpellicular microtubules (), numbers of non-membrane bounded cavities together with normal mitochondria (). In addition, absence of the nuclear envelop and abnormal nuclear chromatin distribution was detected as well (). Moreover, pyknosis of the nucleus of the phagocytic cell was further observed ().
Plate 5 Transmission electron micrograph (TEM) of cutaneous lesions of infected mice (subgroup Ib) at the 6th week post-infection. Fig. (10) TEM of leishmanial lesion of infected untreated mouse (subgroup Ib) showing (a) three intracellular amastigotes (arrow) and an extracellular one (EC) (×7500), (b) normal amastigote plasma membrane (M), nucleus (N) with its heterochromatin (arrow), and nucleolus (n) (×20000), (c) High magnification showing parasite plasma membrane (M), subpellicular microtubules (MT) (x50000), (d) Parasite mitochondria (MC), flagellar pocket (FP) (x20000).
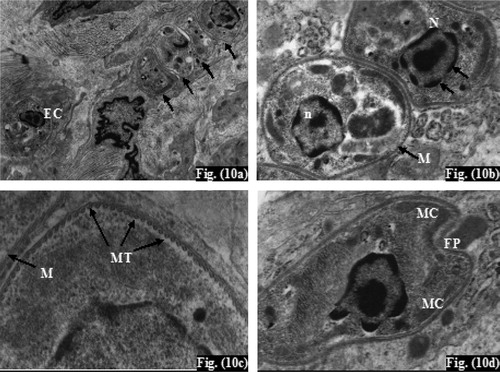
Plate 6 TEM of cutaneous lesions of infected treated mice (subgroup IIa) at the 6th week post-infection. Fig. (11): TEM of leishmanial lesion of infected treated mouse (subgroup IIa) showing (a) Leishmania amastigote with disintegration of plasma membrane (arrow) (x20,000), (b) disappearance of subpellicular microtubules (arrow) (×50,000), (c) non-membrane bounded cavities (C), normal parasite mitochondria (MC) (×13,000), (d) absent amastigote's nuclear envelope (arrow) with abnormal heterochromatin distribution (HC) (×20,000), (e) Pyknosis of the phagocytic cell nucleus (P) (×7500).
4 Discussion
For extending the range of leishmanial species that may potentially be targeted by alkyl-PC drugs, the effect of miltefosine on cutaneous leishmaniasis caused by L. major (MHOM/IL/81/FEBNI) was demonstrated experimentally in this study.
In the present work, oral treatment regimen with MIL started three weeks post-infection, a time interval that allowed establishment of the infection and during which the inoculated sites became swollen and inflamed. Besides, subsequent pathological changes would be irreversible. The timing for treatment initiation can significantly influence the disease outcome as stressed by previous studies [Citation34]. By the end of treatment course, cutaneous lesions became grossly normal with the reduction of parasite burden. One month after treatment stoppage, no clinical sings of relapse were observed, and the parasite density continued to decrease significantly denoting that the leishmanicidal effect of MIL extended for a long period after the end of treatment course. This could be attributed to the long elimination half-life of MIL. It was reported that it has a first elimination half-life of about seven days and a terminal one of about 30 days [Citation35]. Aguiar et al., 2009, investigated the activity of MIL alone, given at a dose of 25 mg/kg/day for the treatment of experimental cutaneous leishmaniasis caused by L. major versus its combination with topical 10% paromomycin gel twice a day for 10 days. They reported that combination therapy induced a statistically significant reduction in lesion size and parasite burden in the skin, with complete healing of ulcers, as compared with those treated with oral miltefosine alone. This difference can be explained by the longer duration of therapy (20 days) used in the present study [Citation36]. This work reported that, parasitological cure could not be achieved in MIL treated L. major-infected mice in spite of development of complete clinical cure. This coincides with Mohebali et al., 2007, who reported parasitologically positive smears for Leishmania amastigotes in four out of 28 patients at the end of treatment of CL caused by L. major with oral MIL in Iran [Citation37]. In contrary, Filho et al., 2008, made a comparative study between oral MIL and parenteral N-methyl glucamine antimoniate for treatment of experimental CL caused by L. amazonensis, and reported that impression smears of all mice treated with MIL were negative for Leishmania amastigotes at the end of treatment [Citation24].
In the current study, N.N.N. cultures were positive for Leishmania promastigotes in all infected subgroups, whether treated or not, at the 6th week post-infection and one month later. These findings are in agreement with Schubach et al., 1998, who isolated Leishmania parasite from a culture medium inoculated with material taken from scar biopsy from two patients eight and 11 years after treatment of American CL with pentavalent antimonials [Citation38]. In addition, Sampaio et al., 2007, reported positivity of cultures inoculated with material aspirated from footpads of L. amazonensis-infected mice after treatment with subcutaneous glucamine associated with topical MIL [Citation39]. In contrary, Filho et al., 2008, reported culture negativity for L. amazonensis promastigotes in all mice treated with MIL [Citation24].
Results of the current study showed that MIL was able to induce clinical cure in all treated mice, however parasitological cure could not be achieved. Clinical evaluation is a method of low precision since the presence or absence of the cutaneous lesions is not always coincides with the presence and the quantity of parasites; big and disfiguring mucous membrane lesions may present small numbers of parasites whereas healed cutaneous lesions may present Leishmania bodies [Citation40]. Besides, Ott et al., 1999, reported that most of Leishmania mexicana-infected mice relapsed after treatment with topical MIL [Citation41]. In addition, Calvopina et al., 2006, demonstrated reappearance of the cutaneous lesions caused by L. mexicana (New World DCL) after stopping MIL in spite of good clinical response during drug administration [Citation40]. Furthermore, Zerpa et al., 2007, revealed the development of new skin lesions following MIL suspension in New World DCL patients after showing dramatic clinical improvement [Citation26]. Sundar et al., 2002, defined parasitological cure of leishmaniasis either as the absence of parasites at the end of therapy, or a parasite density of grade 1+ with no parasites on repeated smears one month later during six months of follow-up [Citation20]. However, Several studies revealed that the intracellular Leishmania organisms could neither be entirely eradicated by anti-leishmanial chemotherapy nor through T-cell-dependent, cytokine-driven acquired resistance mechanisms [Citation42,Citation38].
Histologically, The evolution of Old World CL in mice was attributed to aggregation of the infected macrophages at the site of intradermal inoculation after which granulocytes and lymphocytes appeared and ulceration occurred as supported by Bray, 1987, and Eissa et al., 2003 [Citation43,Citation44], Besides, Verkataram et al., 2001, identified four different histopathological patterns for localized CL in Oman, among them the pattern of diffuse dermal infiltrate composed predominantly of macrophages with an admixture of few polymorphs, lymphocytes and occasionally plasma cells [Citation45]. Congested dilated blood vessels were observed, this could be a compensatory mechanism to overcome the interference of the blood supply by the inflammatory cellular infiltration.
Hematoxylin and eosin-stained sections taken from footpads of infected treated mice showed an intact epidermis with remarkable reduction in terms of the inflammatory response as well as the number of detected parasites. These findings were similar to those reported by Eissa et al., 1997, and 2011, where they revealed reduction in the number of amastigotes and the inflammatory reaction in experimental Old World CL caused by L. major after treatment with dapsone and tamoxifen respectively [Citation46,Citation30]. However, none of researchers who conducted studies on the efficacy of MIL in CL reported its histopathological impacts upon the cutaneous lesions.
Electron microscopy is an important approach for confirming the postulated drug–target interactions. Indeed, Leishmania have special organelles that are involved in metabolic pathways, which are very distinct from those in mammalian cells; these organelles are potential drug targets. Scanning and transmission electron microscopy can identify not only the target organelles but also alterations to the cell surface and ultrastructural changes that characterize distinct forms of programed cell death [Citation47]. Like other alkyl-PCs the mechanism of action of MIL is only partly known; most data are in tumor cell lines as an anti-cancer agent where these compounds can trigger programed cell death (apoptosis) [48–50]. In tumor cells, the lipophilic alkyl-PCs primarily influence membrane physiology in a pleiotropic ways, without interacting with DNA. They could modulate membrane permeability and fluidity, membrane lipid composition, metabolism of phospholipids and proliferation signal transduction [Citation51,Citation52]. Prior studies provided evidence that apart from its direct toxicity against the Leishmania parasite, MIL also modulates immune cells such as macrophages, leading to parasite elimination via oxidative radicals [Citation53].
Data obtained in this study showed that amastigote forms of L. major underwent remarkable morphological and ultrastructural alterations upon treatment, compared with untreated groups. In infected treated mice, TEM examination revealed disintegration of the amastigote plasma membrane with partial disappearance of subpellicular microtubules and absence of the nuclear envelop. This could be attributed to the inhibitory action of MIL on sterol biosynthesis, phospholipid and ether–lipid metabolism which are important components of the plasma membrane and so the nuclear membrane of Leishmania parasite [Citation54,Citation24]. In contrary, Santa-Rita et al., 2004, found that MIL affected the membrane integrity with no effect on subpellicular microtubules of L. amazonensis amastigotes [Citation55]. All detected amastigotes were mono-nucleated with abnormal chromatin distribution, this coincides with Verma and Dey, 2004, who suggested that condensation of nuclear material in L. donovani parasite represents a part of process leading to apoptosis-like death in response to MIL treatment [Citation42]. On the other hand, Santa-Rita et al., 2004, reported the presence of multinucleated amastigotes within L. amazonensis-infected macrophages which were treated in vitro with MIL [Citation55]. In the present study, TEM examination of the parasite mitochondrion showed that its size and membrane integrity were preserved. In contrary, Santa-Rita et al., 2004, revealed the swelling of the mitochondrion with loss of its inner membrane organization in MIL-in vitro-treated L. amazonensis amastigotes, which denoted the structural affection of the parasitic mitochondrion in response to MIL therapy [Citation55]. Current results are in agreement with Luque-Ortega and Rivas, 2007, who reported no structural alteration of the parasite mitochondrion although that it could be functionally affected (mitochondrial dysfunction) by MIL [Citation56]. In their study, they revealed inhibition of cytochrome oxidase enzyme and intracellular ATP synthesis, together with reduction of oxygen consumption and mitochondrial depolarization after overnight in vitro incubation of L. donovani promastigotes with MIL [Citation56].
In the present study, pyknosis of infected macrophage nucleus was observed. It appeared as shrinkage of nucleus with condensation of the nuclear chromatin that denotes degeneration of the affected cell. Such finding might reveal extension of MIL activity to involve Leishmania infected host cells by induction of macrophage cell degeneration. However, in a study conducted by Verma and Dey, 2004, to clarify the possible mechanism of MIL-mediated death of L. donovani, they reported that MIL causes no damage to infected macrophage nuclei [Citation42].
In conclusion, the present study revealed activity of MIL against experimental Old World CL in the mouse model caused by L. major (MHOM/IL/81/FEBNI), one of the causative species of CL in the Middle East.
Notes
Peer review under responsibility of Alexandria University Faculty Medicine
Available online 21 June 2012
References
- World Health Organization (WHO). Leishmaniasis: disease burden and epidemiological trends. Special Programme for Research and Training in Tropical Diseases. Geneva: WHO; 2002.
- P.DesjeuxLeishmaniasis: current situation and new perspectivesComp Immunol Microbiol Infect Dis272004305318
- M.A.Vannier-SantosA.MartinyW.De SouzaCell biology of Leishmania spp.: invading and evadingCurr Pharm Des82002297318
- World Health Organization (WHO). The global burden of disease: update. World Health Organization, Geneva, Switzerland; 2004.
- A.C.CunninghamParasitic adaptive mechanisms in infection by LeishmaniaExp Mol Pathol722002132141
- F.J.Pérez-VictoriaM.P.Sánchez-CañeteK.SeifertS.L.CroftS.SundarS.CastanysMechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical useDrug Resist Updat91–220062639
- J.ShawThe leishmaniases – survival and expansion in a changing world, a mini-reviewMem Inst Oswaldo Cruz1022007541547
- M.BoelaertD.Le-rayP.Van-der StuyftHow better drugs could change kala-azar control. Lessons from a cost-effectiveness analysisTrop Med Int Health72002955959
- R.HadighiP.BoucherA.KhamesipourA.R.MeamarG.RoyM.OuelletteGlucantime-resistant Leishmania tropica isolated from patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugsParasitol Res101200713191322
- M.K.MittalS.RaiRavinderAshutoshS.GuptaS.SundarN.GoyalCharacterization of natural antimony resistance in Leishmania donovani isolatesAm J Trop Med Hyg762007681688
- H.A.AhasanM.A.ChowdhuryM.A.AzharA.K.RafiqueuddinK.A.AzadDeaths in visceral leishmaniasis (Kala-azar) during treatmentMed J Malaysia5119962932
- E.A.M.FleerC.UngerD.J.KimH.EiblMetabolism of ether phospholipids and analogs in neoplastic cellsLipids221987856861
- P.HilgardT.KlennerJ.StekarC.UngerAlkylphosphocholines: a new class of membrane active anticancer agentsCancer Chemother Pharmacol3219939095
- A.KuhlencordT.ManieraH.EiblHexadecylphosphocholine: oral treatment of visceral leishmaniasis in miceAntimicrob Agents Chemother36199216301634
- S.L.CroftD.SnowdonV.YardleyThe activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma bruceiJ Antimicrob Chemother38199610411047
- S.SundarTrial of oral miltefosine for visceral leishmaniasisLancet352199818211823
- C.UngerE.FleerW.DamenzP.HilgardG.NagelH.EiblHexadecylphosphocholine: determination of serum concentrations in ratsJ Lipid Mediat319917178
- S.L.CroftG.H.CoombsLeishmaniasis – current chemotherapy and recent advances in the search for novel drugsTrends Parasitol192003502508
- S.SundarL.P.OlliaroMiltefosine in the treatment of leishmaniasis: clinical evidence for informed clinical risk managementTher Clin Risk Manag352007733740
- S.SundarT.K.JhaC.P.ThakurJ.EngelH.SindermannC.FischerOral miltefosine for Indian visceral leishmaniasisN Engl J Med34722200217391746
- J.BermanP.LaneTreatment of leishmaniasis with miltefosine: 2008 statusExpert Opin Drug Metab Toxicol49200812091216
- J.SotoB.A.AranaJ.ToledoN.RizzoJ.C.VegaA.DiazMiltefosine for New World cutaneous leishmaniasisClin Infect Dis389200412661272
- J.SotoJ.ReaM.BalderramaJ.ToledoP.SotoL.ValdaEfficacy of miltefosine for Bolivian cutaneous leishmaniasisAm J Trop Med Hyg7822008210216
- A.V.C.FilhoI.C.LucasR.N.R.SampaioComparative study between oral miltefosine and parenteral antimonate of N-methyl glucamine in the treatment of experimental leishmaniasis caused by Leishmania amazonensisRev Soc Bras Med Trop4142008340353
- P.EscobarS.MatusC.MarquesS.L.CroftSensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), Et-18-och3 (eldefosine) and amphotericin BActa Trop8122002151157
- O.ZerpaM.UlrichB.BlancoM.PolegreA.AvilaN.MatosDiffuse cutaneous leishmaniasis responds to miltefosine but then relapsesJ Dermatol1566200713281335
- Y.KeynanO.E.LariosM.C.WisemanM.PlourdeM.OuelletteE.RubinsteinUse of oral miltefosine for cutaneous leishmaniasis in Canadian soldiers returning from AfghanistanCan J Infect Dis Med Microbiol192008394396
- P.A.M.Van-ThielT.LeenstraP.A.KagerH.J.De-VriesM.Van-VugtA.BartMiltefosine treatment of Leishmania major infection; an observational study involving Dutch military personnel returning from Northern AfghanistanClin Infect Dis5020108083
- C.McCartry-BurkeP.A.BatesD.M.DwyerLeishmania donovani: use of two different commercially available chemically defined media for the continuous in vitro cultivation of promastigotesExp Parasitol731991385387
- Eissa MM, Amer EI, El-Sawy SMF. Leishmania major: activity of tamoxifen against experimental cutaneous leishmaniasis. Exp Parasitol 2011, in press. http://dx.doi.org/doi:10.1016/j.exppara.05009.
- M.P.BarretJ.C.Mott-ramG.H.CommbsRecent advances in identifying and validating drug targets in Trypanosomes and LeishmaniasTrend Microbiol719998288
- J.D.ChulayA.M.BrycesonQuantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasisAm J Trop Med Hyg3231983475479
- M.A.HayatPrinciples and techniques of EM: biological application2nd ed1981Pock Press, Union New Jersey University
- T.GarnierA.MantylaT.JarvinenJ.LawrenceM.BrownIn vivo studies on the antileishmanial activity of buparvaquone and its prodrugsJ Antimicrob Chemother602007802810
- T.P.DorloP.P.Van ThielA.D.HuitemaR.J.KeizerH.J.De VriesJ.H.BeijnenPharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patientsAntimicrob Agents Chemother52200828552860
- M.G.AguiarD.L.SilvaF.A.NunanE.A.NunanA.P.FernandesL.A.M.FernandesCombined topical paromomycin and oral miltefosine treatment of mice experimentally infected with Leishmania major leads to reduction in both lesion size and systemic parasite burdensAntimicrob Agents Chemother64200912341240
- M.MohebaliA.FoutohiB.HoshmandZ.ZareiB.AkhoundiA.RahnemaComparison of miltefosine and meglumine antimonite for treatment of zoonotic cutaneous leishmaniasis by randomized clinical trial in IranJ Acta Trop103120073340
- A.SchubachM.C.MavzochiT.Cuzzi-MayaA.V.OliveiraM.L.AraujoA.L.OliveiraCutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cureAm J Trop Med Hyg5861998427824
- R.N.R.SampaioI.C.LucasH.L.TakamiInefficacy of the association N-methyl glucamine and topical miltefosine in the treatment of experimental cutaneous leishmaniasis by Leishmania leishmania amazonensisJ Venom Anim Toxins1332007598606
- M.CalvopinaE.A.GomezH.SindermannP.J.CooperY.HashiguchiRelapse of New World diffuse cutaneous leishmaniasis caused by Leishmania mexicana after miltefosine treatmentAm J Trop Med Hyg756200610741077
- S.R.OttT.KlennerP.OverathT.AebischerTopical treatment with hexadecylphosphocholine (Miltex®) efficiently reduces parasite burden in experimental cutaneous leishmaniasisTrans R Soc Trop Med Hyg9319998590
- N.K.VermaC.S.DeyPossible mechanism of miltefosine-mediated death of Leishmania donovaniAntimicrob Agents Chemother48200430103015
- R.S.BrayExperimental leishmaniasis of mammalsW.PetersR.Killick-KendrickThe leishmaniasis in biology and medicine1987Academic PressLondon, Orlando425463
- M.M.EissaA.S.SolimanS.O.NassarUltrastructural and immunological features of experimental cutaneous leishmaniasis after treatment with intralesional hypertonic sodium chloride and CO2 laser raysJ Egypt Soc Parasitol3312003329352
- M.VenkataramM.MoosaL.DeviHistopathological spectrum in cutaneous leishmaniasis: a study in OmanIndian J Dermatol Venereol Leprol6762001294298
- M.M.EissaL.K.YounisE.A.BadawyUltra structure study of experimental cutaneous leishmaniasis before and after dapsone intakeJ Egypt Soc Parasitol2721997539552
- C.M.AdadeT.Souto-PadrónContributions of ultrastructural studies to the cell biology of trypanosmatids: targets for anti-parasitic drugsOpen Parasitol J42010178187
- T.WiederW.ReutterC.E.OrfanosMechanisms of action of phospholipid analogs as anticancer compoundsProg Lipid Res381999249259
- M.RybczynskaM.SpitalerN.G.KnebelEffects of miltefosine on various biochemical parameters in a panel of tumor cell lines with different sensitivitiesBiochem Pharmacol622001765772
- M.M.WrightA.G.HoweV.ZarembergCell membranes and apoptosis: role of cardiolipin, phosphatidylcholine, and anticancer lipid analoguesBiochem Cell Biol8220041826
- C.C.GeilenT.WiederW.ReutterHexadecylphosphocholine inhibits translocation of CTP: choline-phosphate cytidylyltransferase in Madin-Darby canine kidney cellsJ Biol Chem267199267196724
- F.ÜberallH.OberhuberK.MalyJ.ZaknunL.DemuthH.H.GrunickeHexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activityCancer Res511991807812
- KlausGriewankCarolineGazeauAndreasEichhornEsthervon StebutMiltefosine efficiently eliminates Leishmania major amastigotes from infected murine dendritic cells without altering their immune functionsAntimicrob Agents Chemother5422010652659
- H.LuxD.T.HartP.J.ParkerT.KlennerEther lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for antileishmanial alkyl phospholipids analoguesAdv Exp Med Biol4161996201211
- R.M.Santa-RitaA.Henriques-PonsH.S.BarbosaS.L.De-CastroEffect of lysophospholipid analogues; edelfosine, ilmofosine, and miltefosine against Leishmania amazonensisJ Antimicrob Chemother542004704710
- J.R.Luque-OrtegaL.RivasMiltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotesAntimicrob Agent Chemother514200713271332