Abstract
Background
The high prevalence of colorectal carcinoma (CRC) is a driver to understand the underlying molecular mechanisms. Chemoprevention strategy using non-steroidal anti-inflammatory drugs (NSAIDs) revealed that these drugs suppress colorectal carcinoma. The best known targets of NSAIDs are cyclooxygenase (COX) enzymes. The function of prostaglandins and cyclooxygenase in cancer pathogenesis is unclear. COX-2 regulation of proliferation, apoptosis, and tumor-blood vessel interaction has been suggested. β-Catenin is a component of the WNT (wingless type) signaling pathway, increased protein concentrations promote transcription of genes important in regulating the cell cycle.
Aim
To determine the significance of COX-2 and β-catenin expression in colorectal carcinogenesis and prognosis.
Patients and methods
Thirty patients with colorectal carcinomas treated by colonic resection were studied for the expression of both COX-2 and β-catenin by immunohistochemistry. Their expression was interpreted in relation to adjacent normal colonic mucosa and analyzed in correlation with various clinicopathologic parameters and patient’s survival after a follow up period of 24 months.
Results
Our results showed that in normal adjacent colonic mucosa, COX-2 was completely absent, whereas β-catenin was specifically located in the plasma membranes. Both proteins were expressed in tumorous tissues, COX-2 showed diffuse cytoplasmic positivity, whereas β-catenin accumulated in both the cytoplasm and nuclei. We established statistically significant relationships between pathological grade and both β-catenin, and COX-2 positivity scores, being at the higher end for poorly-differentiated tumors. β-Catenin expression also correlated significantly with higher tumor stage and LN metastasis. Both COX-2 and β-catenin expression correlated with a higher incidence of shorter disease free survival.
Conclusion
Both β-catenin and COX-2 expression may play an important role in the evolution of colon carcinogenesis. Increased expression of both could be used as a marker of tumor progression and poor prognosis. This might be of therapeutic value for allocating patients with colorectal carcinoma to different treatment modalities.
1 Introduction
Adenocarcinoma of the colon and rectum is the second leading cause of death from cancer in the industrialized world.Citation1 In Egypt, Colorectal carcinoma is the third most common tumor in males after urinary bladder and lymphohemopoietic malignancies, and in females it ranks fifth after breast, lymphohemopoietic, cervical, and urinary bladder cancers.Citation2 Recently interest in Egyptian CRC has been raised when clinical studies revealed a high incidence of the disease among the young Egyptian population.Citation3 Occurrence of colorectal carcinoma at young age in Egypt could reflect the presence of clinically inapparent inherited syndromes, furthermore there is a high prevalence of consanguinity in Egypt because of the tradition of interfamilial marriages and this cultural characteristic could contribute to non-syndromic inherited predisposition.Citation4
Several epidemiological researches reported a 40–50% decrease in the relative risk of colorectal cancer in persons chronically using non-steroidal anti-inflammatory drugs (NSAIDs) indicating that these drugs might have a chemoprotective and possibly chemotherapeutic effect.Citation5–Citation6Citation7Citation8Citation9 The best known targets of NSAIDs are cyclooxygenase (COX) enzymes, which convert arachidonic acid to prostaglandins (PGs) and thromboxane. Among these PGs, prostaglandin E2 (PGE2) can promote tumor growth by binding its receptors and activating signal pathways which control cell proliferation, migration, apoptosis, and angiogenesis. Therefore, COX inhibition is a promising approach for chemoprevention of colorectal cancer.Citation10 Unlike COX-1, which is found constitutively in tissues, COX-2 expression is induced by a variety of mediators, including among others β-catenin.Citation11
β-Catenin plays an important role in the WNT signaling pathway. Mutations in Wnt/adenomatous polyposis coli (APC)/β-catenin (CTNNB1) signaling pathway members have been found in many colorectal carcinomas.Citation12 Wnt ligands bind to transmembrane Frizzled receptors and their co-receptors, leading to phosphorylation and sequestration of the complex composed of APC, casein kinase 1, glycogen synthase kinase 3, and axin. The resultant stabilization of intracellular CTNNB1 facilitates its translocation to the nucleus, where it interacts with transcription factors of the T-cell factor/lymphoid enhancer-binding factor, activating the targets controlling cell growth and differentiation. Therefore, the interaction with CTNNB1 has been considered to be essential for the tumor suppressor activity of APC.Citation13 In addition, CTNNB1 is a multifunctional signaling protein, which also binds to E-cadherin, linking E-cadherin to actin filaments and promoting cell adhesion and differentiation and epithelial membrane transition (EMT).Citation14 Down-regulation of the epithelial molecule E-cadherin, is a critical event in tumor invasion and a master programer of EMT. The molecules involved in EMT represent potential targets for pharmacological agents and open new avenues for the control of metastatic spread in the treatment of malignancies.Citation15
There is increasing evidence that COX-2 and β-catenin are often co-expressed in cancer cells.Citation16 However, this coordinated over-expression and its role in colorectal carcinoma needs further investigations.
The present study was undertaken on 30 cases of colorectal carcinoma. The expression and cellular localization of both COX-2 and β-catenin using immunohistochemistry was performed to determine the correlation between them and their relation to various clinicopathological parameters and patient’s survival after a follow up period of 24 months.
2 Patients and methods
2.1 Patients
The current study was carried out on a total of thirty patients with colorectal cancer admitted to the department of surgery, Medical Research Institute, Alexandria University from February 2009 to February 2012. The patients were 21 male and 9 female with a male/female ratio of 2.33:1 and a mean age of 52 years (range, 32–70 years).
Thirteen patients had right colon cancer (5 patients had cancer coecum and 8 patients had ascending colon cancer) and 17 patients had left colon cancer (7 patients had descending colon cancer, 7 patients had sigmoid cancer and 3 patients had rectosigmoid cancer). None of the patients had received neoadjuvant therapy prior to surgery and none had a known family history of colorectal cancer. All patients included in this study underwent routine laboratory investigations, radiological investigations (Barium enema, C.T. abdomen) as well as colonoscopic examination and biopsy to confirm malignancy. The surgical procedures performed to our patients were right hemicolectomy for patients with right sided colon cancer, left hemicolectomy for patients with left sided colon cancer and anterior resection for patients with sigmoid and rectosigmoid cancer.
After surgery, all patients were referred to cancer management and research department for adjuvant treatment which was given based on the pathological stage and different risk factors and were followed up for 24 months at the Oncology unit, while their corresponding colectomy specimens were examined at the Pathology department; all departments are affiliated to the Medical Research Institute, Alexandria University, Egypt.
2.2 Methods
In the pathology department, formalin-fixed, paraffin-embedded paired tissue sections from colon carcinoma and adjacent non-malignant mucosa were obtained from each case. Hematoxylin and eosin-stained tissue sections were examined under a light microscope and the histological type of colorectal cancer was determined according to the World Health Organization criteria.Citation17
Tumors were classified into well, moderately and poorly differentiated tumors (>50% solid areas) and the T classification based on the criteria of classification of the American Joint Committee on Cancer (AJCC).Citation18 was performed. According to the AJCC classification, T1 (tumor has invaded the lamina propria or muscle layer), T2 (tumor has invaded the perimuscular connective tissue; no extension beyond the serosa or into the liver), T3 (tumor has perforated the serosa or has directly invaded one adjacent organ or both) T4 (tumor extends more than 2 cm into the liver, and/or into two or more adjacent organs), respectively.
Respective tissue blocks were then sectioned at 4 μm and mounted on glass slides coated with 3-aminopropyltrimethoxysilane and stained immunohistochemically.
2.2.1 Primary antibodies
Two primary antibodies were used; anti-COX-2 rabbit polyclonal antibody (Thermo Scientific, Fermont, USA), used at a 1:50 dilution and anti- β-catenin rabbit monoclonal antibody (clone E247; Thermo Scientific, Fermont, USA), used at a 1:20 dilution.
2.2.2 ImmunohistochemistryCitation19
Prior to immunohistochemical staining, sections were deparaffinized and rehydrated using standard procedures. Antigen retrieval was performed using heat treatment; endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 10 min. To block non-specific antigen sites, tissue sections were incubated for 1 h in 1.5% bovine serum albumin at room temperature. Incubation with the primary antibodies was performed at room temperature for 30 min with anti-COX-2 and anti- β-catenin. After the primary antibody incubation step, a secondary antibody from a streptavidin biotin complex peroxidase kit (LSAB® + kit, Dako, Copenhagen, Denmark) was used according to the manufacturer’s instructions. Peroxidase activity was developed with the substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB; Dako) by incubating the sections in DAB for 10 min. Sections were then rinsed gently with distilled water and counterstained with hematoxylin.
Appropriate positive and negative controls were included in each run of immunohistochemistry. Negative controls were prepared simultaneously for all 30 samples by replacing the primary antibody with distilled water. Positive staining controls for COX-2 included sections of lung carcinoma. For β-catenin normal colonic epithelial cells served as internal positive controls with membrane staining. External positive control was sections from breast carcinoma.
2.2.3 Immunohistochemical scoring
Processed specimens were scored under the light microscope.
A method taking into consideration both intensity and distribution of COX-2 immunoreactivity was employed.Citation20,Citation21 The distribution was scored according to the number of positive cells; none (not stained), 0; focal (<1/3 of cells stained), 1; multi-focal (1/3–2/3 of cells stained), 2; and diffuse (>2/3 stained), 3. The staining intensity was scored as: none (not stained), 0; mild (between 0 and 2), 1; and strong, 2. The distribution and intensity scored were added to produce the following grades for the staining: 0, negative; 2–3 weakly positive; and 4–5, strongly positive.
As previously described by Jass et al.,Citation22 scoring of β-catenin was based upon the distribution of β-catenin within the cell membrane (0–1), cytoplasm (0–2), and nuclei (0–2). We also calculated β-catenin activation score as the sum of nuclear score (+2 = positive expression; +1 = weak expression; 0 = no expression), cytoplasmic score (+2 = positive expression; +1 = weak expression; 0 = no expression), and membrane score (0 = positive membrane expression; +1 = negative membrane expression). Total scores were then collapsed into three grades (grade I, 0–1; grade II, 2–3; grade III, 4–5). with a total score of 0 reflecting cell membrane staining only, similar to that seen in normal colonic mucosa, up to an aggregate score of 5 for tumors with strong nuclear staining (2), diffuse cytoplasmic staining (2), and loss of cell membrane staining (1).
2.2.4 Statistical analysisCitation23
Statistical analysis was performed using SPSS for Windows version 10.0 (SPSS Inc, Chicago, IL, USA).
The relation between COX-2 and β-catenin immunohistochemical scores and various clinicopathological features as well as survival rates were analyzed using the Fisher Exact test, Monte Carlo test or chi- squared test. The Spearman rank correlation test was used to analyze the correlation of β-catenin and COX-2 expression with tumor grade. An association between COX-2 and β-catenin expressions was tested using the chi-squared test. Significant difference between β-Catenin expression-grades in regard to high COX-2 expression scores was identified using Z test.
Disease free survival (DFS) was calculated from the date of diagnosis to the date of death/progression or the date of last seen. The prognostic significance of Cox-2 and β-catenin was analyzed by univariate and multivariate analysis for prognostic significance for 24 months of DFS. Multivariate analysis was carried out using the Cox proportional hazard model.
A p value of less than 0.05 was considered significant.
3 Results
Thirty patients with colorectal carcinoma were included in the present work. They consisted of 21 males and 9 females with ages ranging from 32 to 70 years (mean 52 years).
shows the clinicopathological features of the 30 patients with colorectal carcinomas studied.
Table 1 clinicopathologic features of the 30 colorectal carcinomas cases studied.
Histopathologic examination of colectomy specimens revealed that 26 cases were adenocarcinomas, three cases were mucinous adenocarcinoma and one case was signet ring cell carcinoma.
Of the 26 adenocarcinomas, 5 (16.6%) were well differentiated, 14 (46.6%) were moderately differentiated, and 7 (23.33%) were poorly differentiated.
3.1 Analysis of COX-2 immunoreactivity
Normal colonic epithelium and stroma adjacent to the tumor tissue showed no staining for COX-2 ().
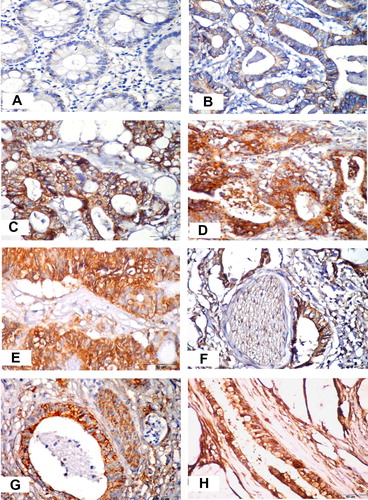
Immunohistochemically detectable COX-2 expression has been reported in all 30 (100%) colorectal carcinomas analyzed. In all positive cases, COX-2 immunoreactivity pattern was located diffusely in the cytoplasm. Weak positivity was found in 7 cases (23.3%) (). Strong positivity with perinuclear accentuation () or granular cytoplasmic pattern ( and E) was observed in 23 cases (76.6%). COX-2 expression in tumourous areas was reported both in the neoplastic epithelial cells, as well as in the surrounding inflammatory cells, vascular endothelial cells, smooth muscle fibers, nerve fibers and fibroblasts (). Two cases of mucinous adenocarcinoma showed strong positivity and one case showed weak positivity (). On the other hand the case of signet ring cell carcinoma showed weak COX-2 positivity.
3.2 Analysis of β-catenin immunoreactivity
In normal tissue adjacent to colorectal carcinoma, β-catenin was mainly localized in the plasma membrane of the cell-to-cell border with a weak expression in the cytoplasm of both the colonic epithelium and goblet cells. No nuclear β-catenin was seen in the normal colonic mucosa ().
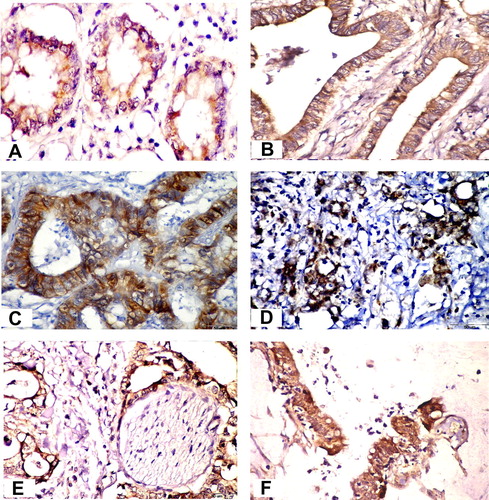
In colorectal carcinoma tissue, β-catenin immunoreactivity was detected in all 30 samples. Complete loss of β-catenin immunoreactivity in the cell-to-cell border was observed in 12 cases (). β-Catenin immunostaining reactivity of grade II was observed in 18 cases (60%) (). Grade III β-catenin immunoreactivity in both the cytoplasm and nuclei of tumor cells was seen in 12 cases (40%) (). Inflammatory cells, stromal cells, nerve fibers and endothelial cells all were β-catenin negative (). The three cases of mucinous adenocarcinoma showed grade II β-catenin immunostaining, whereas the case of signet ring cell carcinoma showed grade III positivity ().
3.2.1 Statistical analysis
The relation between both COX-2 and β-catenin expression and various clinicopathological parameters is summarized in .
Table 2 Relation of COX-2 and β-catenin expression with various clinicopathologic parameters studied.
A significant increase in number of cases having stronger COX-2 and β-catenin positivity was found in higher histologic grades of colorectal carcinoma (p = 0.026 and 0.032, respectively). However no significant relation was found between COX-2 scores and other clinicopathological parameters, including, depth of invasion, lymph node metastasis, or tumor stage. Grade III β-catenin expression was significantly more frequent in male patients (p = 0.004), Lt sided tumors (p = 0.002), higher histologic grade (p = 0.032) and stage (p = 0.002) tumors as well as in node positive tumors (p = 0.002).
A significant correlation was found between both COX-2 and β-catenin expression on one hand and histologic grade on the other hand (p = 0.013 and 0.014, respectively).
COX-2 expression was significantly associated with β-catenin expression (χ2 test = 5, p = 0.05). All Grade III β-catenin immunostaining were strongly positive for COX-2 and all weakly positive cases for COX-2 had grade II immunostaining for β-catenin. β-Catenin grade III positive tumors showed a significantly higher frequency of strong positive expression of COX-2 (scores > 4) than did β-catenin grade II positive tumors (100% vs. 66.6%, Z = 2.24, P = 0.05).
3.2.2 Prognosis in relation to immunostaining results
The follow-up time was 24 months after surgery.
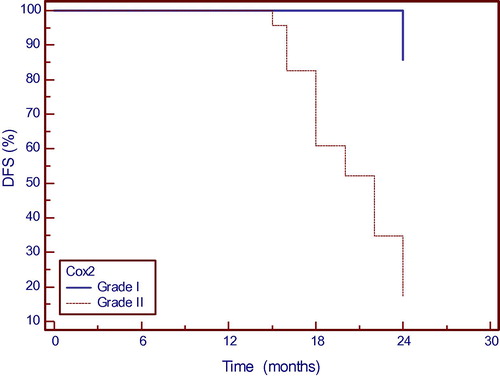
Disease free survival and COX-2 expression; weakly versus strongly positive ():
Survival analysis for all patients showed that COX-2 expression was associated with disease free survival; i.e., shorter disease free survival was identified in patients with strong COX-2 expression scores (p < 0.002).
DFS curves according to COX-2 expression scores.
The higher the score of COX-2 immunohistochemical expression, the lower the percentage of DFS.
Disease free survival and β-catenin expression; Grade II versus Grade III ():
Survival analysis for all patients showed that β-catenin expression was associated with disease free survival; where shorter disease free survival was noted in patients with β-catenin grade III scores (p < 0.005).
DFS curves according to β-catenin expression grades.
The higher β-catenin grade of immunostaining, the lower the percentage of disease free survival.
shows the relation between COX-2 and β-catenin expression and patient’s outcome by the end of follow up period of 24 months.
Table 3 Relation between COX-2 and β-catenin and patient’s outcome at the end of 24 months.
Both COX-2 and β-catenin expressions were related to disease free survival with significantly higher rates of recurrence and death in the patients with strong COX-2 and grade III β-catenin expression (p < 0.006 and 0.001, respectively).
Univariate analysis showed a highly significant difference in DFS between weak and strong COX-2 expression and also between Grade II and III β-catenin expression (p = 0.002). However the statistical significance in univariate analysis could not be confirmed through multivariate analysis (p = 0.390 and 0.129, respectively) ().
Table 4 Univariate and multivariate analysis of clinicopathologic parameters and COX-2 and β-catenin status as predictors of DFS in colorectal carcinoma patients.
4 Discussion
Carcinogenesis and development of colorectal cancer are multi-step and multi-stage processes involving cumulative effects of many genes and epigenetic alterations (e.g., DNA hypomethylation).Citation24
In this work the expression of both COX-2 and β-catenin was studied in 30 patients with colorectal carcinoma by immunohistochemistry. Their expression was correlated with various clinicopathologic parameters and with patient’s disease free survival after a follow up period of 24 months.
Evidence suggests that NSAIDs reduce the risk of CRC and that this effect is mediated through COX-2 inhibition.Citation5,Citation10 COX-2 has fatty acid oxygenase activity for the synthesis of prostaglandins from arachidonic acid. Previous studies have reported that prostaglandin production is generally enhanced in cancer cells, and that prostaglandins promote the proliferation and metastasis of cancer cells. Thus, COX-2 induction has the potential to promote tumor growth and progression.Citation25
Several studies have shown that the COX-2 expression is elevated in colorectal cancer when compared with normal mucosa.Citation26,Citation27 Our results confirmed these previous observations where, COX-2 protein was detected in all of colorectal carcinomas – with different scores of positivity – and was absent in the adjacent normal colorectal tissue. In the literature, increased COX-2 expression was detected in other tumors, and was believed to be involved in their pathogenesis Citation28–Citation29Citation30Citation31Citation32. Therefore our results suggest that increased expression of COX-2 protein correlates with colorectal carcinogenesis.
In agreement with others Citation25, no significant correlation was found in this work, between COX-2 expression scores and many clinicopathological parameters, including gender, age, tumor localization, depth of tumor invasion, lymph node metastasis, or stage. However, we did find a significant negative correlation between the expression of COX-2 and the degree of tumor differentiation (p = 0.026). Others reported that COX-2 expression is related to lymph node involvement and Duke’s stage.Citation32 These discrepancies may be related to the use in this study of the TNM staging system or to the different scoring systems and antibodies employed in immunohistochemistry.
In line with previous publications;Citation33 we did find in this study a strong positivity of stromal cells of the tumorous areas to COX-2 in contrast to their complete negativity in the normal adjacent mucosa. This important finding suggests that COX-2 might be a mediator of tumor epithelial-stromal interactions in colorectal carcinoma. Therefore it could be possible to eliminate the growth and invasive progression promoting effects of stromal fibroblasts by the use of NSAIDs especially COX-2 inhibitors for treatment and prevention of colorectal carcinoma.
It has been demonstrated that COX-2 is one of several genes that are transcriptionally activated by β-catenin.Citation34
In this study, β-catenin localization was different in cancer cells and normal mucosal cells. Normal colonic epithelial cells showed strong uniform membranous β-catenin immunostaining at the cell–cell junction. The localization of β-catenin immunoreactivity to the plasma membrane and cell to cell border of the normal colonic mucosa is consistent with the findings of Iwamoto et al.Citation35 where they stated that the cytoplasmic tail of β-catenin binds to E-cadherin and, indirectly, to the cytoskeleton, so it is localized to the adherens’ junction of the cell-to-cell plasma membrane. Formation of multiprotein complexes consisting of proteins such as APC, axin and β-catenin makes β-catenin a target for degradation, so that no cytoplasmic or nuclear β-catenin will be detected in normal tissue.Citation36 Other workers,Citation37,Citation38 stated that localization of β-catenin, primarily to the apical-lateral cell membrane, signifies its role in cell adhesion.
In our series, β-catenin expression was altered in all primary colorectal carcinomas studied (maintained or lost membranous + cytoplasmic or nuclear positivity). This aberrant distribution of β-catenin inferred from cytoplasmic or nuclear distribution which was sometimes also cell–cell adhesion-deficient reflects either an ineffective β-catenin or loss of β-catenin connection to the cytoskeleton. Observations that are documented in other reports,Citation39 where they thought that an abnormally high amount of β-catenin in the cytoplasm and not in the intercellular boundary seems to indicate a β-catenin protein with oncogenic potential. This cytoplasmic and nuclear accumulations of β-catenin suggest enhanced transcription and activation of the target genes (such as c-myc, cyclin D1 and matrilysin) which are responsible for tumor proliferation and malignant progression through interaction with members of the TCF/LEF DNA-binding family.Citation40,Citation41
In agreement with other workersCitation42 we reported a higher expression of β-catenin in males (p = 0.004). On the other hand we did not find any correlation between β-catenin expression and patient’s age. A significant association between Lt sided tumors and stronger β-catenin expression was noted in our study (p = 0.002) a finding previously reported by others.Citation43
Grade III expression of β-catenin that we observed correlated significantly with deteriorating tumor grade [p = 0.032], findings that are supported by other publications.Citation44 However othersCitation42 reported lower expression of β-catenin in high grade tumors. These discrepancies could be due to the use in our study of a different β-catenin scoring system of immunostaining. Furthermore, a significant association between grade III β-catenin expression in colorectal carcinomas, and advanced tumor stage (depth of invasion) was noted [p < 0.002]. Where, the more deeply invasive tumors (IIIB) showed higher grade expression of β-catenin than superficial tumors (IIA). Similar findings have been reported previously both in CRC and in other types of human carcinoma.Citation42,Citation16 In the present series also and in agreement with others,Citation34 high grade expression of β-catenin was associated with lymph node metastasis [p = 0.002].
In line with our results some workers.Citation45 recommended the use of β-catenin as a marker of colorectal tumor progression and explained it by loss of β-catenin-mediated cell–cell adhesion, and polarity and activation of genes necessary for invasion and dissociation.
A positive association between the COX-2 and β-catenin was observed in the current work (p < 0.05). Furthermore, β-catenin grade III positive tumors showed a significantly higher frequency of strongly COX-2 positive tumors than did β-catenin grade II positive tumors (P = 0.05). In addition their expressions were correlated with higher colorectal adenocarcinoma grade. These relationships are supported by another studyCitation43 who stated that colonic adenocarcinoma is characterized by correlated cellular expression of COX-2 and β-catenin and that a neoplastic COX-2/β-catenin positive phenotype may be linked to colorectal cancer progression. The PGE2, produced by COX-2, rapidly causes transactivation of EGFR, which triggered the ERK2-mitogenic signaling pathway in a colon cancer cell line.Citation46 Furthermore, PGE2 increases the invasiveness of colon cancer cells by trans-activating c-Met and increasing the tyrosine phosphorylation of β-catenin.Citation47 A recent study has also shown that β-catenin stabilizes COX-2 mRNA by interacting with AU-rich elements in a 3 V untranslated region.Citation48 OthersCitation49 concluded that the pCOX2-0.8 minimal promoter contains a novel functional T-cell factor/lymphoid enhancer factor (TCF/LEF)-response element (TBE Site II; -689/-684) that responds directly to enhanced Wnt/β-catenin signaling and which may be important for the onset/progression of gastric cancer. Considering these data, it may be possible that COX-2 and β-catenin may form a positive feedback loop.
In agreement with othersCitation42,Citation50–Citation51Citation52 a strong COX-2 and grade III β-catenin expression were associated with shorter disease free survival (p < 0.002 and 0.001, respectively); where patients with higher scores of expression had poorer disease free survival at the end of a follow up period of 24 months. Both COX-2 and β-catenin expression were related to patient’s disease free survival in the present study. A significantly lower frequency of disease free survivors was noted among patients having strong COX-2 and grade III β-catenin expression (p < 0.006 and 0.001, respectively). Furthermore, in univariate analysis patients with a strong COX-2 expression and grade III have a shorter DFS than those with weak COX-2 and grade II β-catenin expression (p = 0.002). However this significance could not be maintained in multivariate analysis. This means either that they are not independent poor prognostic factors or it might simply be due to the restricted number of patients in our study (30 cases).
5 Conclusion
From the present work, it can be concluded that, the detection of diffuse COX-2 cytoplasmic expression only in colorectal carcinoma and increased expression scores in higher tumor grades implicate that COX-2 might be an early marker of neoplastic transformation involved in both initiation and progression of colorectal carcinoma.
The alterations in the expression and cellular localization of β-catenin occurs early in colorectal tumorigenesis and becomes more strongly expressed in high-grade, deeply invasive and lymph node metastatic tumors suggesting that β-catenin expression may add prognostic information to standard clinicopathological parameters that can be used in selecting candidates for closer follow up and aggressive adjuvant therapy.
In our present study, COX-2 showed significant association with the expression of β-catenin in colorectal carcinoma, suggesting a coordinated local interaction between these molecules to potentiate the growth and invasion of colorectal carcinoma. Therefore they could be used to select patients that might benefit from new pharmacological agents and gene therapy targeting both prostaglandin and β-catenin mediated growth pathways.
At present, whether COX-2 and β-catenin expressions are a valid prognostic marker for colorectal carcinoma remains controversial. Their relation to DFS was significant only in univariate but not multivariate analysis. So they might have the potential to be a poor predictor of prognosis. To elucidate this relationship, further investigations are required on a larger number of cases.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 17 July 2013
References
- R.MidgleyD.KerrColorectal cancerLancet3531999391399
- A.A.Abou-ZeidW.KhafagyD.M.MarzoukA.AlaaI.MostafaM.A.ElaColorectal cancer in EgyptDis Colon Rectum45200212551260
- A.S.SolimanM.L.BondyB.LevinColorectal cancer in Egyptian patients under 40 years of ageInt J Cancer7119972630
- A.S.SolimanM.L.BondyB.LevinS.El-BadawyH.KhaledA.HablassInt. J. Cancer771998811816
- M.J.ThunM.M.NamboodiriC.W.HeathJr.Aspirin use and reduced risk of fatal colon cancerN Engl J Med325199115931596
- J.M.HerendeenC.LindleyUse of NSAIDs for the chemoprevention of colorectal cancerAnn Pharmacother3711200316641674
- R.BenamouzingB.UzzanJ.LittleS.ChaussadeLow dose aspirin, COX-inhibition and chemoprevention of colorectal cancerCurr Top Med Chem552005493503
- E.FlossmannP.M.RothwellEffect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studiesLancet3699573200716031613
- K.CooperH.SquiresC.CarrollD.PapaioannouA.BoothR.F.LoganChemoprevention of colorectal cancer: systematic review and economic evaluationHealth Technol Assess143220101206
- S.J.MyungI.H.KimRole of prostaglandins in colon cancerKorean J Gastroenterol5152008274279
- R.PoonR.SmitsC.LiS.Jagmohan-ChangurM.KongS.CheonCyclooxygenase two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumour)Oncogene202001451460
- J.GrodenA.ThliverisW.SamowitzM.CarlsonL.GelbertIdentification and characterization of the familial adenomatous polyposis coli geneCell661991589600
- N.BarkerH.CleversMining the Wnt pathway for cancer therapeuticsNat Rev Drug Discov520069971014
- K.WillertKAJonesWnt signaling: is the party in the nucleus?Genes Dev20200613941404
- M.GuarinoB.RubinoG.BallabioThe role of epithelial-mesenchymal transition in cancer pathologyPathology3932007305318
- W.S.MoonH.S.ParkH.LeeR.PaiA.S.TarnawskiK.R.KimCo-expression of COX-2, C-met and beta-catenin in cells forming invasive front of gallbladder cancerCancer Res Treat3732005171176
- S.R.HamiltonL.A.AaltonenWorld Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System2000IARC PressLyon
- S.B.EdgeC.C.ComptonThe American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNMAnn Surg Oncol176201014711474
- S.M.HsuM.RanteH.FaugerUse of Avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) proceduresJ Histochem Cytochem291981577580
- M.YukawaT.FujimoriS.MaedaM.TabuchiK.NagasakoComparative clinicopathological and immunohistochemical study of ras and p53 in flat and polypoid type colorectal tumoursGut35199412581261
- M.P.CharalambousT.LightfootV.SpeirsK.HorgansN.J.GooderhamExpression of COX-2, NF-kB-p65, NF-kB-p50 and IKKa in malignant and adjacent normal human colorectal tissueBr J Cancer1012009106115
- J.R.JassK.G.BidenM.C.CummingsL.A.SimmsM.WalshE.SchochCharacterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathwaysJ Clin Pathol521999455460
- E.LeslieJ.GeoffreyM.JamesStatistical Analysis, Interpretation and Uses of Medical Statisticsfourth ed.1991Oxford Scientific Publications pp. 411–416
- A.W.WuJ.GuJ.F.JiZ.F.LiG.W.XuRole of COX-2 in carcinogenesis of colorectal cancer and its relationship with tumor biological characteristics and patients’s prognosisWorld J Gastroenterol99200319901994
- Y.-E.JooH.-S.KimS.-W.MinW.-S.LeeC.-H.ParkC.-S.ParkExpression of Cyclooxygenase-2 protein in colorectal carcinomasInt J Gastroint Cancer311–32002147154
- J.DimbergA.SamuelssonA.HuganderDifferential expression of cyclooxygenase-2 in human colorectal cancerGut451999730732
- F.A.SinicropeM.LemoineL.XiReduced expression of cyclooxygenase 2 proteins in hereditary nonpolyposis colorectal cancers relative to sporadic cancersGastroenterology1171999350358
- D.HwangD.ScollardJ.ByrneExpression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancerJ Natl Cancer Inst901998455460
- H.WolffK.SaukkonenS.AnttilaExpression of cyclooxygenase-2 in human lung carcinomaCancer Res58199849975001
- K.UefujiT.IchikuraH.MochizukiExpression of cyclooxygenase-2 protein in gastric adenocarcinomaJ Surg Oncol691998168172
- K.C.ZimmermannM.SarbiaA.A.WeberCyclooxygenase-2 expression in human esophageal carcinomaCancer Res591999198204
- K.M.SheehanK.SheahanD.P.O’DonoghueThe relationship between cyclooxygenase-2 expression and colorectal cancerJAMA282199912541257
- H.SanoY.KawahitoR.L.WilderA.HashiramotoS.MukaiK.AsaiExpression of cyclooxygenase-1 and -2 in human colorectal cancerCancer Res55199537853789
- S.-J.KimD.-S.ImS.-H.KimJ.-H.RyuS.-G.HwangJ.-K.SeongB-Catenin regulates expression of cyclooxygenase-2 in articular chondrocytesBiochem Biophys Res Commun2962002221226
- M.IwamotoD.J.AhnenW.A.FranklinT.H.MaltzmanExpression of beta-catenin and full-length APC protein in normal and neoplastic colonic tissuesCarcinogenesis21200019351940
- S.K.HongY.A.GulH.IthninA.TalibH.F.SeowExpression of β-catenin, COX-2 and iNOS in colorectal cancer: relevance of COX-2 and iNOS inhibitors for treatment in MalaysiaAsian J Surg27120041017
- B.M.GhadimiJ.BehrensI.HoffmannW.HaenschW.BirchmeierP.M.SchlagImmunohistological analysis of E-cadherin, alpha-, beta- and gamma-catenin expression in colorectal cancer: implications for cell adhesion and signalingEur J Cancer35119996065
- J.WlodarczykM.StolteJ.MuellerE-cadherin, beta-catenin and stromelysin-3 expression in de novo carcinoma of the colorectumPol J Pathol5232001119124
- D.E.AustJ.P.TerdimanR.F.WillenbucherK.ChewL.FerrellC.FlorendoAltered distribution of beta-catenin, and its binding proteins E-cadherin and APC, in ulcerative colitis-related colorectal cancersMod Pathol14120012939
- N.A.WongM.PignatelliBeta-catenin–a linchpin in colorectal carcinogenesis?Am J Pathol1602002389401
- S.YachidaS.MudaliS.A.MartinE.A.MontgomeryC.A.Iacobuzio-DonahueBeta-Catenin Nuclear Labeling is a Common Feature of Sessile Serrated Adenomas and Correlates with Early Neoplastic Progression Following BRAF ActivationAm J Surg Pathol3312200918231832
- S.C.Cesar WongE.S.Fong LoK.C.LeePrognostic and diagnostic significance of β-Catenin nuclear immunostaining in colorectal cancerClin Cancer Res10200414011408
- T.KawasakiK.NoshoyM.OhnishiY.SuemotoG.J.KirknerzR.DehariCorrelation of B-catenin localization with cyclooxygenase-2 expression and CpG island methylator phenotype (CIMP) in colorectal cancerNeoplasia972007569577
- T.KitagawaK.MatsumotoA.NagafuchiS.TsukitaH.SuzukiCo-expression of E-cadherin and alpha-catenin molecules in colorectal cancerSurg Today2961999511518
- D.HorstS.ReuL.KrieglT.KirchnerA.JungThe intratumoral distribution of nuclear beta-catenin is a prognostic marker in colon cancerCancer11510200920632070
- R.PaiB.SoreghanSzaboIL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinalhypertrophyNat Med82002289293
- R.PaiT.NakamuraW.S.MoonA.S.TarnawskiProstaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptorsFASEB J17200316401647
- H.K.LeeS.JeongBeta-catenin stabilizes cyclooxygenase-2 mRNA by interacting with AU-rich elements of 3V-UTRNucleic Acids Res34200657055714
- F.NuñezS.BravoF.CruzatM.MontecinoG.V.De FerrariWnt/β-Catenin Signaling Enhances Cyclooxygenase-2 (COX2) Transcriptional Activity in Gastric Cancer CellsPlos One642011e18562
- Z.H.PengD.S.WanL.R.LiG.ChenZ.H.LuX.J.WuExpression of COX-2 MMP-2 and VEGF in stage II and III colorectal cancer and the clinical significanceHepatogastroentrology581062011369376
- M.KobayashiT.HonmaY.MatsudaY.SuzukiR.NarisawaY.AjiokaNuclear translocation of beta-catenin in colorectal cancerBr J Cancer8210200016891693
- Z.G.LiX.Y.WangJ.L.ChangW.B.XieT.F.LiiuQ.L.ZhangThe establishment of supramolecular immunobead real-time PCR and the identification of Cox-2 as a metastasis-related marker in colorectal carcinomaOncol Rep2832012977984