Abstract
The aim of this study is to evaluate the value of high resolution CT scan (HRCT) in diagnosis and management of congenital hearing loss.
Patients and methods
This is a prospective study including 60 patients, 24 males and 36 females aged from 1 to 7 years, who were presented by unilateral or bilateral congenital conductive (CHL) or/and sensorineural hearing loss (SNHL). All patients were evaluated by HRCT scan with post-processing multiplanar reconstruction (MPR). Only three Results: External auditory canal atresia (EACA) was diagnosed in 10 patients, while both EACA and middle ear anomalies were diagnosed in another 6 patients. Variable inner ear anomalies were diagnosed in the patients with SNHL. One patient with SNHL had cochlear nerve aplasia that was missed by HRCT and was diagnosed by MRI, in addition to the CT diagnosed vestibulocochlear anomalies.
Conclusion
Although HRCT scan can be used as a solitary diagnostic imaging tool for diagnosis of pure CHL, combined CT and MRI examination is crucial for preoperative evaluation of SNHL patients.
1 Introduction
Congenital hearing loss is one of the developmental disorders that may be not clearly identifiable at birth. Delayed identification and lack of appropriate early management of hearing loss in children have potential impact on child educational, cognitive and social development. The goal of early diagnosis and correction of congenital hearing loss is to maximize perception of speech and the resulting attainment of linguistic-based skills.
2 Physiology of hearing
Hearing is a complex physiological process, which is mediated through interaction of many fine structures. External auditory canal (EAC) is the first station; it serves as collecting apparatus that collects sound energy focusing it on tympanic membrane. The tympanic membrane transmits this sound energy as vibrations, through the ossicles of middle ear. Middle ear is a conductive way that transmits the sound vibrations to the inner ear through foot plate of stapes in the oval window. The vibrating foot plate causes fluid motion in the membranous labyrinth of the inner ear. Finally, the inner ear cochlea is the converter operator, which converts these vibrations into nerve impulses. These impulses are transmitted by the cochlear nerve, through the brain stem into the cerebral auditory area, which is located in the temporal lobe.Citation1,Citation2
3 Embryology of the ear
EAC develops from the ectoderm of the first branchial groove, where epithelial cells proliferate forming a solid core of cells, known as meatal plug. This solid core will soon recanalize to form the epithelial lined EAC. Middle ear develops from the endoderm of the first branchial pouch and maintains its pharyngeal communication through the auditory (Eustachian) tube. First and second pharyngeal arches mesoderm are responsible for ossicles’ generation. The first arch is precursor of epitypanic ossicles that are head of malleus, body and short process of incus. The second arch forms the mesotympanic ossicles that are long process malleus, long process incus, stapes head, neck and crura, while stapes footplate is formed from the otic capsule.Citation3
Inner ear begins to develop at the 4th week of gestation, as localized ectodermal thickening in the developing embryo known as otic placode. The otic placode subsequently invaginates to form otocyst. At the fifth week, a diverticulum buds from the otocyst, forming the endolymphatic sac, followed by the cochlea and vestibule. The membranous cochlea achieves 1–1.5 turns at the end of 6 weeks, while 2.5 turns are formed at the end of the 7th week. The semicircular canal develops from the otocyst at 7–8 gestational weeks. Full embryological development of inner ear structures occurs at the end of 8 weeksCitation3,Citation4
4 Incidence of congenital hearing loss
Records of prevalence of congenital hearing loss vary, depending on the criteria used to define the different degrees of hearing loss and the characteristics of the studied population. Congenital hearing loss may be conductive and/or sensorineural, unilateral or bilateral, symmetrical or asymmetrical, progressive or stable. In some published studies,Citation5 profound congenital hearing loss is estimated to occur in approximately 1 in 1000 births. In other researchers’ records, the incidence of congenital bilateral hearing loss (greater than 40 dB) was 2–4 per 1000 live births, in developed countries. In developing countries, the incidence was estimated to be not less than 6 per 1000 live births.Citation6
5 Etiology
Congenital hearing loss may occur as a result of genetic or non-genetic factors. Non-genetic factors (35%) may be due to maternal infections, such as rubella or cytomegalic or herpes simplex viruses, prematurity, low birth weight, birth injuries, toxins including drugs and alcohol consumed by the mother during pregnancy, complications associated with the Rh factor incompatibility, maternal diabetes, toxemia during pregnancy and perinatral anoxia.Citation7
Hearing loss due to genetic factors (more than 50%) can present at neonatal period or develop later on childhood period. This type may be due to autosomal recessive or autosomal dominant inheritance. Some other uncommon types are inherited via X-linked roots. There are many genetic syndromes that include hearing loss as one of their symptoms e.g. Down syndrome, Treacher Collins syndrome, Crouzon syndrome, Alport syndrome, Klippel-Feil, Norrie disease and Waardenburg syndrome.Citation7
6 Role of imaging
Early diagnosis through a clear accurate imaging tool is a cornerstone for management planning. It guides the selection of candidates, who will gain benefits from hearing aid implants or corrective surgeries. The advent of high resolution multislice computed tomography (HRCT) scan of temporal bone was expected to play a very valuable role in preoperative assessment and postoperative evaluation. Post processing 2D and 3D multiplanar reconstruction is the discerning gain of multislice CT application in temporal bone imaging.Citation8
7 Radiological anatomy of the ear
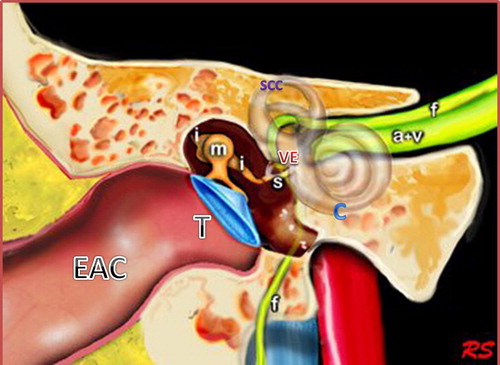
As known, the ear consists of external, middle and inner parts. External ear is formed of the auricle and EAC, which is divided into membranous and bony channels, while middle ear is formed of tympanic cavity and the antrum. The antrum is the air space located superior and posterior to the tympanic cavity having a communicating channel with the epitympanic recess called aditus ad antrum. The middle ear space contains three articulating ossicles (malleus, incus and stapes); that-together with the tympanic membrane-play the major role in sound wave conduction. These conducting structures transfer sound waves to the stapes footplate, which lies in contact with the base of the cochlea in the oval window.Citation9,Citation10
The inner ear consists of the osseous labyrinth which encloses the membranous labyrinth. The osseous labyrinth consists of the vestibule, cochlea, semicircular canals, vestibular aqueduct, and cochlear aqueduct. There is a space between the osseous labyrinth and membranous labyrinth, which is filled with perilymph, in contrast to the membranous labyrinth which is filled with endolymph. The vestibule is the central rounded portion of the osseous labyrinth, which has communication with the cochlea anteriorly, while it communicates the semicircular canals and the vestibular aqueduct posteriorly. It envelopes utricle and saccule, that are parts of the membranous labyrinth. The vestibular aqueduct is a thin linear tube, which contains the endolymphatic duct and sac and communicates the utricle and saccule, it measures 1.5 mm in diameter. The semicircular canals are three canals; superior, lateral and posterior. The cochlea consists of a spiral canal that has 2½ turns around a central column of bone (modiolus). The cochlear aqueduct is a thin osseous canal just inferior and parallel to the internal auditory canal (IAC) ( and ).Citation10,Citation11
8 Patients and methods
This is a prospective temporal bone HRCT scan study, for 60 patients presented with congenital hearing loss, in AL-Mana General Hospital – Saudi Arabia (AGH), from March 2010 to November 2012. The study protocol was approved by the scientific and ethics committee of the hospital, with signed consents from all study candidates’ parents or responsible relatives. First, audiometry studies were performed in audiology department and interpreted by experienced audiologists under the supervision of the Otolaryngology author. All CT images were interpreted, reported and adopted with consensus of the three radiology authors.
Reports were edited carefully fulfilling standardized check list formula, covering all possible affected parts of different ear segments (external, middle and inner). This reporting formula represents expansion of the standardized international scoring system developed by Jahrsdoerfer et al.,Citation12 that was widely applied for external and middle ear anomalies, in attempt for obtaining guidelines for management of these anomalies. These guidelines help selection of candidates with high scores, who were in need for surgical repair with expected good prognostic values.
Scans were made on a 64-section spiral CT scanner (Somatom Sensation; Siemens Medical Solutions, Malvern) and Toshiba Aquillion CX 128 (Toshiba Medical Systems Europe B.V., Zilverstraat 12718 RP Zoetermeer – The Netherlands). The CT scan studies were initially done with axial cuts, using parameters of: 120 kV, 180 mAs, 1-s rotation time, 0.5-mm section thickness, 0.5-mm collimation, 0.2 reconstruction increment, 1-mm table feed and rotation, high resolution algorithm with 512 × 512 matrix, and 9-cm field of view. Bismuth eye shield was routinely used for all patients (AttenuRad; F&L Medical Products Co, USA) for lens protection.
Only three patients who got profound sensorineural hearing loss did additional MRI petrous examination under anesthesia, before cochlear implant procedure. MRI examinations were done through MRI Philips Intera 1.5 T, Closed Magnet, Philips. Medical Systems, 5656 AE Eindhoven – Netherlands.
9 Imaging post-processing
Axial images were transferred and stored through the RIS (Radiology information system) and PACS (Picture archiving and communication system). In the radiology department reporting room, post-processing was done through work station Osirix software (Osirix Foundation California, USA). Multiplanar reconstruction was made to evaluate the different segments and fine structures of the ossicular chain, stapedial footplate, oval and round windows, cochlea, vestibular aqueduct, cochlear aqueduct, semicircular canals and facial nerve and skull base vessels. Evaluation of these structures in the three orthogonal planes (axial, coronal, and sagittal) limits missing of subtle anomalies. For obtaining perfect reconstructed images, the axial cuts were initially done with 1-mm section thickness and an overlap of 0.5 mm and images were displayed at average window values of 800 HU and a window width of 4000 HU.
10 Results
The study included 36 female and 24 male children with age 1–7 years; 19 (31.6%) patients (1–3 y), 15 (25%) patients (3–5 y) and 26 (43.3%) patients (5–7 y) (). 10 patients (16.6%) were presented with conductive hearing loss (CHL), due to EAC atresia, while, another 6 patients (10%) were suffering from CHL due to mixed EAC and middle ear anomalies. 44 patients (73.3%) were complaining of sensorineural hearing loss (SNHL) due to inner ear different part anomalies. Children with CHL were presented at mean age of 1.1 years, in comparison with children with SNHL who were presented at mean age of 4.3 years with significant mean age susceptibility difference (P < 0.005). ()
Table 1 Patients’ age and sex.
Table 2 Type of the deficits.
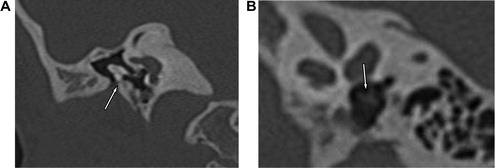
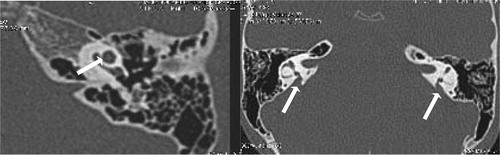
External auditory canal atresia (EACA) was purely found in 10 patients (16.7%) (), while combined EACA and middle ear anomalies were diagnosed in six patients (10%) (–) (). The middle ear anomalies were mainly expressed in the form of ossicular dysplasia/aplasia, however additional important items should be fulfilled in the report. These items include size and aeration of the tympanic cavity, aeration of mastoid cells, any aberrant courses of the facial nerve (found in one patient ()) or the skull base vessels and the degree of development of the round and oval windows (). In SNHL patients – 44 (73.3%) – the findings detected by CT scan were semicircular canal dysplasia or hypoplasia, vestibular aqueduct dilatation, vestibular and cochlear abnormalities, seen in 30%, 63.3%, 31.6%, and 46.6% of patients, respectively. These different inner ear anomalous parts were presented separately or in different combinations. (, –).
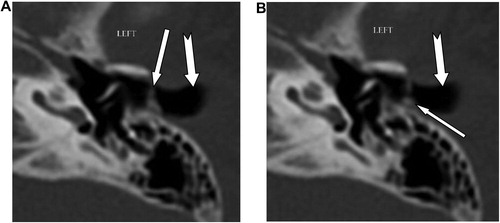
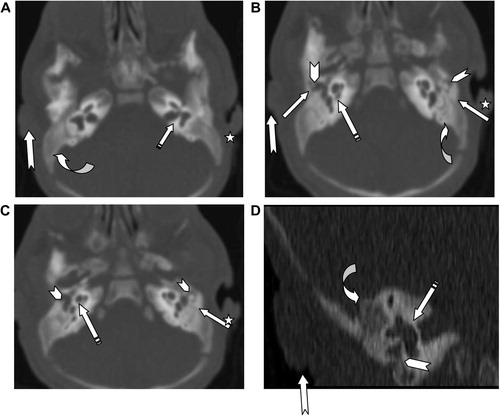
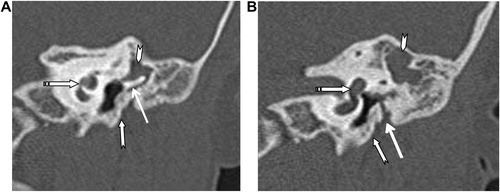
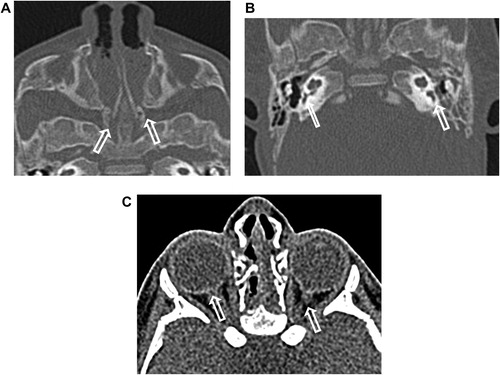
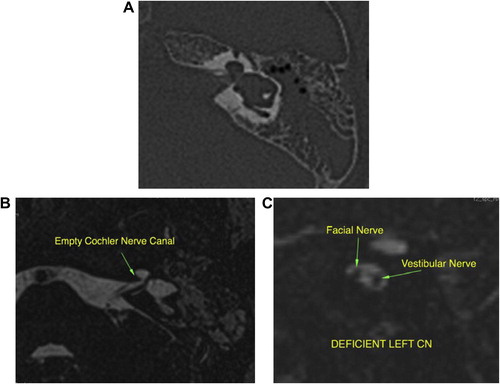
Table 3 Detailed CT findings. CT scan findings of different affected segments of external, middle and inner ears in patients with conductive or/and hearing loss (60 patients).
Using Jahrsdoerfer’s scoring system we classified the severity of conductive hearing loss anomalies in our study, into severe with score <5 in 3 patients (5%), intermediate with score 5–8 in 4 patients (6.7%) and mild with score >8 in 9 patients (15%) ().
Table 4 Jahrsdoerfer scoring of patients with aural atresia and/or Middle ear anomalies.Citation12
Comparative data analysis of the different groups was done through the online website (http://www.graphpad.com/quickcalcs/contingency1.cfm), with application of Fisher’s exact test to estimate P-values that reflect the susceptibility difference of the study groups. P values less than 0.05 are consistent with statistically significant difference.
11 Discussion
Congenital hearing loss is not uncommon congenital anomalies, if not early diagnosed and perfectly managed; it will impede the child speech, language, and cognitive development. This study was planned to check the diagnostic utility of HRCT scan as an imaging tool, in diagnosis and management of children with congenital hearing loss. As expected, children with SNHL were presented at significantly later age groups in comparison with CHL patients. This may be explained by the alarming auricle deformity or the membranous atresia, easily noticed in patients with aural atresia.Citation13
In patients with congenital aural atresia, regardless of the fact that clinical examination is diagnostic for absent external auditory canal and associated microtia, the important role of CT scan examination before surgical repair is the evaluation of the middle ear structures to exclude associated anomalies, that were detected in six patients (10%). So, HRCT scan can perfectly differentiate if it is only external auditory atresia (), or associated with anomalous middle ear structures (–). This could be simply explained by the common embryological branchial origin of these structures. External auditory canal forms from first branchial cleft, malleus and incus from first branchial arch while, mastoid air cells, tympanic cavity and Eustachian tube forms from first branchial pouch.Citation14
In middle ear anomalies, whatever in conjunction with external ear anomaly or isolated, there is a broad variety of ossicular aplasia/dysplasia like bony bridges with malleus and incus fixation, fusion, disjunction or defective parts of the ossicular chain. HRCT scan with post-processing multiplanar reconstruction is highly sensitive for demonstration of these anomalous fine defects of the ossicular structures (–). Patients with severe CHL – 3 patients (5%) – were advised for insertion of titanium prosthesis, which is considered better compared to gold or Teflon, as stated by Singh.Citation15 Reconstructive surgery was not encouraged in such patients due to expected improper prognosis.Citation16
In addition to this ossicular anomaly diagnostic sensitivity, one of the major benefits of preoperative HRCT scan is clear demonstration of the course of the tympanic and mastoid segments of facial nerve. Normally, the tympanic portion of the facial nerve lies below the horizontal semicircular canal and above the anterior margin of the oval window. An aberrant course of facial nerve or skull base vascular structures can be infrequently associated with external and middle ear anomalies, as seen in one of our candidates (). Aberrant intratympanic course of the facial nerve, limits the insertion of prosthesis needed for treatment of middle ear anomaly conductive hearing loss, especially in cases with hypoplastic tympanic cavity. Careful detailed description of the aberrant course of the skull base vessels, is mandatory to protect against life threatening iatrogenic injury of these major vessels.Citation17–Citation19
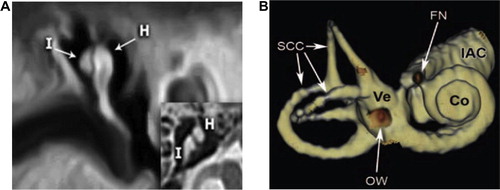
In patients with inner ear anomalies ( and ), cochlear implantation is indicated for severe or profound sensorineural hearing loss in patients who are refractory to conventional hearing augmentation tools. A cochlear implant processes the sound wave received by the device and converts it into an electrical impulse. This process bypasses the severely degenerated hair cells in the organ of Corti and then travels normally along the remainder of the auditory pathway, hence the crucial need to check intact cochlear nerve before implantation. It was previously considered that hypoplastic IAC, which could be easily diagnosed by HRCT scan, is the indicator of cochlear nerve aplasia, as reported by Shelton et al.Citation20
However, as we faced in one patient, it is not uncommon to get inner ear canal of normal caliber, despite cochlear nerve aplasia (). This could be explained by the presence of other components of the vestibulocochlear nerve (vestibular nerve) that still can promote the development of normal caliber of internal auditory canal. High resolution axial and sagittal MRI T2w sequence is the reliable imaging sequence for demonstration of four nervous components of the IAC (facial, cochlear, superior and inferior vestibular nerve). Also, MRI can help in the diagnosis of other associated cranial, ocular, or cervical anomalies that may be found in syndromic congenital SNHL e.g. Apert disease, Cruozon disease, Kleipel Feil syndrome, Hurler syndrome and Waardenburg syndrome.
So, combined HRCT scan and high resolution MRI of the temporal bone are considered a mandatory preoperative investigation tool, for facilitating accurate diagnosis, which guides the ideal management tool. This is consistent with similar data published by Varsha M. Joshi et al.Citation21
12 Conclusion
| - | Regaining the ability to hear has an immeasurable positive psychological and emotional impact on the self-esteem of a young child with congenital hearing loss. | ||||
| - | Temporal bone CT is a good reliable single imaging modality for identifying bony abnormalities of the external auditory canal and middle ear as well inner ear bony labyrinth anomalies. | ||||
| - | The deficiency of cochlear nerve could not be constantly diagnosed by CT scan detected hypoplasia of the bony internal auditory canal, as it may be normal if vestibular nerve components are spared, however it can be clearly diagnosed by MRI. | ||||
| - | Combined HRCT scan and MRI petrous bone are an essential preoperative investigation tool, before any surgical repair procedure. | ||||
13 Limitation of the study
Lack of MRI examination for all candidates—only three did MRI examination—represents limitation of this study as regards radiological evaluation of neural defects in all candidates.
Conflict of interest
None declared.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 January 2014
References
- J.O.PicklesAn introduction to the physiology of hearing4th ed.2012Emerald Group Publishing LimitedBrisbane, Queensland (QLD), Australiap.123–87
- http://alexorl.edu.eg/alexorlfiles/DownLoad%20Lectures/Ear/Physiology%20of%20hearing%20(A.%20Prof.%20Hesham%20Kozou).pdf.
- H.D.CurtinP.C.SanelliP.M.SomTemporal bone: embryology and anatomy4th ed.P.M.SomH.D.CurtinHead and neck imagingvol 22003MosbySt Louis, MO10571075
- R.S.Z.YiinP.H.TangT.Y.TanReview of congenital inner ear abnormalities on CT temporal boneBrit J Radiol842011859863
- N.E.MortonGenetic epidemiology of hearing impairmentAnn N Y Acad Sci63019911631
- Robert Traynor. The Incidence of Hearing Loss Around the World. http://hearinghealthmatters.org/hearinginternational/2011/incidence-of-hearing-loss-around-the-world/.
- Timothy C.Hain. Congenital deafness. http://www.dizziness-and-balance.com/disorders/hearing/cong_hearing.html.
- Girish M.FatterpekarAmish H.DoshiMohitDugarBradley N.DelmanThomas P.NaidichPeter M.SomRole of 3D CT in the evaluation of the temporal boneRadioGraphics262006S117S132
- G.E.ValvassoriImaging of the temporal boneM.F.MafeeImaging of the head and neck2nd ed.2005ThiemeStuttgart–New York67
- Bee E, Smithuis R. Temporal boneAnatomy. http://www.radiologyassistant.nl/en/43facba0911f5 [published: 15.7.2006 and updated: 21.2.2007].
- Grace S.PhillipsSung E.LoGerfoMichael L.RichardsonYoshimiAnzaiInteractive web-based learning module on ct of the temporal bone: anatomy and pathologyRadioGraphics322012E85E105
- R.A.JahrsdoerferJ.W.YeakleyE.A.AguilarR.R.ColeL.C.GrayA grading system for the selection of patients with congenital aural atresiaAm J Otol1311992612
- K.R.BillingsM.A.KennaCauses of pediatric sensorineural hearing loss: yesterday and todayArch Otolaryngol Head Neck Surg1251999517521
- J.M.JohnsonG.MoonisG.E.GreenR.CarmodyH.N.BurbankSyndromes of the first and second branchial arches, part 1: embryology and characteristic defectsAJNR Am J Neuroradiol321 January20111419
- Singh PP. Initial experience with titanium MVP clip prosthesis. In: 4th International Symposium on Middle Ear Mechanics in Research and Otology Zurich July 27–30; 2006.
- A.TjellströmB.HåkanssonG.GranströmBone-anchored hearing aids: current status in adults and childrenOtolaryngol Clin North Am342 (April)2001337364
- R.A.JahrsdoerferTransposition of the facial nerve in congenital aural atresiaAm J Otol1631995290294
- KonradSchwagerReconstruction of middle ear malformationsOtorhinolaryngol Head Neck Surg62007 ISSN 1011-865
- E.SauvagetJ.ParisS.KiciR.KaniaAberrant internal carotid artery in the temporal bone. Imaging findings and managementArch Otolaryngol Head Neck Surg132120068691
- C.SheltonW.M.LuxfordL.L.TonokawaW.W.LoW.F.HouseThe narrow internal auditory canal in children: a contraindication to cochlear implantsOtolaryngol Head Neck Surg1001989227231
- Varsha M.JoshiShantanu K.NavlekarG.Ravi KishoreK.Jitender ReddyE.C.Vinay KumarCT and MR Imaging of the Inner Ear and Brain in Children with Congenital Sensorineural Hearing LossRadioGraphics322012683698