 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A sensitive, reliable and relative fast method has been developed for the determination of total zinc in insulin by atomic absorption spectrophotometer. This designed study was used to optimize the procedures for the existing methods. Spectrograms of both standard and sample solutions of zinc were recorded by measuring the absorbance at 213.9 nm for determination of total zinc. System suitability parameters were evaluated and were found to be within the limits. Linearity was evaluated through graphical representation of concentration versus absorbance. Repeatability (intra-day) and intermediate precision (inter-day) were assessed by analyzing working standard solutions. Accuracy and robustness were experimented from the standard procedures. The percentage recovery of zinc was found to be 99.8%, relative standard deviation RSD 1.13%, linearity of determination LOD 0.0032 μg/mL, and limit of quantization LOQ 0.0120 μg/mL. This developed and proposed method was then validated in terms of accuracy, precision, linearity and robustness which can be successfully used for the quantization of zinc in insulin.
1 Introduction
Insulin is a hormone produced by the pancreas, a large leaf like gland that lies in the duodenum. This hormone is necessary for the metabolism of aldose sugars. Diabetes occurs when the pancreas does not make enough insulin to meet human body needs. Insulin helps to keep blood glucose level nearly at normal.Citation1,Citation2
Zinc plays its role in all stages of insulin metabolism, from production through secretion to utilization and storage. Zinc protects pancreatic beta cells from destruction and its deficiency affects their ability to produce and secrete insulin. Blood glucose level increases if the pancreas does not produce or secrete enough insulin. Decreased zinc concentration in the body has been implicated in lack of insulin sensitivity. In other words the insulin receptors on the beta cells are being inhibited, that means not enough glucose is entering into the beta cells.Citation3,Citation4
The techniques nowadays used to determine zinc in insulin include colorimetry, neutron activation analysis, polarography, X-ray fluorescence, emission spectroscopy, fluorometry and atomic absorption spectrophotometry. Atomic absorption spectrophotometric technique is preferred due to its specificity, sensitivity, precision, simplicity and relatively low cost per analysis.Citation5–Citation6Citation7Citation8
The objective of analytical measurement is to obtain consistent, reliable and accurate data. Validated analytical methods play a major role in achieving this goal. The results from method validation can be used to judge the quality, reliability and consistency of analytical results, which is an integral part of any good analytical practice. Validation of analytical methods is also required by most regulations and quality standards that impact laboratories. The validation measures the different effects in the whole analytical system which influences the result and ensures that there are no other effects which have not been considered.Citation9,Citation10
The purpose of the present study is to realize validation (system suitability, linearity, accuracy robustness and precision) of an existing analytical procedureCitation11 for total zinc determination present in human insulin.
2 Materials and methods
2.1 Reagents and materials
All the reagents used were chemically pure, analytical reagent grade and were used without further purification. Triple distilled water was used throughout this study. Zinc standard stock solution (1000 μg Zn/mL), 6 M and 0.01 M hydrochloric acid solutions were prepared according to the standard procedure. Zinc working solution (0.20–1.20 μg/mL of Zn) was freshly prepared by diluting zinc standard solution (1 mg/mL Zn) with 0.01 M hydrochloric acid. Humulin N Vial (5 mL, 100 IU/mL) of Lilly Pharmaceuticals, Egypt was used. Well shaken insulin vial (1.0 mL containing 100 IU of insulin) was dilute to 100.0 mL with 0.01 M hydrochloric acid.
2.2 Instrumentation
The atomic absorption spectrophotometer (Hitachi model A-1800) was used during this study. For simultaneous analysis, it consists of eight turret lamps with a wavelength range of 190–900 nm. For analysis precision, it has D2 and self reversal background correction with a grating of 1800 gooves/mm. Manufacturer brand Win 2.1 software was used for data integration and processing. The spectroscopic conditions were these; bandwidth 0.4 nm with a 1.0 filter factor and deuterium (D2) background correction. The integration time was 3.0 s set at 5.0 mA lamp current. The analytes were detected at 213.9 nm.
2.3 Method development
2.3.1 Assay method
With the optimized spectroscopic conditions a steady base line was recorded. Standard and sample solutions of zinc were aspirated and spectrograms were recorded by measuring the absorbance at 213.9 nm using a zinc hollow-cathode lamp as a source of radiation and an air-acetylene flame with fuel flow rate of 1600 mL/min. The concentration of the zinc was calculated using the following formula.
(1)
(1)
where ASample = sample absorbance, AStandard = standard solution absorbance, CStandard = concentration of standard solution measured before sample (μg/mL), a = sample volume pipetted for analysis (mL), and b = final volume of sample solution (mL).
2.4 Method validation
2.4.1 System suitability
Standard solutions (0.20–1.20 μg/mL of Zn) were prepared by using a zinc working standard (1 mg/mL Zn) and aspired into the flame of an atomic absorption spectrophotometer. For each sample at least five readings were noted. System suitability parameters were evaluated and found to be within the limits. The purpose of the system suitability test was to ensure that the complete testing system (including instrument, reagents and analyst) is suitable for the intended application. The % relative standard deviation for absorbance from five readings of each zinc standard is presented in . This shows the suitability of atomic absorption spectrophotometer for the determination of zinc in insulin.
Table 1 Evaluation for system suitability of zinc analysis in insulin using AAS.
2.4.2 Linearity
Linearity is evaluated through a graphical representation of ‘concentration’ versus ‘absorbance’, the measured absorbance at λ = 213.9 nm depending on total zinc concentration of zinc standard solutions. shows the necessary data for obtaining the calibration curve, limit of detection and limit of quantization. shows the regression line in the prediction interval. The linearity verifies for total Zn determination through flame atomic absorption spectrometry.
Figure 1 The linearity verification for total Zn determination through flame atomic absorption spectrometry.
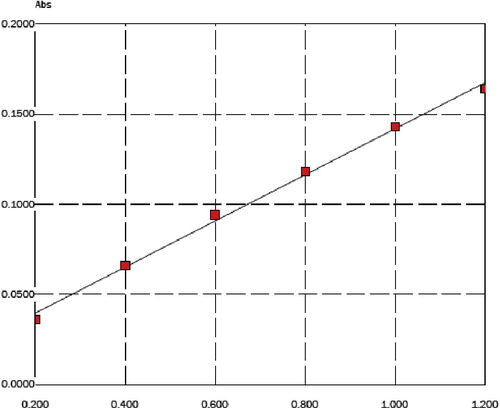
Table 2 Linearity data for analysis of zinc in insulin using AAS.
2.5 Precision
2.5.1 Repeatability (intra-day)
Repeatability is studied by calculating the relative standard deviation (RSD) for five determinations of the concentration of zinc of 0.80 μg /mL, performed on the same day with an interval of an hour under the same experimental and lab conditions. The experimental data with RSD of 0.78% showed that the method is precise. The calculated results of zinc determination in the working standard solution with the relative standard deviation are shown in .
Table 3 Repeatability (intra-day) data for zinc analysis.
2.5.2 Intermediate precision (inter-day)
Intermediate precision includes the estimation of variations in analysis when a method is used within laboratories, on different days. The intermediate precision is assessed by analyzing two working standard solutions on three different days. The obtained RSD values are shown in .
Table 4 Intermediate precision (inter-day) data for zinc analysis.
2.6 Accuracy
In each of the three chosen days, 0.5 mL sample of insulin is correspondingly diluted to 50 mL with 0.01 M hydrochloric acid and the solution is spiked with 0.5, 1.0 and 2.0 mL of 10 μg Zn/mL reference stock solutions. The spiked sample solutions are analyzed according to the analytical procedure and the recovery is calculated with the following equation:
(2)
(2) where SSpiked = calculated zinc in spiked sample (μg/mL) and RReal = zinc in the real sample solution (μg/mL).
The obtained results in the accuracy (recovery) study at total zinc determination from insulin using the flame atomic absorption spectrometry technique are shown in .
Table 5 Percentage recovery of zinc in spiked sample, using spiking levels of 0.100, 0.200 and 0.400 μg/mL.
2.7 Robustness
The spectroscopic conditions are slightly variable like bandwidth from 0.4 nm to 0.2 nm with a filter factor 1.0 and deuterium (D2) background correction. The integration time is changed from 3.0 to 2.0 s. The lamp current is set at 5.0 mA. The analytes are detected at 213.9 nm.
3 Results and discussion
The flame atomic absorption spectrophotometer procedure is optimized to develop a stable assay method. The method is tested for system suitability by aspirating five replicates of zinc samples. An atomic absorption spectrophotometer method is proposed as a suitable method for the determination of zinc in insulin. The best spectroscopic conditions are adequately selected.
The calibration curves for zinc are constructed by plotting the absorbance versus concentration. Linearity is observed in a concentration range from 0.02 to 1.20 μg/mL of zinc with limit of detection 0.0032 μg/mL of zinc and limit of quantitation 0.012 μg/mL of zinc. A linear regression by the least squares method is then applied. The value of the determination coefficient (R2 = 0.99842) showed excellent linearity of the calibration curve for the method.
The precision of an analytical method is the degree of agreement among the individual test results when the method is applied repeatedly to multiple sampling of homologous samples.
Repeatability is studied by calculating the relative standard deviation-RSD for a total of eleven determinations of the concentration of zinc (five 0.80 μg/mL zinc for repeatability and three 0.80 μg/mL zinc and 1.00 μg/mL zinc each for intermediate precision) performed under the same experimental conditions. The results of zinc determinations in the working standard solution with the relative standard deviation calculated are shown in and . Intermediate precision includes the estimation of variations in analysis when a method is used within laboratories but on different days.
The intermediate precision is assessed by analyzing two working standard solutions on three different days (inter day); the RSD values obtained are given in .
The accuracy of an analytical method is based on the closeness of the test results obtained using the proposed method and the true value.Citation12–Citation13Citation14 The accuracy is assessed from five replicate determinations of three different solutions containing 0.10, 0.20 and 0.40 μg/mL of zinc. The absolute means obtained are 98.00%, 101.00% and 100.75% respectively, with a RSD of 1.8217%, 1.3652% and 0.1970% and LOD 0.0032 μg/mL, LOQ 0.0120 μg/mL. These results are given in . It is evident that the method is accurate within the desired range.
The robustness is determined by analyzing the same sample under a variety of conditions. The factors considered were: variation in integration time and band width. The results and the experimental range of the selected variables are given in , together with the optimized values. There were no significant changes in the spectroscopic pattern when the above modifications were made in the experimental conditions, showing that the method is robust. 22.8 μg/mL zinc was determined in insulin marketed by Lilly pharmaceuticals, Egypt.
Table 6 Robustness data for zinc analysis.
4 Conclusion
The data validation shows that the AAS method is accurate, robust and possesses excellent linearity and precision characteristics. The direct dilution method presented here requires less than 2 min per sample. This method can be successfully used for the quantification of zinc as active substance, in dissolution studies and in liquid dosage forms. The dissolution data are reliable and precise. This approach is valid not only for the zinc, but also for other liquid dosage forms, therefore, contributing for the establishment of procedures to assure the quality, safety and efficacy of the developed drug product. This method is cheap and can be used in each laboratory. On the other hand, studies are still going on about its application to auto analytic equipment and routine use.
Conflict of interest
There is no conflict of interest between the authors.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 April 2014
References
- P.KurtzhalsHow to achieve a predictable basal insulin?Diab Metab312005 4S25–33
- J.BrangeL.LangkjoerInsulin structure and stabilityPharm Biotechnol51993315350
- M.J.SalgueiroM.ZubillagaA.LysionekM.I.SarabiaR.CaroT.D.PaoliZinc as an essential micronutrientNutr Res202000737755
- F.VignoliniF.NobiliE.MengheriInvolvement of interleukin-1β in zinc deficiency-induced intestinal damage and beneficial effect of cyclosporineLife Sci621997131141
- G.I.SpielholtzG.C.ToralballaThe determination of zinc in crystalline insulin and in certain insulin preparations by atomic-absorption spectroscopyAnalyst94196910721074
- F.W.SundermanJrAtomic absorption spectrometry of trace metals in clinical pathologyHum Pathol41973549582
- J.C.SmithJr.G.P.ButrimovitzW.C.PurdyDirect measurement of zinc in plasma by atomic absorption spectroscopyClin Chem25197914871491
- Tănase IG, Popescu IL, Pană A. An analytical method validation for atomic absorption spectrometry analysis of total zinc from insulin. Analele UniversităŃii din Bucuresti – Chimie, Anul XV (serie nouă), 2006;1:45–50.
- I.TaverniersM.D.LooseE.V.BockstaeleTrends in quality in the analytical laboratory: I. Traceability and measurement uncertainty of analytical resultsTrends Anal Chem232004480490
- I.TaverniersM.D.LooseE.V.BockstaeleTrends in quality in the analytical laboratory: II. Analytical method validation and quality assuranceTrends Anal Chem232004535552
- European Pharmacopoeia, European Pharmacopoeia Commission, Council of Europe, European Department for the Quality of Medicines, Published by Strasbourg: Council of Europe, 2005.
- ICH harmonised tripartite guideline: validation of analytical procedures: text and methodology, International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, 1996. Retrieved on February 23, 2013, from <http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf>.
- Paul De Bièvre, Helmut Günzle, Validation in chemical measurement. Berlin, Heidelberg, New York: Springer; 2005.
- S.H.L.ValeL.D.LeiteC.X.AlvesM.M.G.DantasJ.B.S.CostaJ.S.MarchiniZinc pharmacokinetic parameters in the determination of body zinc status in childrenEur J Clin Nutr682014203208
