Abstract
Background
C3 complement plays a pivotal role in the complement cascade, subserves several critical functions in human immune response and enhancing bacterial killing and its levels correlate with infectious diseases. However, the association of C3 with recurrent urinary tract infection (UTI) is still debatable.
Aim
The aim of this study was to assess the correlation of serum C3 levels and recurrent UTI among young women.
Materials and methods
Thirty-four recurrent UTI patients whose diagnosed based on Society of Obstetricians and Gynecologists of Canada and European Association of Urology criteria and 34 healthy young women, aged 15–50 years old, were included in this study. Risk factors and clinical manifestations were evaluated and serum C3 levels were measured by ELISA. Correlations of risk factors, clinical manifestation and C3 levels with recurrent UTI were analyzed with chi-square test and Fisher’s exact test or t-test as appropriate with data.
Results
This study found that some risk factors (age [p = 0.000], sexual intercourse frequency [p = 0.00], marital status [p = 0.000] and intrauterine device contraception [p = 0.000]) and clinical manifestations (fever [p = 0.000], dysuria [p = 0.000], frequent urination [p = 0.000], supra-pubic discomfort [p = 0.000] and flank pain [p = 0.006]) were correlated with recurrent UTI. Although this study found that serum C3 levels were significantly different between recurrent UTI patients and healthy young women group (mean 42.08 μg/ml ± 1.20 vs 42.75 μg/ml ± 0.71, p = 0.008), this difference is not clinically relevant.
Conclusion
In this study setting, the levels of C3 among young women with recurrent UTI were lower than healthy women but were not significant clinically.
1 Introduction
Urinary tract infection (UTI) is one of the most common bacterial infections.Citation1 It is estimated that 150 million UTI cases occur per year worldwide, resulting in high direct health care expenditure.Citation2 In general, UTI is self limiting, but has a propensity to recur in particular conditions.Citation3 Young women, even with normal function or anatomy of the urinary tract, commonly suffer from UTI with an estimated incidence of 0.5–0.7 infections per year and 25–30% will develop recurrent UTI.Citation4 Recurrent UTI is an episode of UTI which occurs at least two times in 6 months or three or more re-infections in 12 months with clinical symptoms. Recurrent UTI is one of the main causes of renal scars and increases renal insufficiency, hypertension and morbidity.
Some hypotheses suggest that recurrent UTI related to host immunity, and activation of complement cascade is one of the major immunity components. Activation of complement cascade occurs via three distinct pathways (the classic, alternative, and mannan binding lectin) which can be activated by invading organisms. Activation by any of these pathways leads to form C3 and C5 convertases and as a result it increases the production of biologically active components including anaphylatoxins, opsonins, and membrane attack complex.Citation4,Citation5
C3 plays a pivotal role in the complement cascade, solubilization of immune complexes, bacterial killing through opsonophagocytosis, formation of membrane attack complex, and humoral immune response potentiation.Citation6 However, the role of C3 in recurrent UTI among young women is debatable. Bacteria have been shown to use host immune responses such as complement activation to its advantage in invading the host cells. For example, a study found that mice deficient in C3 gene, when compared with wild-type mice, had a lower infection rate upon inoculation with Escherichia coli in the urinary bladder.Citation7 Furthermore, the study revealed that bacteria opsonization by C3 assisted bacteria invasion to urinary tract cellsCitation4 and inhibition of complement activation reduced tissue damage.Citation7–Citation10 However, studies confirmed that C3 deficient individuals had higher susceptibility to recurrent bacterial,Citation6,Citation11,Citation12 fungalCitation13 and viralCitation14 infection. Therefore, the aim of this study was to determine the association between total serum C3 level and recurrent UTI among young women.
2 Materials and methods
2.1 Subjects
Thirty-four young women, aged 15–50 years old, with recurrent UTI which have been confirmed and 34 healthy young women were included in this study. Recurrent UTI criteria based on Society of Obstetricians and Gynecologists of CanadaCitation15,Citation16 and European Association of UrologyCitation17 were applied. Recurrent UTI criteria were: (a). Uncomplicated UTI that occurred three times or more in 12 months or two times or more in 6 months; (b). No structural and/or functional abnormality of the urinary tract; (c). Mid-stream sample of urine <103 cfu/mL of uropathogen, and (d). Infection was confirmed with mid-stream urine culture. Patients who had ⩾3 urinary symptoms such as dysuria, frequency, urgency, hematuria, back pain, self-diagnosis of UTI, nocturia, costovertebral angle tenderness were anamnesed for deeper investigation. Patients with diabetes mellitus, liver cirrhosis, immunosuppressive diseases, use of immunosuppressive drug, pregnancy, post manipulated bladder, post catheterization, post kidney transplant, and a negative urine culture result were excluded from the study. Healthy young women group were defined as a group of women with no clinical symptom of UTI in the last 3 years and had no sign and symptom of infection at the enrollment time of this study. Healthy young women group consists of doctors, nurses and medical students. The subject recruitment and sample collection were done only after obtaining written informed consent of the participants. The work was carried out in accordance with The Code of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
2.2 ELISA for quantification of total serum C3
During an episode of UTI — after initial diagnose and before any treatment — vein blood samples of recurrent UTI patients were collected. Blood samples from healthy young women group were collected during no sign and symptom of infection. Serums were prepared as described previously.Citation18 Briefly, 2.5 ml blood from each participant was placed in an empty vacutainer tube for serum preparation. Blood samples were allowed to clot at room temperature for 2 h and then tubes were centrifuged. Serums were removed and stored at −70 °C and used before 3 months.
Serum C3 levels were measured by ELISA principle as described previously.Citation19 In brief, 96-well plates were coated overnight at 4 °C with 100 ml of sheep anti-human C3 diluted 1/200 in PBS. After washing, they were blocked with PBS, 2% BSA, at 37 °C for 1 h. Serum samples, diluted 1/200,000 in sample buffer, were added in duplicate. Then rabbit anti-human C3 diluted 1/3000 in sample buffer (PBS, 2% BSA, 0·05% Tween). After incubation at 37 °C for 1 h, horseradish peroxidase-conjugated goat anti-rabbit IgG diluted 1/5000 in sample buffer. The enzyme activity was read after incubation with o-phenylenediamine by measuring absorbance at 490 nm. A pooled normal human serum of known C3 concentration was used to generate a standard curve.
2.3 Statistical analysis
Correlations of risk factors and clinical manifestations with recurrent UTI were analyzed with chi-squared test and Fisher’s exact test or Student’s t-test as appropriate with data. Student’s t-test for comparison was used to compare serum C3 levels between recurrent UTI and healthy young women groups. Values of p ⩽ 0.05 were regarded as significant.
3 Results
3.1 Sample characteristic and risk factors
A total of 68 young women (34 patients with recurrent UTI, mean age was 32 years old; 34 healthy women, mean age was 26.1 years old) were enrolled in this study. Risk factors of recurrent UTI are presented in . Age (p = 0.000), sexual intercourse frequency (p = 0.000), marital status (OR = 13.9, 95% CI: 4.2–46.3) and intrauterine device contraception (OR = 8.6, 95% CI: 0.9–73.9) were correlated with recurrent UTI. In addition, this study found that recurrent UTI had a significant association with fever (OR = 2.7, 95% CI: 1.9–3.8), dysuria (OR = 5.8, 95% CI: 2.9–11.5), frequent urination (OR = 3.4, 95% CI: 2.2–5.3), supra-pubic discomfort (OR = 3.4, 95% CI: 2.2–5.3) and flank pain (OR = 2.3, 95% CI: 1.7–2.9), but not with hematuria (p = 0.120).
Table 1 Risk factors, clinical manifestation and C3 levels between recurrent UTI patients and healthy young women.
3.2 Serum C3 levels and recurrent UTI
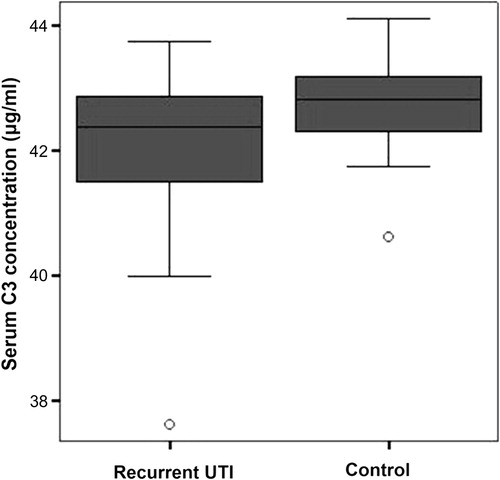
Serum C3 concentrations were measured by using ELISE microplate reader. The average concentrations of serum C3 in recurrent UTI were 42.08 μg/ml ± 1.20, while the average concentrations of serum C3 among healthy young women were 42.75 μg/ml ± 0.71. Statistic analysis found that serum C3 levels of recurrent UTI group and healthy young women group were significantly different (p = 0.008) as shown in . Serum C3 concentrations from recurrent UTI patients and healthy young women are presented in detail in .
4 Discussion
Previous studies found that there were several risk factors of recurrent UTI such as sexual intercourse frequency, use of contraception (intrauterine device, diaphragm and spermicidal), UTI history, use of antibiotic and estrogen, and the anatomy of urinary tract.Citation16,Citation17,Citation20–Citation22 In a six-month cohort study by Foxman et al.Citation23 it was found that recurrent UTI increased among patients who had high intercourse frequency (OR = 1.6, 95% CI: 1.2–2.1) and the usage of intrauterine contraception acceptor (OR = 1.5, 95% CI: 0.9–2.5). This study found that age, marital status, sexual intercourse frequency and the usage of intrauterine device were correlated with recurrent UTI.
Besides those risk factors, host immunity component might be one of the important risk factors of recurrent UTI and one of the important host immunity components is C3. Several studies have been conducted to assess the role of C3 in infectious diseases.Citation6,Citation11,Citation13,Citation14,Citation24 Previous studies found that C3-deficient individuals tend to suffer from infections caused by Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, Neisseria meningitidis, Haemophilus influenza, Enterobacter aerogenes and E. coli.Citation6,Citation11,Citation24 A study, by using mice lacking C3 model, found that C3 was essential for Candida albicans and Candida glabrata resistance and absence of C3 impaired fungal clearance.Citation13 In addition, another study showed that C3 deficient mice (C3−/− mice) were highly susceptible to influenza virus, delayed viral clearance and increased viral titers in the lungs.Citation14 All of these evidences reveal that C3-deficient individuals have higher risk to be infected by microorganisms and infection tends to be recurrent and more severe.
Although there is no study existing in terms of the role of C3 in recurrent UTI, it seems that C3 has detrimental roles in UTI, especially in ascending UTI. Evidence reveals that within the urinary tract system, the presence of bacteria alone is not sufficient to cause ascending infection because bacteria have to traverse urinary tract mucosal before infection can become established. Bacteria might achieve this step by causing epithelial detachment or shedding, or by direct invasion of the epithelial cells.Citation7 Interestingly, this step might be correlated with host complement activation. Studies found that complement activation caused damage of host urinary tract system tissuesCitation25,Citation26 and inhibition of complement activation reduced the inflammatory response and, therefore, reduced the degree of tissues damage.Citation7–Citation10 In addition, study by Springall et al.Citation7 found that C3 deficiency protected mice from ascending renal infection. Their study revealed that renal epithelial cells internalized fewer bacteria in the absence of C3. Moreover, bacteria might use host C3 to invade the renal epithelium via the surface receptors Crry or CD46.Citation7,Citation27
These evidences seem to contradict each other. On one hand, it is clear that C3 opsonizes bacteria, C3 deficiency leads to ineffective clearance of the bacteria and causes severe infection and low serum C3 level increases risk factor for recurrent infections. On the other hand, activation of C3 correlates with higher urinary tract tissue damage and the rate of renal infection. The possible reason for this condition is C3 might have bifunctional property that is dependent on its concentration, similar to bifunctional property of some main cytokines.Citation28
This study found that the levels of C3 among recurrent UTI patients were significantly lower compared with healthy young women. As mentioned in previous studies, low C3 level was one of the risk factors for viral, fungal and bacterial infections.Citation6,Citation11,Citation13,Citation14,Citation24 However, in this study, the levels of C3 either from recurrent UTI and healthy young women groups were very low compared to C3 reference valueCitation29 and the difference of C3 levels between recurrent UTI patients and healthy young women were not significant clinically.
Moreover, in this study, the blood samples from recurrent UTI patients were collected during acute episodes of infection. It means the comparison was made between symptomatic recurrent UTI patients and asymptomatic healthy young women. Previous study found that some cytokines, which were produced during inflammation or infection such as interleukin-1, interleukin-6, and tumor necrosis factor, inferred C3 synthesis within days of the insult.Citation29 Therefore, it seems that our comparison in this study did not appropriate to draw conclusions regarding the association of C3 as risk factor of recurrent UTI but rather C3 dynamic the infection per se. To determine the role of C3 as risk factor of recurrent UTI, serum C3 levels of recurrent UTI patients should be measured after an UTI acute period (during asymptomatic period). In addition, other inflammatory biomarkers such as proinflammatory cytokines and acute phase proteins are important to be measured in order to determine whether the level of C3 in recurrent UTI is correlated with inflammation process or not.
Interestingly, this study revealed that acute episode of UTI among recurrent UTI patients did not increase C3 level. Because C3 plays a central role in activation of classical and alternative complement pathways; therefore, we speculate that complement activation system is low in recurrent UTI. The other possible reasons are complement level can fall to a very low level within a few hours due to the development of immune complexes.Citation29
This study is not, however, without limitations. First, in this study, single nucleotide polymorphisms that can affect C3 levels were not determined. Single nucleotide polymorphisms should be considerate because previous studies found that serum C3 level was affected by the genetic make-up of the individual.Citation30,Citation31 The others, the sample size of this study was relatively small and urine C3 level was not measured. Therefore, further study comparing C3 levels with our recommendation design as mentioned before, with large sample size is required to investigate the potential roles of C3 in recurrent UTI.
5 Conclusion
In conclusion, this study found serum C3 levels are lower among young women with recurrent UTI compared to healthy young women.
Conflicts of interest
Funding: no funding source.
Competing interests: none.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 29 May 2014
References
- B.FoxmanThe epidemiology of urinary tract infectionNat Rev Urol7122010653660
- R.MittalS.AggarwalS.SharmaS.ChhibberK.HarjaiUrinary tract infections caused by Pseudomonas aeruginosa: a minireviewJ Infect Public Health22009101111
- W.E.StammS.R.NorrbyUrinary tract infections: disease panorama and challengesJ Infect Dis183Suppl. 12001S1S4
- R.KucheriaP.DasguptaS.H.SacksM.S.KhanN.S.SheerinUrinary tract infections: new insights into a common problemPostgrad Med J8120058386
- D.RicklinG.HajishengallisK.YangJ.D.LambrisComplement: a key system for immune surveillance and homeostasisNat Immunol112010785797
- S.RamL.A.LewisP.A.RiceInfections of people with complement deficiencies and patients who have undergone splenectomyClin Microbiol Rev2342010740780
- T.SpringallN.S.SheerinK.AbeV.M.HolersH.WanS.H.SacksEpithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coliNat Med772001801806
- M.P.GlauserJ.M.LyonsA.I.BraudePrevention of chronic experimental pyelonephritis by suppression of acute suppurationJ Clin Invest611978403407
- J.A.RobertsJ.K.RothJr.G.DomingueImmunology of pyelonephritis in the primate model. VI. Effect of complement depletionJ Urol1291983193196
- T.ShimamuraMechanisms of renal tissue destruction in an experimental acute pyelonephritisExp Mol Pathol3419813442
- E.S.ReisD.A.FalcãoL.IsaacClinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor HScand J Immunol632006155168
- M.TotanRecurrent pneumococcal meningitis in homozygous C3 deficiencyIndian J Pediatr6972002625626
- S.V.TsoniA.M.KerriganM.J.MarakalalaN.SrinivasanM.DuffieldP.R.TaylorComplement C3 plays an essential role in the control of opportunistic fungal infectionsInfect Immun779200936793685
- M.KopfB.AbelA.GallimoreM.CarrollM.F.BachmannComplement component C3 promotes T-cell priming and lung migration to control acute influenza virus infectionNat Med842002373378
- A.EppA.LarochelleD.LovatsisJ.E.WalterW.EastonS.A.FarrellSociety of Obstetricians and Gynaecologists of Canada. Recurrent urinary tract infectionJ Obstet Gynaecol Can32201010821101
- S.DasonJ.T.DasonA.KapoorGuidelines for the diagnosis and management of recurrent urinary tract infection in womenCan Urol Assoc J552011316322
- Grabe M, Bjerklund-Johansen TE, Botto H, Çek M, Naber KG, Tenke P, et al. Guidelines on urological infections. European Association of Urology 2010. Available from: http://www.uroweb.org/gls/pdf/Urological%20Infections%202010.pdf. [accessed on 01.08.2012].
- N.HussainG.JafferyS.HasnainSerum complement C3 and C4 levels in relation to diagnosis of lupus nephritisTrop J Pharm Res74200811171121
- S.TangW.ZhouN.S.SheerinR.W.VaughanS.H.SacksContribution of renal secreted complement C3 to the circulating pool in humansJ Immunol1627199943364341
- D.ScholesT.M.HootonP.L.RobertsA.E.StapletonK.GuptaW.E.StammRisk factors for recurrent urinary tract infection in young womenJ Infect Dis1824200011771182
- A.V.FrancoRecurrent urinary tract infectionBest Pract Res Clin Obstet Gynaecol1962005861873
- S.GhazalM.MusmarM.AL-TellEpidemiology of aerobic bacterial infections among IUD (intrauterine device) users in the Northern West BankAn-Najah Univ J Res (N Sc)18120041324
- B.FoxmanRecurring urinary tract infection: incidence and risk factorsAm J Public Health801990331333
- J.E.FigueroaP.DensenInfectious diseases associated with complement deficienciesClin Microbiol Rev41991359395
- Y.HoriK.YamadaN.HanafusaT.OkudaN.OkadaT.MiyataCrry, a complement regulatory protein, modulates renal interstitial disease induced by proteinuriaKidney Int56199920962106
- Y.MoritaA.NomuraY.YuzawaK.NishikawaN.HottaF.ShimizuThe role of complement in the pathogenesis of tubulointerstitial lesions in rat mesangial proliferative glomerulonephritisJ Am Soc Nephrol8199713631372
- K.LiM.J.FeitoS.H.SacksN.S.SheerinCD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coliJ Immunol1774200625432551
- H.HarapanJ.K.FajarN.WahyuniatiJ.R.AnandL.NambaruK.F.JamilNon-HLA gene polymorphisms and their implications on dengue virus infectionEgypt J Med Hum Genet142013111
- R.F.RitchieG.E.PalomakiL.M.NeveuxO.NavolotskaiaT.B.LedueW.Y.CraigReference distributions for complement proteins C3 and C4: a practical, simple and clinically relevant approach in a large cohortJ Clin Lab Anal181200418
- M.HeurichR.Martínez-BarricarteN.J.FrancisD.L.RobertsS.Rodríguez de CórdobaB.P.MorganCommon polymorphisms in C3, factor B, and factor H collaborate to determine systemic complement activity and disease riskProc Natl Acad Sci USA10821201187618766
- X.YangJ.SunY.GaoA.TanH.ZhangY.HuGenome-wide association study for serum complement C3 and C4 levels in healthy Chinese subjectsPLoS Genet892012e1002916