Abstract
Background: Cell death pathway can occur under physiological or pathological conditions. In vitro and in vivo studies have shown that d-galactosamine (DGA) induces hepatocyte damage. Objective: The present study aims to evaluate the protective effect of prostaglandin E1 (PGE1) on DGA-induced apoptosis, necrosis and oxidative stress in primary culture of human hepatocytes. Methods: Normal human hepatocytes were obtained from the safety margin of liver specimens, removed during hepatectomy operation to liver cancer patients, and isolated using the classical collagenase perfusion method. After culture stabilization, PGE1 (1 μM) was added 2 h before DGA (5 mM). Cultures were maintained for 24 h before the parameters for apoptosis, necrosis and oxidative stress were measured. Apoptosis was studied by DNA-fragmentation, neutral (nSMase) and acid (aSMase) sphingomyelinase and caspase-3 activity. Necrosis was investigated by lactate dehydrogenase (LDH) and transaminases (ALT & AST) enzymes. The oxidative stress was assessed by malondialdehyde (MDA), glutathione (GSH), oxidized glutathione (GSSH), glutathione S-transferase (GST), glutathione peroxidase (GSPx), catalase (CAT), superoxide dismutase (SOD) and nitric oxide (NO). Results: The hepatotoxin DGA induced apoptosis and enhanced all parameters related to necrosis and intracellular oxidative stress. On the other hand, PGE1 reduced the measured values for the parameters indicative for the DAG induced apoptosis, necrosis and oxidative stress. In addition, PGE1 proved also to prevent GSH depletion. The obtained results provided evidences for the biochemical hepatotoxic effects of DGA (5 mM) especially through the induction of apoptosis, necrosis and oxidative stress alterations in the cultured human hepatocytes. Conclusion: PGE1 could be a useful protective treatment against DGA-induced hepatocyte cell death.
1 Introduction
The liver is the principle organ involved in the metabolism and detoxification of biological toxins and medicinal agents. Such metabolism is always associated with the disturbance of hepatocyte biochemistry and generation of reactive oxygen species (ROS).Citation1 The oxidative stress is an important mechanism responsible for dysfunction of liver.Citation2 There is a wide range of liver pathologies ranging from sub-clinical hepatitis up to necro-inflammatory hepatitis, cirrhosis and carcinoma that have been proved to be associated with the redox imbalance and oxidative stress.Citation3
Apoptosis and necrosis are two different types of cell death. Necrosis is a non-controlled cellular disruption as a consequence of extreme noxious irreversible conditions.Citation4 In contrast, apoptosis is a genetically controlled cell death pathway that can occur under physiological or pathological conditions. It plays an important role in removing redundant, damaged or infected cells and has a vital role in maintaining homeostasis of liver tissue as well as during the pathogenesis of liver diseases.Citation5
DGA is an amino-sugar with unique hepatotoxic properties in mammals.Citation6 The mechanism for DGA-induced liver injury, although poorly understood, seems to be partly related to the immune system.Citation7 Its toxic effects are connected with insufficiency of UDP (uracil nucleotides)-sugar “UDP-glucose and UDP-galactose”, release of pro-inflammatory mediators (such as TNF-α from Kupffer’s cells), loss of intracellular calcium homeostasis and altering uridine-pool in hepatocytes.Citation7,Citation8 These changes affect cell membranes, organelles and the synthesis of proteins and nucleic acids. DGA, at higher dose, inhibits the energy metabolism of hepatocytes, destroys the enzymes involved in the transport of substrates to the mitochondria and modifies the phospholipid composition of membranes.Citation9
DGA is commonly used in experimental model for induction of hepatocellular injury. A 5 mM dose of DGA has been established as the dose which potentially induces apoptosis, necrosis and alters cultured human hepatocytes functions.Citation10,Citation11
Prostaglandins are biologically active polyunsaturated fatty acids derived from arachidonic acid present in most mammalian tissues. They have been suggested to be involved in membrane stabilization. In the liver, prostaglandins were found to be involved in hepatocyte proliferation and vital defence processes such as inflammation, tissue repair and immune responses.Citation12,Citation13 PGE1 was found to be effective as delivering drug selectively to hepatocytes.Citation14 Several studies have been conducted to assess the ability of PGE1 to reduce in vivo and in vitro DGA-induced cell death in cultured rat hepatocytes.Citation15 There were growing evidences that changes in intracellular redox state appear to regulate critical biological responses. ROS influence signals transduction and transcription-factors, and play a central role in the pathophysiology burden of liver injury in this sense. Propagation of the oxidative stress induced intracellular reactions further shifts cell death from apoptotic to necrotic pathways in DAG-treated rats’ hepatocytes.Citation16
The present study aimed at exploring the intracellular biochemical alterations in cultured human hepatocytes that are involved in the induction of apoptosis, necrosis and oxidative stress by 5 mM of the hepatotoxin DAG. It also aimed at assessing the possible protective effect of PGE1 against the progression of the DAG-induced damage in the cultured human hepatocytes.
2 Materials and methods
This study was approved by the Ethics Committee of Medical Research Institute, Alexandria University. All reagents used in this study were supplied by Sigma–Aldrich Chemical (St. Louis, MO, USA) unless otherwise stated.
Human hepatocytes were isolated from normal hepatic parenchyma at the apparently free margin around lesions obtained during hepatectomies done at the department of Surgery, Medical Research Institute, Alexandria University. A written informed consent was obtained from all participants before surgery including consent to hepatectomies and to use their resected specimen in this study. All patients were thoroughly investigated assessing the hepatic functions, abdominal ultrasound and triphasic multidetector C.T. for abdomen and pelvis were done. The included patients were six; three men and three women with 40 ± 8 years old. Hepatectomies were done in three cases of synchronous non-anatomical resection of colorectal liver metastasis (one case with cancer sigmoid and solitary lesion at segment IV b and the other two cases with cancer rectum and having metastasis at segments V and VIII) and the other cases; anatomical left lateral segmentectomy was done for two patients who had hepatocellular carcinoma (HCC) involving segments II and III and the last case had an adenoma at segment IV b. We excluded patients with Hepatitis-C virus, Hepatitis-B, patients with liver cirrhosis or periportal fibrosis.
A crush clamp techniqueCitation17 using Kelly clamp was used for parenchymal transection to fracture the parenchyma and expose the vessels. Sealing of the vessels was done using monopolar diathermy, bipolar diathermy or harmonic scalpel (Ethicon EndoSurgery, Cincinnati, OH, USA) and wide caliber vessels were secured with ties or clipping. Bleeding from hepatic parenchyma was secured using monopolar diathermy and Argon Plasma Coagulation.Citation18
2.1 Preparation of primary hepatocytes and cell culture
The human hepatocytes were isolated (in immunology lab.) by in situ collagenase perfusion of liver samples according to the method described by Ferrini et al.Citation19 Liver samples were first perfused with a non-recirculating washing solution I, pH 7.4 at a flow of 75 ml/min in order to remove blood cells. Afterward, they were perfused with a non-recirculating chelating solution II, pH 7.4 at a flow of 75 ml/min for 10 min then with recirculating isolation solution III at a flow of 75 ml/min. for 10 min. Cell suspension was filtered through Nylon mesh (250 μm) and washed three times at 50 g for 5 min at 4 °C in culture medium (DEM:Ham-F12 and William’s E mediums (1:1) supplemented with 26 mM NaHCO3, 15 mM HEPES, 0.2 g/l glutamine, 50 mg/l vitamin C, 0.04 mg/l dexa-methasone, 2 mg/l insulin, 200 mg/l glucagon, 50 mg/l transferrin, 4 ng/l ethanolamine).
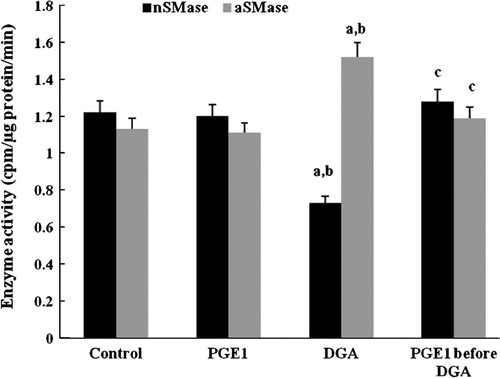
Cell viability was consistently more that 85% as determined by trypan blue dye exclusion technique, based on impermeability of viable cells to trypan blue. The number of viable lymphocytes/ml was calculated according to the following equation: % of viable cells = (no of viable cells/total no counted) × 100. Contamination of hepatocyte cultures with kupffer cells was not detected morphologically, through latex bead ingestion (3 μm). Hepatocytes (150,000 cells/cm2) were seeded in type I collagen coated dishes (Iwaki, Gyouda, Japan) and cultured in medium containing 5% fetal calf serum for 4 h. Afterward, the medium was removed and replaced by fresh culture medium without fetal bovine serum. The study was initiated 24 h after seeding of the cells to allow stabilization of the culture. After stabilization of cell culture, PGE1 (1 μM) was added 2 h before DGA (5 mM) and the cultures were maintained for 24 h before the determined biochemical parameters were measured. Apoptosis was studied by DNA-fragmentation, neutral (nSMase) and acid (aSMase) sphingomyelinase and caspase-3 activity. Necrosis was investigated by lactate dehydrogenase (LDH) and transaminases (ALT & AST) enzymes. The oxidative stress was assessed by malondialdehyde (MDA), glutathione (GSH), oxidized glutathione (GSSH), glutathione S-transferase (GST), glutathione peroxidase (GSPx), catalase (CAT), superoxide dismutase (SOD) and nitric oxide (NO).
2.2 DNA fragmentation in hepatocytes
The fragmentation of DNA in hepatocytes was evaluated using electrophoresis as described by Siendones et al.Citation20 The whole hepatocyte population was treated with 1 ml of lysis buffer, pH 8.0, at 4 °C for 10 min. Samples were incubated with RNAse (50 μg/ml) at 37 °C for 2 h and proteinase K (100 μg/ml) at 48 °C for 45 min. DNA was obtained by phenol:chloroform:isoamyl alcohol (25:24:1) (Sigma Chemical Co.) extraction and precipitated with cold isopropanol (1:1) at 20 °C for 12 h. DNA was recovered by centrifugation of the sample at 10,000g at 4 °C for 10 min. Thereafter, the precipitate was washed with 70% ethanol, dried, and resuspended in Tris–EDTA buffer (10 mM Tris, 1 mM EDTA) at pH 8.0. Samples (100 μg DNA) were analyzed on 1% agarose gel with ethidium bromide.
2.3 Caspase-3-associated activity
Hepatocytes were treated with 1 ml of lysis solution at 4 °C for 10 min, transferred to eppendorf tubes, and centrifuged at 10,000g at 4 °C for 5 min. Caspase-3 associated activity was measured in samples (25 μg protein) by a fluorometric assay using the peptide-based substrate ac-N-acetyl-Asp-Glu-Val-Asp-AFC (Ac-DEVD-AFC) (Bachem, Bubendorf, Switzerland).Citation21
2.4 Assays for sphingomyelinase activities
The measurement of neutral (nSMase) and acid (aSMase) sphingomyelinase activities was carried out following the procedure described by Martin et al.Citation22
2.5 Measurement of LDH-release and transaminases (ALT & AST) activities
Lactate dehydrogenase (LDH) was measured by a colorimetric routine laboratory method.Citation23 Volume of cell lysate ranging from 50 to 200 ml was incubated with 0.2 mM β-NADH and 0.4 mM pyruvic acid diluted in PBS, pH 7.4. LDH concentration in the sample was proportional to the linear decrease in the absorbance at 334 nm. LDH concentration was calculated using commercial standard. Alanine transaminase (ALT) and aspartate transaminase (AST) were estimated according to Bergmeyer and Bernt.Citation24
2.6 Evaluation of lipid peroxidation and quantification of glutathione (GSH) and oxidized glutathione (GSSG) longer
The presence of malondialdehyde (MDA) in culture medium was used as an index of lipid peroxidation in hepatocytes following TBARS “thio-barbituric acid reactive substances” assay.Citation25 GSH, GSSH were quantified in hepatocytes following the procedure described by Teare et al.Citation26 Liver samples (∼100 μg) were homogenized in ice-cold 0.1 M phosphate buffer (pH 7.4). For GSH content, the homogenate was immediately mixed with sulfosalicylic acid, shaked well, centrifuged, and 100 μl of the supernatant was added to 9.9 ml water and spectrophotometric absorbance was recorded at 412 nm. For GSSG, 200 μl supernatant was added to 3.78 ml water to which 40 μl of 2-vinylpyridine was mixed to mask the GSH and left at room temperature for 3 h before estimation.
2.7 Glutathione S transferase (GST) and glutathione peroxidase (GSPx) assays
GST and GSPx activities were measured as described by Habig et al.Citation27 and Awasthi et al.Citation28, respectively. GST activity was assayed, using 1-chloro-2, 4-dinitrobenzene (CDNB) and 3, 4-dichloronitrobenzene (DCNB), by spectrophotometer at room temperature by monitoring the change in absorbance at 344, 270, and 344 nm.Citation27 The activity of GSPx was assayed in a l-ml system containing 0.1 M potassium phosphate buffer, 0.2 mM NADPH, 1 i.u. glutathione reductase, 4 mM GSH, 4 mM EDTA, 4 mM sodium azide, and 0.02 ml glutathione peroxidase. The mixture was incubated at 37 °C for 10 min after which 10 μl of 10 mM t-butyl hydroperoxide was added and the rate of reaction was measured using spectrophotometer.Citation28
2.8 Catalase (CAT) and superoxide dismutase (SOD) activities
CAT was assayed colorimetrically at 620 nm as described by Tawfik et al.Citation29 SOD activities were determined according to the methods of Pottathil et al.Citation30
2.9 Nitric oxide (NO) metabolite measurement
The release of NO was indirectly assessed by measuring the accumulated nitrite using the Griess reaction as described by Green et al.Citation31 Nitrite was detected and analyzed by formation of a red pink color upon treatment of a NO2−-containing sample with the Griess reagent.
2.10 Protein assay
Total protein concentration was determined by the Bio-RadCitation32 assay, using bovine serum albumin as standard. Measurements were done on UV Spectrophotometer at 595 nm.
3 Statistical analysis
The Data were collected and entered into the personal computer. Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) version 20. Statistical significance was set at (P-value <0.05). Results are expressed as means ± SD of duplicate experiments. Differences between groups were assessed by one-way analysis of variance (ANOVA) using the least significant differences (LSD) test as multiple comparison analysis according to Berenbaum.Citation33
4 Results
The pathological examination of the resected specimen revealed the same pathology expected preoperatively with free safety margin in all cases. The histopathological reports revealed; metastatic adenocarcinoma from colorectal cancer in three cases, Fibrolamellar variant of HCC in two cases and an adenoma in the last case.
The effect of DGA-treatment (5 mM) revealed a general increase in the biochemical quantitation of the parameters for apoptosis, in the cultured hepatocytes. DGA increased the measured arbitrary units from apoptosis expressing DNA fragmentation by gel electrophoresis (: 1232 ± 114, 1241 ± 117, 2689 ± 141a,b, and 1312 ± 141c, for control, PEG1 treatment, DGA treatment and PEG1 treatment before DGA addition, respectively). Caspase-3-associated activity in cultured hepatocytes was expressed with the highest values in cultures treated with DGA. These values were reduced nearly by 50% when PGE1 was added before treatment with DAG ().
Figure 1 1.5% agarose gel showing the effect of PGE1-treatment on DNA-fragmentation due to DGA-induced injury in cultured human hepatocytes. “C” refers to control.
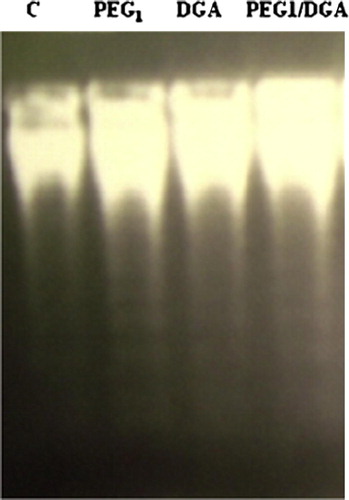
Figure 2 Effect of PGE1-treatment on caspase-3-associated activity (absorbance/h/mg protein) due to DGA-induced injury in cultured human hepatocytes (a significantly different among control group, b significantly different among PGE1 group, c significantly different among DGA group).
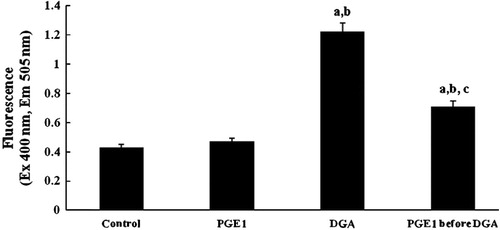
Sphingomyelinase (nSMase and aSMase) activities denote cellular levels of ceramide at the indicated times. They decreased significantly in DGA-group. PGE1-treatment restored sphingomyelinase activities within normal range ().
Figure 3 Effect of PGE1-treatment on nSMase and aSMase (cpm/μg protein/min) activities due to DGA-induced injury in cultured human hepatocytes (a significantly different among control group, b significantly different among PGE1 group, c significantly different among DGA group).
Levels of transaminases (ALT, AST) and LDH were used as indicators to evaluate the attribution of PGE1 to the structure damage of the hepatocytes. In the present study, the enzyme assays of transaminases showed that a toxic dose of DGA significantly raised the both levels of ALT and AST. PGE1 could inhibit the enzyme activities effectively. The ALT and AST level decreased significantly compared to control group ().
Figure 4 Effect of PGE1-treatment on LDH (mIU/L), ALT and AST (IU/L) activities due to DGA-induced injury in cultured human hepatocytes (a significantly different among control group, b significantly different among PGE1 group, c significantly different among DGA group).
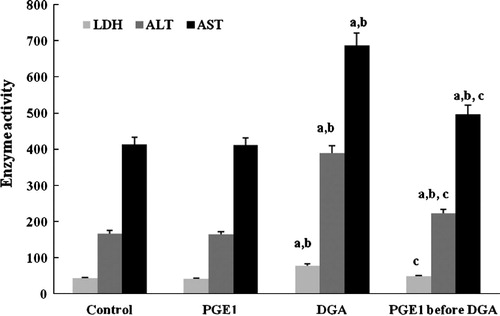
Depletion of intracellular-GSH and the increase in lipid peroxidation as MDA accumulation and GSSH in cells () were used to assess the onset of oxidative injury induced by DGA. PGE1 abolished GSH depletion and exhibited remarkable lessening of raised MDA and GSSH ().
Figure 5 Effect of PGE1-treatment on MDA, GSH and GSSH (nmol/μg protein) contents due to DGA-induced injury in cultured human hepatocytes (a significantly different among control group, b significantly different among PGE1 group, c significantly different among DGA group).
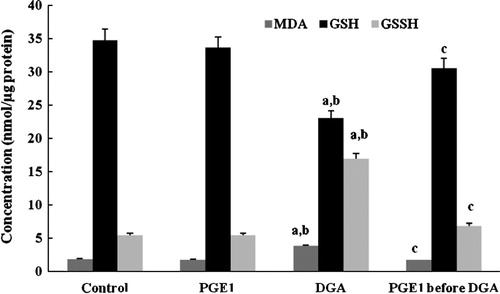
As shown in and , a single dose of DGA induced severe oxidative damage and the GST, GSPx, CAT and SOD activities decreased markedly. PGE1 proved to normalize the enzyme activities of the four indexes.
Figure 6 Effect of PGE1-treatment on total GST and GSPx (U/mg protein) activities due to DGA-induced injury in cultured human hepatocytes (a significantly different among control group, b significantly different among PGE1 group, c significantly different among DGA group).
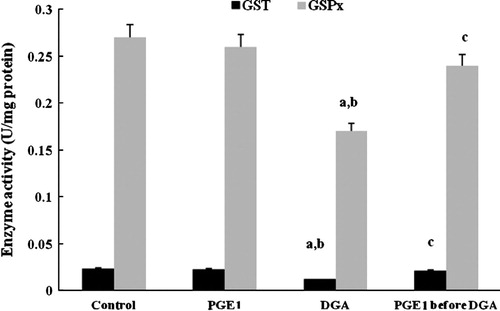
Figure 7 Effect of PGE1-treatment on CAT (a) and SOD (b) (U/μg protein) activities due to DGA-induced injury in cultured human hepatocytes (a significantly different among control group, b significantly different among PGE1 group, c significantly different among DGA group).
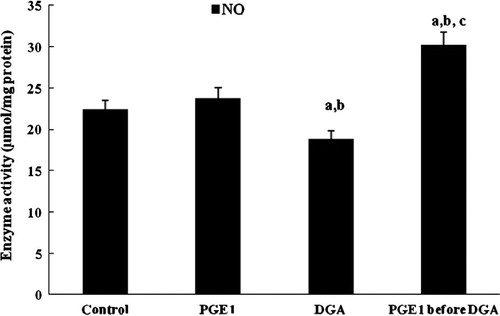
Similar results were obtained in case of the nitric oxide (NO) concentration (), PGE1 restored nitric oxide depletion caused by DGA hepatotoxin.
5 Discussion
ROS, products of normal metabolism, cause oxidant injury if they accumulate in pathological amounts.Citation34 Under normal conditions, where the levels of ROS are tightly regulated and play a role in normal metabolism, the body’s antioxidant defences are able to successfully manage non-pathological amounts of exogenous or endogenous oxidants from reactive oxygen/nitrogen species.Citation35 Among therapeutics for liver diseases, protective drugs have attracted more and more attentions, such as antioxidant prevention approaches. The environment can be a source of excess oxidants, which can cause both acute and chronic cellular injury.Citation36 Moreover, ROS were thought to be involved in several cellular mechanisms including apoptosis.Citation37
Hepatic injury induced by DGA is a suitable experimental model of human liver failure.Citation10 It induces an intense oxidative stress in hepatocytes.Citation16 The present work shows that DGA-induces apoptosis followed by necrosis in cultured human hepatocytes. The identification of DNA fragmentation and caspase-3 activity modifications induced by DGA in human hepatocytes can help in the insight of the causal molecular mechanisms responsible for the induction of apoptosis and necrosis.
Apoptosis, known as programmed cell death, is a cellular self-destruction mechanism involved in a variety of biological events such as developmental remodeling, tissue homeostasis and the removal of unwanted cells in most living tissues, including the mammalian liver. Similar to other organs, it plays a role in the pathogenesis of hepatic failure and organ dysfunction after liver ischemia/reperfusion injury.Citation38 Siendones et al.Citation5 found that generation of ROS with perturbation of pro- and anti-oxidant ratio caused alteration in mitochondria structure and hepatocytes membrane potential.
Activation of caspases has been demonstrated to be involved during in vivo and in vitro hepatocytes’ apoptosis.Citation39 Induction of apoptosis by DGA was related to a significant increase in caspase-3 processing and activity observed in hepatocytes.Citation5 Genetic and biochemical studies have uncovered a functional network of cell death regulators.Citation38 It has shown that mammals have two distinct apoptosis signaling pathways. One pathway is activated by “death receptors” (a subgroup of the tissue nuclear factors family (TNF)). The other is initiated by certain cytotoxic stress.Citation4 Excessive generation of ROS and caspase activation have been shown to be essential in cell apoptosis.Citation40
Mammalian sphingomyelinase (SMase) hydrolyzes the phosphodiester bond in sphingomyelin (SM), yielding ceramide and phosphorylcholine. SMase is transiently activated in response to a variety of extra cellular stimuli, which leads to increased cellular levels of ceramide. Ceramide is a member of a group of lipid signaling molecules and has a specific role in the onset of different stress responses, including cell growth arrest, inflammation, and apoptosis.Citation41 In the present study, sphingomyelinase decreased markedly in DGA group. Garcia et al.Citation42 have reported that GSH has a role in the regulation of nSMase so GSH depletion sensitizes hepatocytes to the aSMase-induced apoptosis. Osawa et al.Citation43 have concluded that activation of aSMase contributed to hepatocyte apoptosis.
MDA level is a main marker of endogenous lipid peroxidation.Citation25 Besides, successful protection of liver damage by efficiently inhibiting MDA formation and decreasing ALT and AST, PGE1 could enhance the activities of antioxidant enzyme system of the hepatocyte cells, including LDH ().
The replenishment of GSH may occur via reduction of GSSG by glutathione reductase or by de novo synthesis of GSH from its constituent amino acids.Citation44 It plays a key protective role against oxidant-induced cell death.Citation45 There was a significant GSH-depletion and GSSG-formation after liver reperfusion injury.Citation2 GSH exhibits a large panel of actions in controlling apoptosis mechanisms or membrane transport.Citation46 Usually GSSG represents only 1–10% of the total liver GSH.Citation47 In the present study, liver GSSG in the DGA treated hepatocytes culture plate accounted almost 50% of total GSH, suggesting that there was a significant intracellular oxidative stress during the DGA treatment.
SOD and GSPx are intracellular antioxidant enzymes that protect against oxidative stress. Hepatocytes showed susceptibility to oxidative injury with depletion of antioxidant defences, exemplified by decreased GSH and CAT and raised MDA.Citation30,Citation48 It has been suggested that induction of GST enzyme was a major cell protective mechanism and accompanied by elevation of intracellular GSH level.Citation13
Our results indicate that PGE1 has a positive hepatoprotective impact on the DGA induced lesion. Since antioxidant enzymes such as SOD and GSPx are considered to be a primary defence system for oxidative damage prevention. PGE1 exerts antioxidant effect not only through its own radical scavenging activity but also, by boosting the host antioxidant enzyme system. On the other hand, we found that several indexes showed a significant change. GST, GSPx, CAT and SOD activities were raised significantly on addition of PGE1 to the cultured hepatocytes.
Massive intervention into the redox state by pharmaceutical doses of exogenous antioxidants should be considered with caution due to the indefinite role of free radicals in regulation of apoptosis and cytotoxicity of injured cells.Citation49 Our results showed that DGA could increase ROS generation, but PGE1 could reverse such effect by lowering the level of intracellular ROS.
It has previously shown that NO mediates apoptosis by DGA in primary culture of rat hepatocytes and has been recognized as a critical mediator in numerous biological processes.Citation50 Some chemical aspects of NO molecule determine its free radical capacity to mediate cell signaling. In addition, it has been shown to exert a noxious effect in the initiation and progression of cell death.Citation51
Similar studies have evidenced that PGE1 pre-administration protects against NO-dependent cell death through a rapid increase of inducible-NO synthase (NOS-2) expression which protected against DGA-induced cell death.Citation50,Citation52 The factors that inhibit hepatic injury may be, those intensifying the hepatic antioxidants or free radical scavengers and those inducing hepatocyte regeneration.
6 Conclusion
PGE1 can effectively protect the hepatocytes from oxidation induced death through scavenging intracellular oxidative damage induced by ROS.
Conflict of interest
There is no conflict of interest or financial ties to include.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 5 January 2015
References
- J.C.Fernandez-ChecaN.KaplowitzHepatic mitochondrial glutathione: transport and role in disease and toxicityToxicol Appl Pharm2042005263273
- T.FilhoC.CorsoM.ZanotelliC.MarroniA.BrandaoE.SchlindweinLiver glutathione depletion after preservation and reperfusion in human liver transplantationActa Cirurgica Brasileira212006223229
- J.VrbaM.ModrianskOxidative burst of kupffer cells: target for liver injury treatmentBiomed Pap14620021520
- A.StrasserL.O’ConnorV.M.DixitApoptosis signalingAnnu Rev Biochem692000217245
- E.SiendonesY.Jimnez-GomezJ.L.MonteroC.Gomez-DiazJ.M.VillalbaJ.MuntanPGE1 abolishes the mitochondrial-independent cell death pathway induced by d-galactosamine in primary culture of rat hepatocytesJ Gastroenterol Hepatol202005108116
- R.SilversteinReview: d-galactosamine lethality model: scope and limitationsJ Endotx Res102004147162
- H.IzuK.HizumeK.GotoM.HirotsuneHepatoprotective effects of a concentrate and components of sake against galactosamine (GalN)-induced liver injury in miceBiosci Biotechnol Biochem712007951957
- T.MorikawaH.MatsudaK.NinomiyaK.YoshikawaMedicinal foodstuffs. XXIX. Potent protective effects of sesquiterpenes and curcumin from zedoariae rhizoma on liver injury induced by d-galactosamine/lipopolysaccharide or tumor necrosis factor-αBiol Pharm Bull252002627631
- R.FerencikovaZ.CervinkovaZ.Drahota1Hepatotoxic effect of d-galactosamine and protective role of lipid emulsionPhysiol Res5220037378
- A.Rodriguez-ArizaL.M.Lopez-SanchezR.GonzalezF.J.CorralesP.LopezA.BernardosAltered protein expression and protein nitration pattern during d-galactosamine-induced cell death in human hepatocytes: a proteomic analysisLiver Int25200512591269
- A.S.SreedharP.CsermelyHeat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive reviewPharmacol Ther1012004227257
- J.MuntaneF.J.RodriGuezO.SegadoA.QuinteroJ.M.LozanoE.SiendonesTNF-α-dependent production of inducible nitric oxide is involved in PGE1 protection against acute liver injuryGut472000553562
- Y.KawamotoY.NakamuraY.NaitoY.ToriiT.KumagaiT.OsawaCyclopentenone prostaglandins as potential inducers of phase ii detoxification enzymesJ Biol Chem275120001129111299
- M.NishikawaDevelopment of cell-specific targeting systems for drugs and genesBiol Pharm Bull282005195200
- A.Abou-ElellaE.SiendonesJ.PadilloJ.L.MonteroM.De la MataJ.MuntaneTNF-alpha and nitric oxide mediate apoptosis by d-galactosamine in primary culture of rat hepatocytes. Exacerbation of cell death by co-cultured Kupffer cellsCan J Gastroenterol162002791799
- A.QuinteroC.A.PedrazaE.SiendonesA.M.ElsaidA.ColellC.Garcia-RuizPGE1 protection against apoptosis induced by d-galactosamine is not related to the modulation of intracellular free radical production in primary culture of rat hepatocytesFree Radical Res362002345355
- N.N.RahbariM.KochT.SchmidtE.MotschallT.BrucknerK.WeidmannMeta-analysis of the clamp-crushing technique for transection of the parenchyma in elective hepatic resection: back to where we started?Ann Surg Oncol162009630639
- R.J.AragonN.L.SolomonTechniques of hepatic resectionJ Gastr Oncol3120122840
- J.B.FerriniJ.C.OurlinL.RichardG.FabreP.MaurelHuman hepatocyte cultureMethods Mol Biol1071998341352
- E.SiendonesD.FouadA.M.AbouelellaA.QuinteroP.BarreraJ.MuntaneRole of nitric oxide in d-galactosamine-induced cell death and its protection by PGE1 in cultured hepatocytesNitric Oxide822003133143
- S.HortelanoM.ZeiniA.CastrilloA.M.AlvarezL.BoscaInduction of apoptosis by nitric oxide in macrophages is independent of apoptotic volume decreaseCell Death Differ962002643650
- S.MartinF.NavarroN.FortofferP.NavasJ.M.VillalbaNeutral magnesium-dependent sphingomyelinase from liver plasma membrane: purification and inhibition by ubiquinolJ Bioenerg Biomembr3322001143153
- R.TaffsM.SitkovskyIn vitro assays for mouse B and T lymphocyte functionJ.E.ColiganA.M.KruisbeekD.H.MarguliesE.M.ShevachW.StroberCurrent protocols in immunology1991Greene Publishing, Wiley InterscienceNew York18
- H.U.BergmeyerE.BerntUV-assay with pyruvate and NADHH.U.BergmeyerMethods of enzymatic analysisVol. 21974Academic PressNew York574578
- T.P.A.DevesagayamK.K.K.BoloorT.RamasarmaMethods for estimating lipid peroxidationIndian J Biochem Biophys402003300308
- J.P.TeareN.A.PunchardJ.J.PowellP.J.LumbW.D.MitchellR.P.ThompsonAutomated spectrophotometric method for the determining oxidized and reduced glutathione in liverClin Chem3941993686689
- W.H.HabigM.J.PabstW.B.JakobyGlutathione S-transferases. The first enzymatic step in mercapturic acid formationJ Biol Chem24922197471307139
- Y.C.AwasthiE.BeutlerS.K.SrivastavaPurification and properties of human erythrocyte glutathione peroxidaseJ Biol Chem25013197551445149
- S.S.TawfikA.M.AbouelellaY.E.ShaheinCurcumin protection activities against γ-Rays-induced molecular and biochemical lesionsBMC Res Notes62013375
- R.PottathilK.A.ChandraboseP.CuatrecasasDJLangEstablishment of the interferon-mediated antiviral state: possible role of superoxide dismutaseProc Natl Acad Sci786198133433347
- L.C.GreenD.A.WagnerJ.GlogowskiP.L.SkipperG.S.WishnokS.R.TannenbaumAnalysis of nitrate, nitrite and [15N] nitrate in biological fluidsAnal Biochem12611982131138
- M.M.BradfordA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem721976248254
- M.C.BerenbaumA method for testing for synergy with any number of agentsJ Infect Dis1371978122130
- H.LiuF.ZhengQ.CaoB.RenL.ZhuG.StrikerAmelioration of oxidant stress by the defensin lysozymeAm J Physiol Endocrinol Metab2902006E824E832
- E.R.StadtmanRole of oxidant species in agingCurr Med Chem1200411051112
- J.MoskovitzE.R.StadtmanSelenium-deficient diet enhances protein oxidation and affects methionine sulfoxide reductase (MsrB) protein level in certain mouse tissuesProc Natl Acad Sci100200374867490
- C.FleuryB.MignotteJ.L.VayssiereMitochondrial reactive oxygen species in cell death signalingBiochimie842002131141
- M.O.HengartnerThe biochemistry of apoptosisNature402000770776
- Y.OsawaY.BannoM.NagakiY.NozawaH.MoriwakiS.NakashimaCaspase activation during hepatocyte apoptosis induced by tumor necrosis factor-a in galactosamine-sensitized miceLiver212001309313
- Y.PuD.C.ChangCytosolic Ca2+ signal is involved in regulating UV-induced apoptosis in hela cellsBiochem Biophys Res Commun28220018489
- Y.A.HannunL.M.ObeidThe ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kindJ Biol Chem27720022584725850
- R.C.GarciaM.MariA.MoralesA.ColellE.ArditeC.J.C.FernandezHuman placenta sphingomyelinase, an exogenous acidic pH-optimum sphingomyelinase, induces oxidative stress, glutathione depletion, and apoptosis in rat hepatocytesHepatology32120005665
- Y.OsawaH.UchinamiJ.BielawskiR.F.SchwabeY.A.HannunD.A.BrennerRoles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-αJ Biolog Chem28020052787927887
- J.LeeJ.KangM.H.StipanukDifferential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivationBiochem J3932006181190
- J.C.Fernandez-ChecaRedox regulation and signaling lipids in mitochondrial apoptosisBiochem Biophys Res Commun3042003471479
- C.L.HammondT.K.LeeN.BallatoriNovel roles for glutathione in gene expression, cell death, and membrane transport of organic solutesJ Hepatol342003946954
- M.BilzerG.PaumgrtnerA.GerbesGlutathione protects the rat liver against reperfusion injury after hypothermic preservationGastroenterology1171999200210
- O.MeuretteO.L.LefeuvreA.RebillardG.D.LagadicB.M.DimancheRole of intracellular glutathione in cell sensitivity to the apoptosis induced by tumor necrosis factor {alpha}-related apoptosis-inducing ligand/anticancer drug combinationsClin Cancer Res11200530753083
- F.Q.LiuJ.R.ZhangX-ray induced L02 cells damage rescued by new anti-oxidant NADHWorld J Gastroenterol98200317811785
- E.SiendonesD.FouadM.J.M.Diaz-GuerraM.De la MataL.BoscaJ.MuntanePGE1 induced nitric oxide reduces apoptosis by d-galactosamine through attenuation of NF-KB and NOS-2 expressionHepatology40200412951303
- D.C.RockeyV.ShahNitric oxide biology and the liver: report of an AASLD research workshopHepatology392004250257
- D.FouadE.SiendonesG.CostanJ.MuntaneRole of NF-kB activation and nitric oxide expression during PGE1 protection against d-galactosamine induced cell death in cultured rat hepatocytesLiver Int242004227236