Abstract
Objective
To evaluate the potential apelin effect on hepatic injury in caerulein (Cn) -induced AP in rats.
Experimental protocol
Thirty male albino rats were divided into three groups, 10 rats each: control group: received 0.9% NaCl solution. AP group: received (Cn, 50 μg/kg/h, i.p.) for 6 h. Apelin-13 treated AP group: received apelin 13, 50 nmol/kg/h, i.p, immediately after each Cn injection, starting after the second Cn dose. 12 h after the last Cn injection, the rats were sacrificed, and serum amylase, lipase, phospholipase A2 (PLA2), interleukin (IL)-6, IL-1β, IL-10, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactic dehydrogenase activity (LDH) were assayed. The hepatic malondialdehyde (MDA), reduced glutathione (GSH) and catalase (CAT) levels, caspase-3 activity and tumor necrosis factor-alpha (TNF-α) were assessed, while myeloperoxidase (MPO) was determined in pancreatic and hepatic tissues.
Results
Cn injection caused severe AP, with marked hepatic damage. The exogenous apelin reduced Cn-induced pancreatic and hepatic injury with reduction in hepatic oxidative, apoptotic and inflammatory markers, pancreatic and hepatic MPO activity with modulation of inflammatory cytokines.
Conclusion
Apelin could be protective in AP associated liver damage, possibly through anti-oxidant, anti-apoptotic mechanisms with modulating the inflammatory mediators.
1 Introduction
AP is a non-infectious inflammatory disease, associated with autodigestion of the pancreas, with sudden onset and rapid progression.Citation1
Figure 1 Effect of apelin 13 (50 nmol/kg/h) on MPO activity in pancreatic (A) and hepatic (B) tissues, in Cn – induced AP group. Data are expressed as mean ± (SD). ∗At P < 0.05 versus control group, #at P < 0.05 versus AP group.
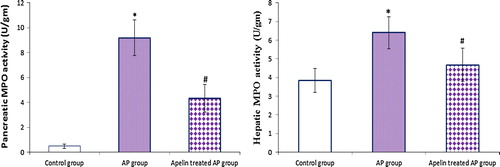
Figure 2 Effect of apelin 13 (50 nmol/kg/h) on serum amylase (A), lipase (B) and phospholipase A2 (C), in Cn – induced AP group. Data are expressed as mean ± (SD). ∗At P < 0.05 versus control group, #at P < 0.05 versus AP group.
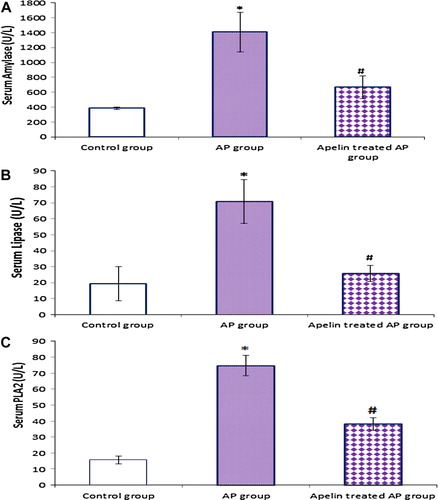
Figure 3 Effect of apelin 13 (50 nmol/kg/h) on serum IL-6 (A), IL-1β (B) and IL-10(C), in Cn – induced AP group. Data are expressed as mean ± (SD). ∗At P < 0.05 versus control group, #at P < 0.05 versus AP group.
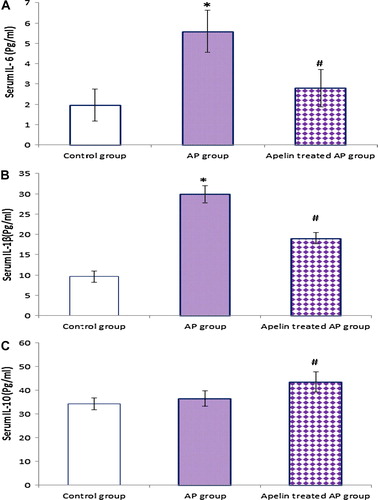
Table 1 Effect of apelin 13 (50 nmol/kg/h) on liver functions in Cn – induced AP group.
Table 2 Effect of apelin 13 (50 nmol/kg/h) on liver apoptotic, proinflammatory oxidative stress markers in Cn – induced AP group.
Clinically ranges from a mild, self-limiting localized disease to severe AP often lead to distant organ dysfunction and high mortality.Citation2
AP induced liver injury is considered to be an important prognostic indicator in AP, and may develop into hepatic failure and even result in death. Thus, it is of importance to protect liver function and block injury-related pathways.Citation3
The pathophysiology of AP and its associated liver injury are heterogeneous and involve a complex cascade of events.Citation4 Recent studies have shown that inflammatory cytokines, and adhesion molecules such as TNF-α, IL-6, and IL-1β produced within the pancreas and systemically, as well as neutrophil activation and adhesion contribute to the development and severity of AP and its complicated organs dysfunction.Citation5
Both necrosis and apoptosis occur in experimental pancreatitis. It is now well known that the severity of the disease is related to the type and the degree of cell death induced by different etiologic factors.Citation6
Several experimentalCitation7 and clinical studies,Citation8 have provided some support for the concept that oxidative stress is the common pathway for the pathogenesis of AP and associated hepatic injury.
Current Cn induced AP model reproduces the cardinal features of human pancreatitis including elevated serum amylase/lipase and pancreatitis-associated complications. Cn induces secretory block which is followed by lysosomal degradation of intercellular organelles within autophagic vacuoles in acinar cells, a marked interstitial edema and premature intracellular protease activation.Citation9
Apelin, a small regulatory peptide, has been identified as the endogenous ligand of the human orphan G protein-coupled receptor APJ, a receptor structurally related to the angiotensin II (ANG II) receptor AT1. It can act via autocrine, paracrine, endocrine, and exocrine signaling.Citation10
Apelin is synthesized as a 77 amino acid prepropeptide that is cleaved into several active isoforms, with apelin-13 is the final active product, being the most potent isoform, more resistant to enzymatic cleavage, with a brief plasma half-life in man and relatively short lived effects.Citation11
The apelin-APJ axis is widely expressed in heart, brain, lung, kidney as well as the gastrointestinal tract, on pancreatic duct, acinar and islets cells and hepatic parenchymal, Kupffer (KCs), stellate and endothelial cells.Citation12
Apelin peptides have been shown to affect many biological functions in mammals including the neuroendocrine and immune systems.Citation10 Apelin signaling is known to play important roles in cardiovascular homeostasis; however, its functions in liver injury associated with AP remain unclear.Citation13
Hans et al. proved upregulated pancreatic apelin expression during Cn-induced AP in mice.Citation14
Multiple therapeutic modalities have been suggested for AP and its related organ dysfunction, which remain largely supportive but none has been unambiguously proven to be effective yetCitation2
The aim of this study was to assess the role of apelin-signaling in the pathophysiology of the AP induced liver injury, and evaluate potential new therapeutic strategies through highlighting the effect of exogenous apelin-13 on liver injury in a rat model of Cn-induced AP and the mechanisms behind apelin’s effect.
2 Material and methods
2.1 Animals and experimental design
This study was carried out on thirty male albino rats weighing about 200–250 g. The rats were housed, four per cage, under standard laboratory conditions at room temperature (24 ± 2 °C), and had free access to water and food. The rats were fasted during the night before the experiment. All animal experiments were undertaken with the approval of Ethical Animal Research Committee of Tanta University.
The rats were randomly divided into three groups (10 rats each):
2.2 Control group
The rats were given hourly, i.p injection of 2 ml 0.9% NaCl saline solution, throughout the experimental period.
2.3 Acute pancreatitis (AP) group
AP was induced by i.p. injection of a supra-maximal concentration of (Cn) (50 μg/kg), (Sigma–Aldrich Chemical, Steinheim, Germany), diluted in 2 mL saline, every hour for a total of 6 h. At the end of Cn injections, the rats were given 2 mL i.p. saline till the end of experiment.Citation15
Cn is a stable cholecystokinin analogue, leading to proteolytic enzyme secretion that causes pancreatic acinar autolysis with progressive interstitial edema just one hour after injection. It is used to induce experimental AP models in rats and mice.Citation9
2.4 Apelin-13 treated acute pancreatitis group
Apelin-13 (Apelin®, Phoenix Pharmaceutical, Belmont, CA, USA), is given (50 nmol/kg/h, i.p.),Citation16 dissolved in 2 ml saline, immediately after each Cn injection starting after the second Cn dose.
At the end of experiment, 12 h after the last Cn injection, the rats were sacrificed and blood samples were collected, immediately centrifuged at 3000g for 10 min, and the supernatant was stored at −20 °C for biochemical assays. Tissue samples of pancreas and liver of all groups were quickly removed and kept frozen at −80 °C for further analysis. Hepatic and pancreatic protein content was determined according to the method of Lowry et al.Citation17
The following parameters were determined.
2.5 Liver function assay
Serum (ALT) and (AST), as indicators of liver functions, were measured according to the method of Rei.Citation18
Serum (LDH) activity, as a marker of tissue injury, was assayed according to the method of Martinek.Citation19
2.6 Liver caspase-3 activity and (TNF-α) assay
Liver caspase-3, the common signal molecule of various apoptotic mechanisms and TNF-α, was measured by the enzyme-linked immunosorbent assay (ELISA) method according to the studies by Janicke et al.Citation20 and Endo et al.Citation21 respectively.
2.7 Hepatic and pancreatic myeloperoxidase activity assay
(MPO) activity, as a marker of tissue leukocyte infiltration, was assessed by the ELISA (MPO ELISA kit, Hycult Biotechnology, Uden, Netherlands), according to the method of Kuebler et al.Citation22 One unit of MPO activity is defined as degrading 1 μmol of hydrogen peroxide at 37 °C; MPO activity was expressed as unit per gram of tissue protein (U/g).
2.8 Serum enzyme activities assay
Serum amylase level was assessed by modified Bernfeld’s methodCitation23 as described by Jamieson.Citation24 Serum lipase was measured as described by Williamson.Citation25 Serum (PLA2) was measured as described by Aufenanger et al.Citation26 The results are expressed as U/L.
2.9 Serum cytokines assay
Serum (IL)-6, (IL-1β), and IL-10 were measured using ELISA kits according to the manufacturer’s instructions (Quantikine®; R&D Systems, Minneapolis, MN, USA). The results of cytokine levels were expressed as pg/ml.
2.10 Liver lipid peroxidation and antioxidant enzymes assay
Hepatic oxidative stress markers were evaluated by the amount of lipid peroxides measured as (MDA) and hepatic antioxidant levels, GSH and CAT. MDA content was measured as described by Esterbauer and CheesemanCitation27 CAT and GSH were measured according to the studies by AebiCitation28 and Nagi et al.Citation29 respectively.
3 Statistical analysis
The results were presented as the mean ± standard deviation (SD). Data were analyzed using the unpaired student’s t-test. Probability values less than 0.05 were considered significant. All the analyses were performed using Graph Pad Prism software release 4.0 (Graph Pad Software, San Diego, CA).
4 Results
4.1 Effect of apelin treatment on liver functions ()
Following Cn injections, our results revealed marked hepatic damage as evidenced by significant increase in ALT, AST and LDH levels in Cn-induced AP group compared to the control group; however, apelin treatment provided marked hepatic protection with significant reduction in serum levels of ALT, AST and LDH as compared with AP group.
4.2 Effect of apelin treatment on liver caspase-3 activity and TNF-α ()
At the end of our experiment, biochemical assessment of hepatic apoptosis and inflammatory markers were performed and revealed significant increase in liver caspase-3 and TNF-α levels in AP rats as compared to control, while apelin treatment induced significant decrease in both parameters in comparison with AP group.
4.3 Effect of apelin treatment on hepatic and pancreatic MPO activity ()
The present study revealed that AP induction was associated with marked hepatic and pancreatic neutrophils infiltration that was improved significantly with apelin treatment.
4.4 Effect of apelin treatment on serum amylase, lipase and PLA2 levels ()
On AP induction, there was marked pancreatic damage, as indicated by significant increase in serum amylase, lipase and PLA2 levels compared with the control group, while apelin treatment caused significant decrease in these parameters as compared with AP group.
4.5 Effect of apelin treatment on hepatic lipid peroxidation and antioxidant enzymes ()
Compared with control group, AP induction was associated with marked oxidative stress with significant increase in liver MDA level and decrease in liver GSH and CAT levels. Apelin treatment induced marked antioxidant activity with significant reduction in liver MDA and elevation in liver GSH and CAT levels compared with AP group
4.6 Effect of apelin treatment on serum cytokines ()
It is obvious from our results that the AP induction was associated with increase in serum IL-6, IL-1β and IL-10 as compared with the control group, while apelin treatment exhibited marked anti-inflammatory effect through abrogating the proinflammatory cytokines (IL-6, IL-1β), and enhancing the anti-inflammatory cytokine, IL-10 compared to AP group.
5 Discussion
AP associated hepatic dysfunction has been described by many authors. It is considered a manifestation of systemic inflammatory response during which the hepatic microcirculatory dysfunction, tissue hypoxia, and inflammatory cytokines could play a central role. Further elucidation of the involved mechanisms and their interactions is critical in developing effective treatment.Citation30
To our knowledge, the present study is the first to delineate the potential role of apelinergic pathway in AP – induced hepatic injury.
On AP induction, there was significant increase in serum lipase, amylase and sPLA2, and advocated the auto digestion theory of pancreas. PLA2 released to blood to attack and decompose the phospholipids ingredient of membrane, destroying cell membrane stability and causing massive out leakage of lysosome enzymes, generating bioactive free fatty acid and soluble lecithin, and destroying the function and structure of systemic cell and organ system.Citation31
The protective effect of apelin in AP recorded in our study is consistent with the published data by Han et al.Citation32 in AP rat model, who suggested that pancreatic apelin expression was up-regulated in AP and functions as an anti-inflammatory factor.
According to our results, Cn-induced AP evoked deleterious effects on the liver. High LDH activity revealed progressive hepatic cell death and high AST and ALT plasma activity indicated a relevant metabolic dysfunction with cell damage.Citation3
Several studies point out that almost all the pancreatic enzymes and mediators released from the pancreas into the plasma during AP pass through the liver before their dilution in the systemic circulation, aggravating hepatic damage with leakage of liver enzymes into circulation.Citation33
The hepatic protective effect of apelin recorded in our study, is consistent with those previously recorded in rat models of hepatic ischemia reperfusion injury (I/RI)Citation34 and renal I/R induced liver injury,Citation35 with improved Serum (ALT) and (AST), liver (MDA) and (GSH), and liver histopathology.
The mechanisms by which distant organs are involved in AP remain obscure. Besides autodigestion and necrosis of pancreatic tissue, serum levels of pro-inflammatory cytokines increase during the course of AP, and positively correlated with its severity and appear to be the driving force for the initiation and propagation of the AP from local pathological changes to systemic multiple organs dysfunction and their blockade attenuates the disease process.Citation2
In the present study, hepatic TNF-α and serum IL-1ß, IL-6, and IL-10 levels increased after induction of pancreatitis.
The Synergy between pro-inflammatory cytokines and oxidative stress in AP, is particularly pronounced with elevated hepatic TNF-α, that amplifies the inflammatory cascade through different mechanisms, such as the activation of mitogen activated protein kinases and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and/or the inactivation of protein phosphatases.Citation36
When the quantity of produced TNF-α exceeds that of tissue TNF receptor, the excessive TNF-α enters blood circulation, triggering the release of cytokines such as IL-1β, IL-8 and IL-6.Citation36
Previous studies have pointed to (KCs), the resident macrophages in the liver, as predominant source of inflammatory mediators, when activated in response to substances released by the pancreas during AP.Citation33
The proinflammatory cytokines promote B and T cell activation, acute period reaction and activation of coagulation cascades. Moreover IL-6, carried by blood to the liver, stimulates the liver to secrete the mannose-bonding protein to bind bacteria and endotoxin. IL-6 also can lead to leukocyte adhesion to the surface of endothelial cells of hepatic vasculature with enhanced radical generation and release of toxic substances such as elastic protease triggering a series of complementary reactions and lead to hepatic injury.Citation37 Furthermore these inflammatory mediators invade the hepatic tissues and destroy the Na+–K+ pump on hepatic cell membrane. The condition further deteriorated when the liver loses its barrier function to prevent endotoxemia, that has led to the excessive release of the endogenous inflammatory mediators, forming a vicious cycle amplifying reactions.Citation38
On the other hands, IL-10 is a potent cytokines inhibitor and has been shown to attenuate the degree of pancreatic and liver injury in mice models of AP.Citation39
We observed that apelin treatment resulted in significant reduction in hepatic TNF-α and serum IL-1ß, and IL-6, with significant increase in plasma IL-10 levels. These results could provide a rationale for the use of apelinergic system as broad anti-inflammatory, through modulating pro- and anti-inflammatory cytokines profile in AP.
The apelins’ anti-inflammatory activity in AP is previously recorded in vitro, in pancreatic acinar cells,Citation40 and in mouse primary hepatocytes.Citation41 It may be linked to apelin reduction of AP-induced elevations in NF-κB activation,Citation32 (MPO) activity or mitigating TNF-α-induced reactive oxygen species (ROS) generation and c-Jun N-terminal kinase (JNK) activationCitation41 and macrophage inflammatory protein-1 (MIP-1α, MIP-1β) expression levels.Citation42
While the mechanism of AP is not fully known, it is believed that oxidative stress is a key pathogenic factor in initiation and propagation of AP.Citation8
The data obtained in our study support the early activation and implication of oxidative stress in AP associated hepatic injury.
In addition to the direct detrimental oxidative effects, ROS can also serve as secondary messengers in intracellular signaling and induce proinflammatory cascades. Free radicals produced by active leukocytes in liver may oxidize the active sites of the antioxidant enzymes, CAT and GSH, causing reduced activity, leading to hepatic cell lytic necrosis, when ROS production exceeds the antioxidant capacity, aggravating the condition.Citation43 Apelin markedly reduced (MDA) level, an indicator of free radical generation, improved the antioxidant enzymes, CAT and GSH, in AP group, trying to restore the oxidant/antioxidant balance, with improved hepatic function. The antioxidant effect of apelin observed in our study could be explained at least partially through its anti-inflammatory and inhibitory effect on hepatic leukocyte infiltration. Moreover apelin behaves as CAT activator as documented previously by Foussal et al.Citation44
Improvement of hepatic antioxidant, GSH, with decreased MDA, under apelin treatment has been recorded previously in vitro,Citation41 and in vivo, in mesenteric I/R induced hepatic injury.Citation45
Increased leukocyte infiltration in AP group compared to the control one observed in our study, is a multistep process, coordinated by specific adhesion molecules and a major characteristic of human and experimental pancreatitis, constituting a critical link in mediating tissue damage in AP. A fact is supported by reduced tissue injury in Cn-induced AP with neutrophils depletion.Citation46
The apelin-induced inhibition of neutrophil recruitment may be one mechanism behind the reduction in pancreatic and hepatic injury with apelin treatment. It could be attributed to reduced levels of cytokines and chemokines.Citation47
The implied inhibitory role of exogenous apelin on neutrophil invasion is bolstered in apelin-deficient mice with pancreatitis, where pancreatic neutrophil recruitment was enhanced significantly, reduced subsequently by apelin treatment, and possibly mediated by an apelin-induced reduction of keratinocyte chemokine, a key chemoattractant for neutrophils and granulocyte colony-stimulating factor secretion, which exerts a variety of trophic actions on neutrophils to increase their density.Citation14
It is evident from our results that there was significant increase in the hepatic caspase 3 activity, a critical executioner of apoptosis, in the Cn – induced AP group. These results have implicated the apoptosis in AP- induced early hepatic damage, consistent with previous report of positive correlation of apoptosis with degree of hepatic damage and hepatic failure in AP.Citation48
In our study, apelin-13 significantly reduced liver caspase 3 activity of AP rats. The anti-apoptosis effect of apelin may be attributed to its anti-oxidant effect or through the APJ/phosphatidylinositol-3 kinase (PI3-K)/Akt signaling pathways.Citation49
Consistent with its role in blocking cell death, Kunduzova et al.Citation50 revealed dose-dependent antiapoptotic effects of apelin, possibly through enhancing the Bcl-2, prosurvival protein expression, or abrogating Bax protein production, a well-known proapoptotic protein.
Aside from its direct effects on the pathophysiology in AP, the apelin-APJ pathway has additional protective actions on AP-induced liver injury through its well-known positive inotropic effect, improving microcirculation/perfusion, aiding to protect tissues. Moreover endogenous apelin is required for the suppression of inflammation-induced vascular hyper permeability and restores the vasoconstrictors/vasodilator imbalance.Citation51
Another mechanism could explain the apelin protective effect in our study suspected by Kapica et al.Citation16 who concluded that intravenous apelin decreased pancreatic-biliary juice volume, protein and trypsin outputs in a dose dependent manner, alleviating the inflamed pancreas.
6 Conclusion
The results of the present study identified a previously unknown role for apelin in abrogating the acute Cn induced pancreatic and hepatic damage, probably by modulating inflammatory cytokines, alleviating leukocytes infiltration, oxidants stress with its anti-apoptotic effect. So targeting the apelinergic system could be a line of treatment in the course of AP associated liver damage. However, the present study may be just the tip of an iceberg and further investigations are needed to fully characterize apelin-mediated protection in AP associated hepatic injury and provide further comprehension for aplinergic signals pathway in AP.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 14 December 2015
References
- G.YuR.WanG.YinJ.XiongDiosmetin ameliorates the severity of cerulein-induced acute pancreatitis in mice by inhibiting the activation of the nuclear factor-κBInt J Clin Exp Pathol75201421332142
- V.PhillipJ.M.SteinerH.AlgülPhase of acute pancreatitis: assessment and managementWorld J Gastrointest Pathophysiol532014158168
- Z.G.LuanH.ZhangX.C.MaR.X.GuoTherapeutic treatment with ethyl pyruvate attenuates the severity of liver injury in rats with severe acute pancreatitisPancreas4152012729737
- A.BakoyiannisS.DelisC.DervenisPatho-physiology of acute and infected pancreatitisInfect Disord Drug Targets10201024
- D.AwlaA.V.ZetterqvistA.AbdullaC.CamelloM.F.GomezH.ThorlaciusNFATc3 regulates trypsinogen activation, neutrophil recruitment, and tissue damage in acute pancreatitis in miceGastroenterology143201213521360
- J.YangA.FierY.CarterG.LiuF.BaiT.P.Loughran et alLiver injury during acute pancreatitis: the role of pancreatitis associated ascitic fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosisJ Gastrointest Surg72003200227
- J.B.ChungJ.H.LeeJ.H.SuhS.W.ParkS.Y.SongK.H.KimRole of oxygen free radicals in patients with acute pancreatitisWorld J Gastroenterol9200322662269
- Q.Q.TangS.Y.SuM.Y.FangZinc supplement modulates oxidative stress and antioxidant values in rats with severe acute pancreatitisBiol Trace Elem Res1591–32014320324
- N.IrreraA.BittoM.InterdonatoF.SquadritoD.AltavillaEvidence for a role of mitogen-activated protein kinases in the treatment of experimental acute pancreatitisWorld J Gastroenterol204420141653516543
- A.M.O’CarrollS.J.LolaitL.E.HarrisG.R.PopeThe apelin receptor APJ journey from an orphan to a multifaceted regulator of homeostasisJ Endocrinol219120131335 11
- J.J.MaguireM.J.KleinzS.L.PitkinA.P.Davenport[Pyr1] Apelin-13 Identified as the Predominant Apelin Isoform in the Human Heart Vasoactive Mechanisms and Inotropic Action in DiseaseHypertension542009598604
- M.H.KwonB.TuvshinturW.J.KimH.R.JinG.N.YinK.M.Song et alExpression of the apelin-APJ pathway and effects on erectile function in a mouse model of vasculogenic erectile dysfunctionJ Sex Med1012201329282941
- A.PrincipeM.P.LesmesG.F.VaroJ.RosThe hepatic apelin system: a new therapeutic target for liver diseaseHepatology48200811931201
- S.HanE.W.EnglanderG.A.GomezJ.F.AronsonC.RastelliniR.KunduPancreatitis activates pancreatic apelin-APJ axis in miceAm J Physiol Gastrointest Liver Physiol30522013139150
- J.L.FrossardA.HadengueL.SpahrP.MorelC.M.PastorNatural history of long-term lung injury in mouse experimental pancreatitisCrit Care Med30200215411546
- M.KapicaA.JankowskaH.AntushevichA.DembinskiR.ZabielskiThe effect of exogenous apelin on the secretion of pancreaticjuice in anaesthetized ratsJ Physiol Pharmacol63120125360
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- R.ReiMeasurement of aminotransferase: part I. Asparate aminotransferaseCRC Crit Rev Clin Lab Sci21198499186
- R.G.MartinekA rapid ultraviolent spectrophotometric lactic dehydrogenase assayClin Chem Acta4019729199
- R.U.JanickeM.L.SpregartM.R.WatiA.G.PorterCaspase-3 is required for DNA fragmentation and morphological changes associated with apoptosisJ Biol Chem273199893579360
- Y.EndoM.ShibazakiK.YamaguchiS.SugawaraH.KikuchiK.KumagaiEnhancement by galactosamine of lipopolysaccharide (LPS)- induced tumour necrosis factor production and lethality: its suppression by LPS pretreatmentBr J Pharmacol1281999512
- W.M.KueblerC.AbelsL.SchuererMeasurement of neutrophil content in brain and lung tissue by a modified myeloperoxidase assayInt J Micro Circ Clin Exp1619968997
- P.BernfeldAmylases alpha and betaMeth Enzymol11955149158
- A.D.JamiesonK.M.PruittR.C.CalwellAn improved amylase assayJ Dent Res481969483
- T.WillamsonThe estimation of pancreatic lipase–a brief reviewMed Lab Sci331976265279
- J.AufenangerW.ZimmerR.KattermannCharacteristics of a radiometric E. coli-based phospholipase A2 assay; modified method for clinical applicationClin Chem391993605613
- H.EsterbauerK.H.CheesemanDetermination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenalMeth Enzymol1861990407421
- H.E.AebiCatalaseH.U.BergmeyerJ.BergmeyerM.GrablMethods of enzymatic analysis1993Weinheim Velag Chemie Gmbh273286 3
- M.N.NagiS.K.SunejaL.CookD.L.CintiDepletion of rat hepatic glutathione and inhibition of microsomal trans-2-enoyl-CoA reductase activity following administration of a dec-2-ynol and dec-2-ynoic acidArch Biochem Biophys29319927178
- I.HritzL.CzakóZ.DubravcsikG.FarkasD.KelemenN.Lásztity et alHungarian Pancreatic Study GroupAcute pancreatitis. Evidence-based practice guidelines, prepared by the Hungarian Pancreatic Study GroupOrv Hetil15672015 Feb 15244261
- K.J.ZhangD.L.ZhangX.L.JiaoC.DongEffect of phospholipase A2 silencing on acute experimental pancreatitisEur Rev Med Pharmacol Sci1724201332793284
- S.HanA.GuillermoG.W.EnglanderC.RastelliniApelin reduces pancreatic inflammation during acute pancreatitisGastroenterology14052011385386
- H.B.LiuN.Q.CuiD.H.LiC.ChenRole of Kupffer cells in acute hemorrhagic necrotizing pancreatitis-associated lung injury of ratsWorld J Gastroenterol1232006403407
- T.SagirogluM.B.AksoyG.SagirogluT.YaltaM.A.YagciA.SezerEffect of leptin and apelin preconditioning on hepatic ischemia reperfusion injury in ratsIndian J Surg7622014111116
- T.SagirogluN.TorunM.YagciT.YaltaS.OguzEffects of apelin and leptin on renal functions following renal ischemia/reperfusion: an experimental studyExp Ther Med352012908914
- J.ChenQ.P.CaiP.J.ShenR.L.YanX.Y.ChenNetrin 1 protects against l-Arginine induced acute pancreatitis in micePLoS One7920124620
- W.SunJ.D.ZhangY.ZhaoY.ZhaoQ.WangExpression of IL-6 and integrin family adhesion molecule in model of rat with acute necrotizing pancreatitis complicated with multi-organ injuryShijie Huaren Xiaohua Zazhi1162003753755
- Y.Z.YangH.WangC.K.ZhanL.M.PengC.X.GuoTreatment of 24 cases of severe acute pancreatitis complicated with multi-organ failureChongqing Med J3210200313881389
- R.M.MinterM.A.FerryM.E.MurdayC.L.TannahillF.R.BahjatAdenoviral delivery of human and viral IL-10 in murine sepsisJ Immunol167200110531059
- Y.CaoC.RastelliniX.GaoS.HanV.BhatiaM.Falzon et alInteraction of BMP. Apelin and PTHrP pathways in pancreatic duct ligation-induced chronic pancreatitisPancreas42820131341
- J.ChuH.ZhangX.HuangY.LinT.ShenApelin ameliorates TNF-a-induced reduction of glycogen synthesis in the hepatocytes through g protein-coupled receptor APJPLoS ONE822013e57231
- Y.CaoX.GaoC.J.DuanC.ChaoG.H.GreeleyM.R.Hellmich et alInduction of gremlin, a bone morphogenetic protein antagonist, during the development of chronic pancreatitisGastroenterology1402011549
- K.BatciogluM.GulA.B.UyumluM.EsrefogluLiver lipid peroxidation and antioxidant capacity in cerulein-induced acute pancreatitisBraz J Med Biol Res4292009776782
- C.FoussalO.LairezA.PathakC.Guilbeau-FrugierP.ValetActivation of catalase by apelin prevents oxidative stress-linked cardiac hypertrophyFEBS Lett58411201023632370
- T.SagirogluS.OğuzG.SagirogluT.YaltaM.B.SayhanThe effects of Apelin on Mesenteric Ischemia and reperfusion damage in an experimental rat modelBalkan Med J2922012148152
- A.AbdullaD.AwlaH.ThorlaciusS.RegnérRole of neutrophils in the activation of trypsinogen in severe acute pancreatitisJ Leukocyte Biol9052011975982
- S.HanD.KolliP.RobertoE.W.EnglanderInhibitory activity of pancreatic Apelin on neutrophil infiltration and systemic cytokine levels during acute pancreatitisGastroenterology14252012318
- X.P.ZhangL.WangJ.ZhangStudy progress on mechanism of severe acute pancreatitis complicated with hepatic injuryJ Zhejiang Univ Sci B82007228236
- R.R.CuiD.A.MaoL.YiC.WangX.B.LiaoH.Zhou et alApelin suppresses apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt signaling pathwaysAmino Acids395201011931200
- O.KunduzovaS.YabanogluP.ValetA.PariniApelin protects against oxidative stress and apoptosis in neonatal rat cardiac myocytesJ Mol Cell Cardiol4432008783
- A.G.JappN.L.CrudenA.B.DavidK.Y.VivienneVascular Effects of Apelin In Vivo in ManJ Am College Cardiol52112008908913
