Abstract
Acute gastrointestinal (GI) bleeding is an important cause of emergency hospital admissions, with its related morbidity and mortality. The availability of endoscopic equipment has had an important effect on the rapid identification and treatment of the bleeding source. Multidetector computed tomography (MDCT) allows direct demonstration and visualization of the bleeding source and its characterization. The information provided by MDCT angiography before attempts at therapeutic angiographic procedures leads to faster selective catheterization of bleeding vessels.
The purpose of this study was to show the role of MDCT and catheter embolization in management of acute GI bleeding.
Materials and methods
This is a retrospective study that included 57 patients referred in 4 years with active gastrointestinal bleeding.
MDCT was performed in 43 cases, while 14 patients didn’t have CT angiography and proceeded straight to angiography.
Results
37 patients had positive MDCT findings, of which 27 cases had positive bleed on angiography, and 10 did not show active bleeding on angiography. 6 patients had a negative MDCT and did not proceed to angiography. 14 patients did not have a MDCT and proceeded straight to angiography; 6 of them showed active bleeding on angiography.
Conclusion
MDCT is an excellent technique before angiography and embolization in cases with acute gastrointestinal bleeding. Transcatheter embolization is an effective tool for control of GI bleed.
1 Introduction
Gastrointestinal (GI) hemorrhage is a medical emergency. Its associated mortality is approximately 10%, a figure which has remained largely unchanged over the past decades. The incidence of upper GI bleeding is approximately six times higher than that for lower GI bleeding.Citation1
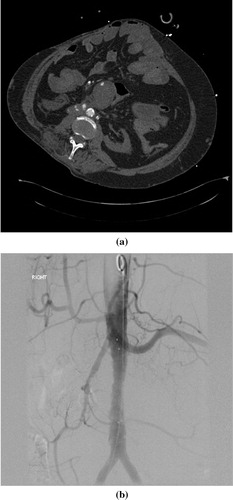
Figure 2 Sigmoid bleeding in 85 yrs old female patient. (a) Contrast enhanced CT in arterial phase showing high density contrast within the sigmoid colon in keeping with active bleeding. (b) Superselective angiography showing severe narrowing of the distal sigmoid branches due to compensatory arterial spasm. (c) Superselective angiography after coil insertion showing complete embolization.
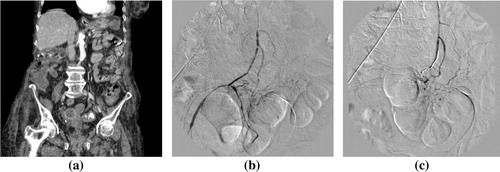
Figure 3 Bleeding from gastric ulcer within hiatus hernia. (a) CT arterial phase showing small focal active bleeding within hiatus hernia. (b) Digital subtraction angiography with selective celiac angiography showing faint spot of active bleeding. (c) Digital subtraction angiography with super-selective short gastric artery arising from splenic artery catheterization showing a spot of active bleeding. (d) Digital subtraction angiography after coil embolization, showing successful control with no active bleeding.
Upper GI bleeding occurs proximal to the ligament of Treitz (which connects the fourth portion of the duodenum to the diaphragm) and arises from the esophagus, stomach, or duodenum.Citation2 About half the cases of upper GI bleeding are caused by gastric and duodenal ulcers.Citation3 Lower GI bleeding involves the small bowel, colon, or rectum. Depending on the amount of blood loss and location, the clinical presentation varies considerably.Citation2
Patients with upper GI hemorrhage may present with hematemesis, coffee ground vomiting, melena, or hematochezia if the hemorrhage is severe. Patients with lower GI bleeding may present with melena, hematochezia, or rectal bleeding. Melena usually indicates blood that has been in the GI tract for at least 8 h. Melena is four times more likely to come from upper GI bleeding than from the lower GI tract. Hematochezia (bright red stool) is the sign of fast-moving active bleeding and is found six times more frequently in lower GI bleeding.Citation4
1.1 Multidetector CT
General advantages of CT scanning include its wide availability and the added diagnostic yield of cross-sectional imaging.Citation5
A recent in vitro study found the threshold for detecting bleeding with contrast material enhanced multidetector CT to be 0.35 mL/min. Multidetector CT allows arterial phase scanning of the whole abdomen, and contrast material extravasation can be revealed in the small bowel, which is an anatomic region not readily amenable to conventional endoscopy.Citation6
The sensitivity of CT for diagnosing the source of GI bleeding has been shown to be higher in patients with active hemorrhage (91–92%) than in those with obscure GI bleeding (45–47%).Citation7–Citation9
1.2 Radionuclide scintigraphy
Radionuclide scintigraphy that is most commonly performed with technetium 99 m (99mTc)–labeled red blood cells (RBCs) is often used as a screening test before angiography because it is noninvasive and more sensitive (bleeding at a rate of 0.05–0.1 cc per minute can be detected). It also can be repeated in the setting of intermittent bleeding. However, the ability of radionuclide scanning to localize the bleeding source correctly varies widely in the literature. A significant disadvantage of radionuclide scintigraphy is the lack of therapeutic possibilities.Citation5
1.3 Angiography and transcatheter embolization
Angiography is the only radiographic modality that can be both diagnostic and therapeutic. Bleeding rates 0.5–1 cc per minute can be detected with angiography. Successful localization of bleeding is highly dependent on the rate of bleeding at the time of the examination.Citation5
Superselective angiography and transcatheter embolization (SATE) has traditionally been indicated in those patients who are considered poor candidates for surgery. Interventional radiologic expertise has improved significantly along with improvements in coaxial catheters and embolic agents (microcoils, gelfoams, and pledgets). These improved systems allow better navigation and localization of arterial bleeding and treatment of small caliber vessels with a decreased risk of ischemic injury. In addition, SATE is a less invasive option than is surgery. For these reasons, radiologists now argue that SATE is a safe and effective treatment option that should be considered in a wider spectrum of patients.Citation10
2 Material and methods
The study included 57 patients referred with active gastrointestinal bleeding in 4 years.
CTA was performed in 43 cases. 37 patients had positive CTA, of which 27 cases had positive bleed on angiography, and 10 did not show active bleeding on angiography. 6 patients had a negative CTA and did not proceed to angiography. 14 patients did not have a CTA and proceeded straight to angiography; 6 of them showed active bleeding on angiography.
The equipment used for CTA was Toshiba Aquillon 128 MDCT unit kV/effective mAs/rotation time (s): 120 kV/225 effective mAs/0.35 s; slice thickness 0.5 mm.
CTA was done without prior oral administration of water or contrast material. Active contrast material extravasation within the bowel lumen is obscured by oral contrast material, leading to false-negative results. Intraluminal water may be helpful in finding the cause of acute GI bleeding, but it might also lead to dilution of extravasated contrast agent, leading to false-negative results.
The CT examination was performed first, with a noncontrast scan of the abdomen, followed by contrast-enhanced phases CT at 45 s, 70 s after an injection of 120–150 mL of nonionic contrast medium (350 mg I/mL) given at the antecubital vein at a rate of 4 mL/s.
The data acquired were transferred to a workstation (Osirix software) for multiplanar reconstruction.
All angiographic procedures were performed with digital subtraction imaging, and standard five French catheters (Simmons I, II or Cobra Glidecath, Terumo Europe, Leuven, Belgium and Simmons II or Cobra, Cook Medical, Bjaeverskov, Denmark) and iopromidum (Ultravist, Schering, Machelen, Belgium) were used as contrast material.
The embolization procedure was performed in all patients with the use of a microcatheter (Progreat 2.7 Fr, Terumo Europe, Leuven, Belgium). The embolic agents used were as follows: microcoils (Boston Scientific or Cook Medical) were used in 32 patients, and a 20 mm × 8 cm stent graft (Cook Medical, Bjaeverskov, Denmark) was used in one patient.
3 Results
This study included 57 patients, 33 men and 24 women.
Their average age was 69.9 and age range from 32 to 91 years.
3.1 CT angiography
Out of the 57 patients in this study, CTA was performed in 43 cases. 37 patients had positive CTA, of which 27 cases had positive bleed on angiography, and 10 did not show active bleeding on angiography. 6 patients had a negative CTA and did not proceed to angiography.
The remaining 14 patients did not have a CTA and proceeded straight to angiography; 6 of them showed active bleeding on angiography.
3.2 Conventional catheter angiography
Out of the 57 patients in this study 51 patients had angiography:
| • | 33 showed active bleeding and were treated with embolization and/or stent graft successfully with no immediate complications. | ||||
| • | 18 patients had a negative angiogram. | ||||
| • | The remaining 6 patients did not proceed to angiography because they had a negative CTA. | ||||
The 33 patients with active bleeding were distributed as following:
| • | 10/33 of the embolized cases were post-operative; the commonest cause was post-ERCP sphincterotomy (3 cases) followed by post-colonoscopy biopsy (2 cases). | ||||
| • | In 5/33 of the embolized cases there was spontaneous GI bleeding in patient on thrombolysis, anticoagulation or hemophilic patients. | ||||
| • | In 3/33 the underlying cause for bleeding was pancreatitis | ||||
| • | 3/33 patients had duodenal ulcer. | ||||
| • | 1/33 had bleeding rectal cancer. | ||||
| • | 1/33 had bleeding gastric ulcer in hiatus hernia. | ||||
| • | In 10/33 patients there was no underlying cause for bleeding. | ||||
Coils were used for embolization in all cases apart from the case of aorto-enteric fistula where a stent-graft was used.
The initial technical success rate (effective embolization of the target vessel) was 100%.
There was one recurrent bleeding in a case of bleeding with no underlying cause in the ascending colon (three days after the initial successful embolization) which required a second embolization, which controlled the bleeding effectively.
No immediate post-procedural complications were encountered regarding puncture site hematoma or coil migration. There were no reported cases of postembolic infarction (see –).
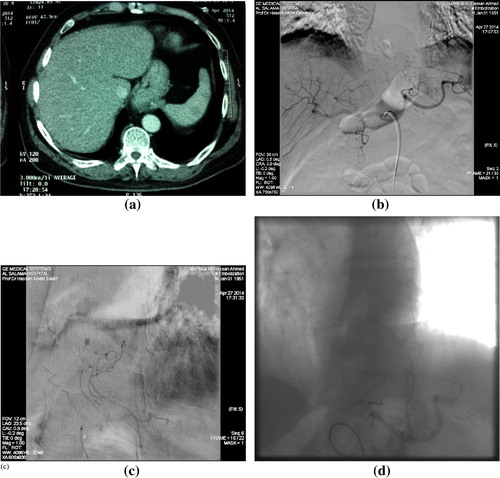
Figure 1 Post left renal biopsy; bleeding from Wandering artery of Drummond (marginal artery). (a) CT angiography showing site of active bleeding at the site of the marginal artery (arrow). (b) Digital subtraction IMA selective angiography showing the site of active bleeding active (arrow). (c) Digital subtraction angiography showing coils deployed proximal and distal to bleeding point. (d) Digital subtraction check angiogram: showing no contrast extravasation, denoting successful embolization.
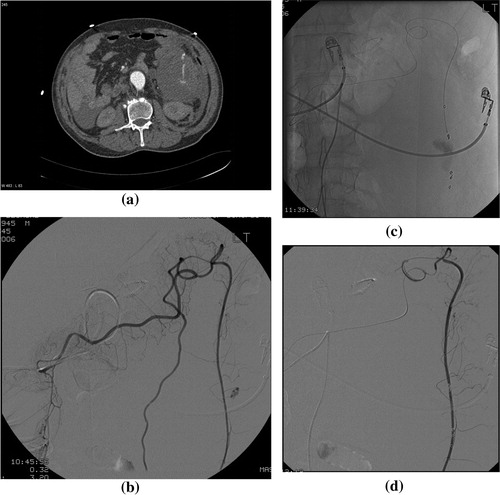
Figure 4 Post retroperitoneal surgery severe hemoglobin drops with aorto-enteric fistula. (a) CT arterial phase showing active bleeding from iatrogenic aorto-enteric fistula with contrast seen in the duodenum. (b) Digital subtraction angiography following stent-graft insertion, showing successful control with no active bleeding.
4 Discussion
Upper GI endoscopy is currently considered the first-line diagnostic and therapeutic modality for direct localization and local treatment of upper GI bleeding.Citation11 However, the distal duodenum and most of the small bowel cannot be adequately assessed with conventional endoscopy.Citation12 Despite adequate colon preparation, urgent colonoscopy may help identify a definitive bleeding source in only 13% of patients and a probable bleeding source in 67%.Citation13
In cases of endoscopic failure, therapeutic options include surgery and transcatheter therapy. Surgery is associated with high mortality rates of 15% up to 30% in emergent cases; therefore less invasive therapies, such as transcatheter embolotherapy have become more and more attractive as a rescue strategy in cases where endoscopic hemostasis is not feasible or unsuccessful.Citation14
Transcatheter embolization was first described as an alternative to surgery in patients with UGI hemorrhage by Rosch in 1972. In the intervening years, there has been strong support in the literature advocating the use of transcatheter embolization as a method to control UGI bleeding. It is most useful in patients who are poor surgical candidates due to advanced age or severe underlying medical conditions, or in patients with less common causes of bleeding, such as hemobilia or ruptured pseudoaneurysm complicating pancreatitis.Citation15
Another advantage of transarterial embolization (TAE) is that most of the patients with recurrent bleeding after initial treatment with surgery or TAE could be effectively treated with TAE, thus avoiding a second surgical procedure.Citation16
In our study scanning was performed with a 128-slice CT system. We used a multi-phase acquisition, which was designed to optimize detection of most of the common causes of GI bleeding. Huprich et al., and Jaeckle et al., have recommended a triple-phase acquisition protocol to increase the sensitivity for the detection of GI bleeding.Citation9,Citation17
In our study contrast material (350 mg I/ml) was injected at the rate of 4–5 ml/s. This agrees with Huprich et al., contrast material (300 mg I/ml) was injected with a flow rate of 4 ml/s, and 64-slice MDCT was able to identify the bleeding source in 45% of patients.Citation9
Our study included 57 patients; CTA was performed in 43 cases. 37 patients had positive CTA (86.05%), of which 27 cases had positive bleed on angiography (62.7%), and 10 did not show active bleeding on angiography. 6 patients had a negative CTA and did not proceed to angiography.
The remaining 14 patients did not have a CTA and proceeded straight to angiography; 6 of them showed active bleeding on angiography
A total of 33 patients with active bleeding were embolized (27 patients had positive CTA plus 6 patients didn’t have CTA and proceeded straight to angiography).
10/33 of the embolized cases were post-operative (30.3%); the commonest cause was post-ERCP sphincterotomy (3 cases) followed by post-colonoscopy biopsy (2 cases). In 5/33 of the embolized cases there was spontaneous GI bleeding (15.15%) in patient on thrombolysis, anticoagulation or hemophilic patients. In 3/33 the underlying cause for bleeding was pancreatitis (9.09%). 3/33 patients had duodenal ulcer (9.09%). 1/33 had bleeding rectal cancer (3.03%). 1/33 had bleeding gastric ulcer (3.03%). In 10/33 patients there was no underlying cause for bleeding (30.3%).
The initial technical success rate (effective embolization of the target vessel) was 100% in our study. Funaki et al.Citation18 achieved 96% hemostasis in a series of 25 patients.
Microcoils have become the agent of choice for many interventionalists. The wide range of available coil sizes allows custom selection of coil to match the vessel diameter. Because these coils are highly radiopaque, they can more accurately be deployed.Citation19 In our study, all cases were embolized using coils apart from one case of aorto-enteric fistula where a stent-graft was used.
Early surgical conversion because of embolization-induced bowel ischemia is between 5% and 15% of cases. Re-bleeding after an initially successful therapeutic embolization is also acceptably low with recurrent lower GI bleeding rates of 10–30% within the first month after successful therapeutic angiography.Citation14 In our study, there was recurrent bleeding in one case (3%) of ascending colon bleeding of no underlying cause. In this case, bleeding recurred three days after the initial successful embolization, and it required a second embolization setting; which controlled the bleeding effectively. None of our cases required surgery post-embolization.
The safety and effectiveness of superselective microcoil embolotherapy are supported in the literature. In our study, no immediate post-procedural complications were encountered regarding puncture site hematoma or coil migration. There were no reported cases of delayed postembolic infarction. We agree with Eriksson et al.Citation16 who reported that none of their patients demonstrated any complications in the short or long term that could be related to the TAE procedure. Kuo et al.Citation19 demonstrated the safety of superselective microcoil embolization in 22 patients with no major ischemic complications.
In one of the cases, the patient had a blind percutaneous left renal core biopsy in the morning and later on in the day he was shocked and hypotensive; the patient was referred to interventional radiology, and an MDCT was organized; the CT showed active bleeding arising from the colon rather than bleeding from the left kidney as would be expected from the history of the patient. The explanation would be that there was transfixation of the left kidney during biopsy with subsequent injury to the arterial supply of the colon. This highlights the value of MDCT for localizing the source of bleeding. In this case, the bleeding site was identified at angiography to be at the marginal artery and hence, distal embolization at that level was done successfully using micro-coils. This agrees with results of Evangelista et al.Citation20, and Kuo et al.Citation19 that a distal site at the level of the vasa recta or marginal artery should be chosen for embolization in order to minimize the region at risk for ischemia.
Eriksson et al. found that the frequency of repeat bleeding might be reduced if the bleeding site can be marked during gastroscopy with a metallic clip.Citation16 In one of our cases, the patient presented with upper GIT bleeding, and endoscopy showed severe hemorrhage from a duodenal ulcer; the endoscopist could not control the bleeding, but inserted a metallic clip at the site of bleeding as a tag to guide embolization. In this case an MDCT was not needed because the site of bleeding was identified and tagged by the radio-opaque metallic clip.
5 Conclusion
MDCT angiography should be considered the primary imaging modality in the diagnosis of active GI bleeding. A positive MDCTA allows earlier targeted embolization, while a negative MDCTA can prevent patients having an unnecessary high-risk interventional procedure.
Transcatheter embolization is an effective tool for control of GI bleed and is less invasive than surgery. It has become more attractive alternative in cases where endoscopic hemostasis is not feasible or unsuccessful.
Conflict of Interest
Author states that there is no conflict of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 18 December 2015
References
- S.C.KrämerJ.GörichN.RilingerEmbolization for gastrointestinal hemorrhagesEur Radiol102000802805
- H.ScheffelT.PfammatterS.WildiP.BauerfeindB.MarincekH.AlkadhiAcute gastrointestinal bleeding: detection of source and etiology with multi-detector-row CTEur Radiol176200715551565
- R.LoffroyB.GuiuP.D’AthisArterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleedingClin Gastroenterol Hepatol72009515523
- D.A.PeuraF.L.LanzaC.J.GostoutP.G.FoutchThe American College of gastroenterology bleeding registry: preliminary findingsAm J Gastroenterol9261997924928
- L.L.StrateC.R.NaumannThe role of colonoscopy and radiological procedures in the management of acute lower intestinal bleedingClin Gastroenterol Hepatol82010333343
- T.JaeckleG.StuberM.H.HoffmannM.JeltschB.L.SchmitzA.J.AschoffDetection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CTEur Radiol187200814061413
- W.YoonY.Y.JeongS.S.ShinAcute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CTRadiology23912006160167
- T.P.JainM.S.GulatiG.K.MakhariaS.BandhuP.K.GargCT enteroclysis in the diagnosis of obscure gastrointestinal bleeding: initial resultsClin Radiol6272007660667
- J.E.HuprichJ.G.FletcherJ.A.AlexanderJ.L.FidlerS.S.BurtonC.H.McCulloughObscure gastrointestinal bleeding: evaluation with 64-slice multiphase CT enterography—initial experienceRadiology24622008562571
- T.H.PatelP.R.CordtsP.AbcarianM.A.SawyerWill transcatheter embolotherapy replace surgery in the treatment of gastrointestinal bleeding?Curr Surg5832001323327
- C.J.LaingT.TobiasD.I.RosenblumW.L.BankerL.TsengS.W.TamarkinAcute gastrointestinal bleeding: emerging role of multidetector CT angiography and review of current imaging techniquesRadioGraphics274200710551070
- E.W.LeeJ.M.LabergeDifferential diagnosis of gastrointestinal bleedingTech Vasc Interv Radiol732004112122
- T.L.AngtuacoS.K.ReddyS.DrapkinL.E.HarrellC.W.HowdenThe utility of urgent colonoscopy in the evaluation of acute lower gastrointestinal tract bleeding: a 2-year experience from a single centerAm J Gastroenterol966200117821785
- G.MaleuxF.RoeflaerS.HeyeLong-term outcome of transcatheter embolotherapy for acute lower gastrointestinal hemorrhageAm J Gastroenterol104200920422046
- S.K.KimV.DuddalwarFailed endoscopic therapy and the interventional radiologist: non-variceal upper gastrointestinal bleedingTech Gastrointest Endosc72005148155
- L.G.ErikssonM.LjungdahlM.SundbomR.NymanTranscatheter arterial embolization versus surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failureJ Vasc Interv Radiol19200814131418
- T.JaeckleG.StuberM.H.HofmannW.FreundB.L.SchmitzA.J.AschofAcute gastrointestinal bleeding: value of MDCTAbdom Imag332007285293
- B.FunakiJ.K.KostelicJ.LorenzSuperselective microcoil embolization of colonic hemorrhageAJR Am J Roentgenol1772001829836
- W.T.KuoD.E.LeeW.E.A.SaadN.PatelL.G.SahlerD.L.WaldmanSuperselective microcoil embolization for the treatment of lower gastrointestinal hemorrhageJ Vasc Interv Radiol14200315031509
- P.T.EvangelistaM.J.HalliseyTranscatheter embolization for acute lower gastrointestinal hemorrhageJ Vasc Interv Radiol112000601606
