Abstract
Background
Mesenchymal stem cells (MSCs) are recruited to the stroma of cancers. They interact with cancer cells to promote invasion and metastasis or to suppress tumor growth. The unique tumor-homing capacity of MSCs makes them a promising vehicle to deliver various anticancer agents.
Aim
The aim of this study was to detect the possibility of using mesenchymal stem cells as a future weapon against breast cancer.
Methods
PubMed, PubMed central, Springer and Cochrane databases were searched using specified terms.
Results
Literature search yielded 17 manuscripts: seven of which suggested the use of MSCs in breast cancer therapy, while six studies raised the possibility that MSCs may promote tumor growth and four other studies assumed a dual role for MSCs.
Conclusions
The role of MSCs in breast cancer therapy is still debatable. We recommend future research in the field of MSCs in Alexandria University as it is our hope in the fight against breast cancer.
1 Introduction
Breast cancer is the most common malignancy among females throughout the world. Combined therapeutic modalities including surgery, chemotherapy, radiotherapy, endocrine and targeted therapies are the mainstay of treatment. However, they may lead to unsatisfactory outcomes, mainly due to the difficulty in accessing tumor sites, the dispersed nature of the disease and the toxicity of the treatment.Citation1
MSCs were originally isolated from bone marrow, and later from adipose tissues and many other organs. These cells are capable of self-renewal and differentiation into bone, fat, or cartilage cells under appropriate conditions.Citation2
MSCs are often involved in tissue remodeling after injury or chronic inflammation. Tumors resemble chronic wounds or “wounds that never heal”.Citation2 The recruited MSCs and their derivative cancer-associated fibroblasts interact with cancer cells to promote invasion and metastasis. On the other hand, the unique tumor-homing capacity of MSCs renders them a promising vehicle for adequate and specific delivery of various anticancer agents.Citation2
This article discusses the potential of using MSCs as a weapon against breast cancer.
2 Methods
PubMed, PubMed central, Springer and Cochrane databases were searched using specified terms. Key words used were mesenchymal stem cells, mesenchymal stem cells and breast cancer, breast cancer.
Inclusion criteria Publications on mesenchymal stem cells were included in the study. Reference lists of reviews and research articles were examined for relevant publications.
Exclusion criteria Case reports, letters, reviews without original data, non-English language papers, abstracts, and articles with incomplete data were excluded.
3 Results and discussion
Various studies have reported contradicting results as regards the role of MSCs in breast cancer. While several studies support the growth promoting effects of MSCs, others suggest that they act as tumor suppressive agents. A dose dependent effect has been proposed to explain these varying roles; at lower cell numbers (relative to the number of tumor cells) human MSCs are more likely to inhibit tumor growth, whereas at higher cell numbers they promote tumor growth.Citation3 Alternative theories state that these varying roles might be related to the presence of different subpopulations among MSCs, individual variations in the physiological immune status of the donors, and differences in MSC isolation and culture methods.Citation4 Other mechanisms have been reported as well including chemokine signaling, modulation of apoptosis, vascular support, and immune modulation.Citation5
3.1 Suppression of tumor growth
The role of MSCs as tumor suppressors depends mainly on their involvement in tissue remodeling after injury or chronic inflammation. MSCs are recruited specifically to the tumor site to form tumor stroma.Citation2
The avid tropism of MSCs to tumors, as well as their ability to engraft, survive, and proliferate in the tumor architecture, renders them prime cellular vehicles for the delivery of anti-neoplastic therapy to both primary tumors and their metastases.Citation4
Brennen et al.Citation1 demonstrated that MSCs engrafted in tumors could act as stromal precursor cells and successfully function as cellular vehicles for gene delivery and contribute to the local production of biological agents.Citation1
A number of approaches have been utilized in this regard. For example the use of genetically modified MSCs to kill malignant cells has shown positive results.Citation6 ()Citation4 Systemically-infused interferon beta (IFN-β) expressing bone marrow-derived MSCs successfully reduced the growth of human breast cancer cells from MDA-MB-231 line upon engraftment into the tumor stroma.Citation6
Figure 1 MSC-based caner therapy. Several approaches have been applied using MSCs as a delivery system for anti-neoplastic agents. These methods culminate in cancer cell killing through different mechanisms. Some of which include enhancing the host’s local immune responses through MSC gene modifications to express IFN-β, IL-2, or IL-12; inhibiting growth of malignant cells through MSC release of oncolytic viruses (CRAd-MSCs); including caspase-8-dependent apoptosis through TRAIL-expressing MSCs; or bystander killing through soluble toxic compounds secreted by MSCs expressing pro-toxin converting enzymes, such as HSV-tk, CD, or 5-FC. Abbreviations: MSCs, mesenchymal stem cells; IFN-β, interferon-beta; IL-2, interlukin-2; IL-12, interlukin-12; CRAd, conditionally replicating adenoviruses; TRAIL, TNF related apoptosis inducing ligand; HSV-tk, human herpes simplex virus thymidine kinase type 1; 5-FC, 5 flucytosine.
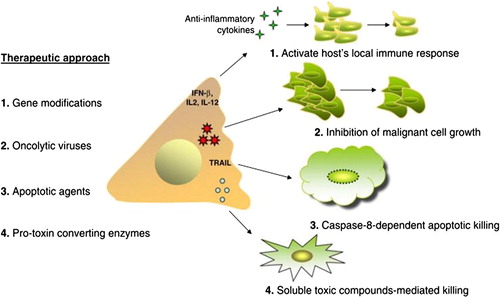
BALB/c murine MSCs home specifically to mouse 4T1 sites and deliver murine IFN-β to the tumors sufficient to inhibit breast cancer growth through inactivation of the Stat3 signaling pathway. In addition, MSC/IFN-β/GFP treatment also showed signs of an improvement in cell-mediated immunity as indicated by the increased numbers of splenic mature dendritic cells and decreased numbers of regulatory T lymphocytes.Citation7
Liu et al. studied the effects of hUMSCs/IL-18 (human umbilical cord MSCs genetically modified with interleukin-18 gene) on the growth, migration and invasion of two breast cancer cell lines in vitro. The results showed that hUMSCs/IL-18, but not hUMSCs, significantly inhibited the growth, migration and invasion of human breast cancer cell lines MCF-7 and HCC1937 in vitro. Flow cytometric analysis showed that hUMSCs/IL-18 significantly increased the percentage of cells in the G0/G1 phase but decreased that in the S and G2/M phase.Citation1
The second approach was the use of MSCs as intermediate carriers for conditionally replicating adenovirus (CRADs) to target metastatic breast cancer in vivo.Citation8 Stoff-Khalili et al. concluded that hMSCs may be an effective platform for targeted delivery of CRAds to distant cancer sites such as metastatic breast.
They investigated the in vivo anti-tumor activity of hMSC-Ad5/3.CXCR4 (C-X-C chemokine receptor 4). Mice injected with hMSC-Ad5/3.CXCR4 had significantly lower mean lung weights than Ad5/3.CXCR4 treated or control untreated mice. Thus, the tumor burden of breast cancer metastases in the lungs was significantly less in animals treated with CRAd loaded hMSCs than with the CRAd alone. Furthermore, treatment with hMSC-Ad5/3.CXCR4 improved survival of mice bearing breast cancer metastases in the lungs.Citation8
MSCs were found to inhibit tumor growth through a variety of other mechanisms including secretion of paracrine factors. Ma et al.Citation4 studied the effect of hUMSCs on cancer stem cells in vitro. The results showed that hUCMSCs inhibited the growth of breast cancer cell lines (MDA-MB-231 and MCF-7), and primary breast cancer stem cells (CSCs) in a dose-dependent manner. The underlying mechanism is likely related to cell cycle arrest and induction of tumor cell apoptosis.Citation4 HUMSCs were also found to inhibit the development of pulmonary metastases from breast adenocarcinoma MDA-MB-231 cell line in vivo in mice. Long-term in vivo bioluminescence imaging of intravenously injected MSCs genetically labeled with luc2 gene showed distribution of MSCs to the lungs and abdominal organs within the first 2–3 weeks and remigration to the lungs in 6–7 weeks. MSCs reduced the proliferative activity of cancer cells in vitro. The mechanism is thought to be through soluble factors.Citation9
HUCMSC transplantation is expected to become a new strategy for tumor therapy. The safety profile of hUCMSCs was satisfactory as well. HUMSCs did not establish colonies in soft agar and did not exhibit tumorigenic ability after transplantation. Therefore, the use of hUCMSCs in clinical therapy is safe and feasible.Citation4
3.2 Promotion of tumor growth by MSCs
On the other hand, several studies have documented the tumor promoting capabilities of MSCs. ().Citation4 This is proposed to occur through several mechanisms. Rhodes et al.Citation10 showed that bone marrow hMSCs have the ability to increase tumor volume, enhance estrogen sensitivity, promote hormone-independent tumor growth, and alter progesterone receptor expression. The addition of hMSC increased the estrogen response by fourfold over that of control tumors.Citation10 Dittmer et al.Citation3 demonstrated that hMSCs interfere with cell–cell adhesion and enhance migration of breast cancer cells by activating ADAM10 (a disintegrin and metalloprotease 10), known to cleave E-cadherin. Recent evidence implies that E-cadherin is the major protein that mediates MCF-7 cell–cell adhesion in spheroids.Citation3
Figure 2 Circulation-derived MSCs migrate to tumors and promote cancer progression. MSC niches (depicted here in the bone, but it could be in other tissues as well) respond to numerous signals generated by the tumor milieu. These signals, including TGF-β, IL-6, Cyclophilin B, HDGF, uPA/uPAR, MCP-1, VEGF, and FGF2, act as chemoattractants for MSCs. At the tumor site, MSCs infiltrate into the stroma and produce bioactive molecules such as CCL5, IL-6, SDF-1, and TGF-β, which promote tumour growth and/or distant metastasis to secondary organs, such as the lungs. Abbreviations: TGF-β, transforming growth factor beta; IL-6, interlukin-6; HDGF, Hepatoma-Derived Growth Factor; uPA, urokinase-type plasminogen activator; uPAR, urokinase-type plasminogen activator receptor; MCP-1, monocyte chemoattractant protein-1; VEGF, vascular endothelial growth factor; FGF2, fibroblast growth factor 2; CCL5, chemokine (C-C motif) ligand 5; SDF-1, stromal cell-derived factor 1.
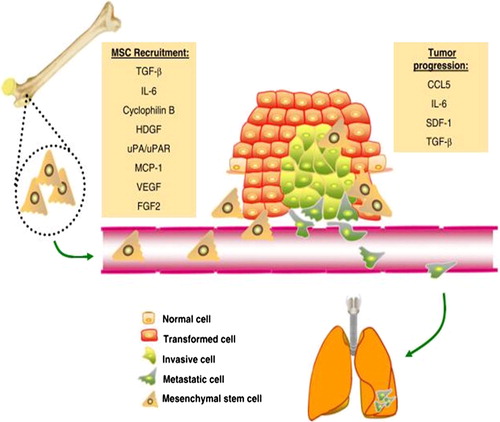
The secretion of soluble factors through exosomes is yet another mechanism by which MSCs promote tumor progression. MSCs were shown to secrete 40–100 nm particles, which have the typical characteristics of exosomes, and these MSC-derived exosomes promoted migration of the breast cancer cell line MCF-7. Global gene expression profiling revealed that several cancer-related signaling pathways were upregulated after exosome treatment in MCF7. In addition, the Wnt signaling pathway was further confirmed to be activated. These findings demonstrated a new mechanism through which MSC-conditioned media may contribute to tumor cell migration.Citation11
Furthermore, MSCs were found to promote breast cancer cell proliferation in vivo. Yan et al. showed that MSCs could enhance mammosphere formation partially via the epidermal growth factor (EGF)/epidermal growth factor receptor (EGFR)/Akt signaling pathway.Citation12
In addition, MSCs may promote breast cancer metastasis through facilitation of epithelial mesenchymal transition (EMT).Citation13,Citation14 EMT is a complex series of cellular reprogramming events which culminates in the loss of epithelial characteristics and the de novo acquisition of a mesenchymal phenotype. During embryonic development, EMT imparts the plasticity and migratory capabilities that enable the extensive cell movements underlying gastrulation and organogenesis.Citation13 It is a critical process underlying the subpopulation of breast cancer cells that is responsible for tumor initiation and for regenerating the tumor after initial bulk tumor regression following therapy.Citation15
The mechanism by which MSCs affect breast cancer cells is thought to be through soluble factors as bone morphogenetic protein 9 (BMP9). BMP 9 may regulate the cross-talk between MDA-MB-231 breast cancer cells and HS-5 bone marrow-derived MSCs in a co-culture system. Wan et al.Citation16 indicated that BMP 9 can reduce receptor activator of nuclear factor Kappa B ligand (RANKL) secretion by HS-5 cells and inhibit the invasion of MDA-MB-231 cells through blocking the AKT signaling pathway. Simultaneously, BMP 9 can promote the osteogenic differentiation and proliferation of human bone marrow stromal cell line HS-5 cells in the tumor microenvironment.Citation16
In summary, MSCs interact with tumor cells in a myriad of ways, which have the potential to support or suppress tumor growth. The heterogeneity in MSCs is likely a major factor contributing to the inconsistent reports about the effects of MSCs on tumors. No evidence of tumor formation has been reported in over 1000 patients treated with MSCs for a variety of indications, so far.Citation5
The use of hUCMSCs in clinical therapy is expected to be safe and feasible.Citation4 However, the possibility of MSCs promoting tumor growth and metastasis raises concerns about the safety of their use as clinical tools.Citation5 Further research in the field of mesenchymal stem cells and breast cancer is required before they can be widely used in the treatment of breast cancer.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 17 February 2016
References
- X.LiuJ.HuS.SunF.LiW.CaoY.WangMesenchymal stem cells expressing interleukin-18 suppress breast cancer cells in vitroExp Ther Med20201511921200
- Q.ZhaoF.LiuMesenchymal stem cells in progression and treatment of cancersFront Biol (Beijing)932014186194
- A.DittmerK.HohlfeldJ.LützkendorfL.P.MüllerJ.DittmerHuman mesenchymal stem cells induce E-cadherin degradation in breast carcinoma spheroids by activating ADAM10Cell Mol Life Sci66200930533065
- Y.MaX.HaoS.ZhangJ.ZhangThe in vitro and in vivo effects of human umbilical cord mesenchymal stem cells on the growth of breast cancer cellsBreast Cancer Res Treat1332012473485
- A.H.KloppA.GuptaE.SpaethM.AndreeffF.MariniConcise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth?Stem Cells2920111119
- C.P.El-HaibiA.E.KarnoubMesenchymal stem cells in the pathogenesis and therapy of breast cancerJ Mammary Gland Biol Neoplasia152010399409
- X.LingF.MariniM.KonoplevaW.SchoberY.ShiJ.BurksMesenchymal stem cells overexpressing IFN-β inhibit breast cancer growth and metastases through Stat3 signaling in a syngeneic tumor modelCancer Microenviron320108395
- M.A.Stoff-KhaliliA.A.RiveraJ.M.MathisN.S.BanerjeeA.S.MoonA.HessMesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinomaBreast Cancer Res Treat1052007157167
- A.V.MeleshinaE.I.CherkasovaM.V.ShirmanovaN.V.KlementievaE.V.KiselevaL.B.SnopovaInfluence of mesenchymal stem cells on the metastases development in mice in vivoStem Cell Res Ther62015110
- L.V.RhodesS.E.MuirS.ElliottL.M.GuillotJ.W.AntoonP.PenfornisAdult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independenceBreast Cancer Res Treat1212010293300
- R.LinS.WangR.C.ZhaoExosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell modelMol Cell Biochem38320131320
- X.L.YanC.J.FuL.ChenJ.H.QinQ.ZengH.F.YuanMesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathwayBreast Cancer Res Treat1322012153164
- N.SphyrisS.A.ManiThe importance of the epithelial-mesenchymal transition in breast cancerCurr Breast Cancer Rep12009229237
- F.T.MartinR.M.DwyerJ.KellyS.KhanJ.M.MurphyC.CurranPotential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT)Breast Cancer Res Treat1242010317326
- C.J.CreightonJ.C.ChangJ.M.RosenEpithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancerJ Mammary Gland Biol Neoplasia152010253260
- S.WanY.LiuY.WengW.WangW.RenC.FeiBMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cellsCell Oncol372014363375
