?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This paper is an attempt to establish the mathematical models to understand the distribution of drug administration in human body through oral and intravenous routes. Three models were formulated based on diffusion process using Fick’s principle and law of mass action. The rate constants governing the law of mass action were used on the basis of the drug efficacy at different interfaces. The Laplace transform and eigenvalue methods were used to obtain the solution of the ordinary differential equations concerning the rate of change of concentration in different compartments viz. blood and tissue medium. The drug concentration in the different compartments has been computed using numerical parameters. The graphs plotted illustrate the variation of drug concentration with respect to time using MATLAB software. It has been observed from the graphs that the drug concentration decreases in the first compartment and gradually increases in other compartments.
1 Introduction
The relation between drug intake and concentration of drug at the target site through various compartments in biological processes is considered to be the subject of great importance. The dosage and inflow and outflow of drug in the processing compartments have both favourable and adverse effects on human body. The researchers in pharmacokinetics studied the behaviour of an administered drug or chemical among various compartments of the human body over a period of time. It helps to understand the relationships between the rates of absorption, distribution and elimination process of the drug within the body and helps to establish the desired therapeutic response. Mathematical modelling for drug diffusion constitutes an influential predictive tool to have the basic understanding of bio-transport processes. Although the mathematical modelling is theoretical in nature; however, the results established lead to realistic outcome once compared and verified empirically. In the absence of experiments, a good number of mathematical models and numerical simulations were carried out with an efficiency up to large extent. Compartment modelling plays a key role in pharmacokinetics due to local processes in each part of the compartment. Compartment model is the mathematical representation of the body or a part of the body created to study physiological or pharmacological kinetic characteristics. The body is represented as a series of compartments arranged either in series or in parallel depending upon the process or transport of material. These models can be used to understand the transport processes between interconnected volumes, such as the flow of drugs or any chemical within the body. A compartment model helps in understanding biological processes involved in the kinetic behaviour of a drug introduced into the body tissues. Depending upon the behaviour of drug, the body is comprising of a single or more compartment system. In one compartment model, the whole human body is considered as a homogeneous unit in which an administered drug diffuses instantaneously within the blood. In case of two compartment model, the body can be represented as two although separate but connected compartments viz. the central compartment and the peripheral one. One of the early uses of the compartment models was studied by Widmark in 1920s to model the propagation of alcohol in the body.Citation1
Feizabadi et al.Citation2 discussed two compartment model interacting with dynamic anti-cancer agents. KochCitation3 discussed the application of mathematics in pharmacokinetics by using one and two compartment models. Olga et al.Citation4 developed a two-compartment mathematical model to investigate cholesterol transport in the circulatory system and its de novo synthesis in the liver. Cherrauault and SarinCitation5 studied three compartment open model with two time lags. Their model deals with the identification of exchange parameters involved in a three compartment open model with two time lags in which elimination occurs from the central compartment. Ardith and TimothyCitation7 studied a mathematical model to compare bolus injection, continuous infusion for various duration, liposomal and thermoliposomal delivery of doxorubicin. Mina et al.Citation8 studied the diffusion process of a drug through a skin-like membrane by making use of transformation group theoretical approach. Earlier, weCitation9 studied the drug distribution in TDD systems by making use of variational finite element method taking absorption rate of drug by the tissue as decreasing function of drug concentration. Further, we used FEM to study the drug distribution in TDD systems in unsteady state case by making use of quadratic shape function.Citation10 Moreover, Khanday and NajarCitation11,Citation12 established the mathematical models on oxygen transport in biological tissues through capillary bed using both analytical and numerical methods. In this study, we extended the diffusion of drug in blood and tissue using three mathematical formulations and the behaviour of drug concentrations in relation with physiological parameters has been studied.
2 Mathematical model
The mathematical analysis always leads to optimal solution to various complex problems. Thus, it is imperative to establish mathematical model to estimate the drug concentration at different sites and within the blood. When the drug is orally administered, it dissolves and releases the medications into the gastrointestinal tract. The medications diffuse from there into the blood and the bloodstream takes medications to the site where it has therapeutic effect. The medications are gradually cleared from the blood by the liver and the kidneys. The flow of drugs within the body is modelled by treating the different parts of the body as compartments and then tracking the medication as it enters and leaves each compartment. The drug leaves one compartment and enters into the another one at the rate proportional to the concentration of drug present in the first compartment. The rate of drug movement between compartments is described by the first order kinetics. The constant of proportionality is mainly determined by the drug, the compartment and general health of the individual. If denotes the concentration of drug in the compartment at time t, then the rate of change of
is
This principle is based on the law of conservation of mass and is known as the Balance Law.Citation6
2.1 Model-I
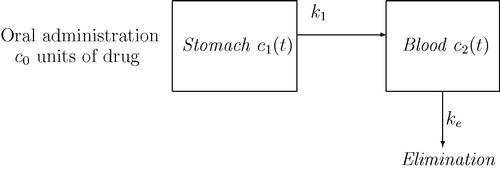
A two compartment model for drug absorption and circulation through gastrointestinal tract and blood has been formulated in the beginning. The first compartment corresponds to the GI tract and from there the drug diffuses into the second compartment namely blood as shown in . Let denote the concentration of drug in stomach or GI tract and blood stream compartments respectively. Let
be the initial concentration of drug dosage. The general form of the two compartment model describing the rate of change in oral drug administration is given as
(1)
(1)
Each equation describes the change in drug concentration in their respective compartments over time. Also the quantities denote the rate constants from one compartment to another and the clearance constant. Eq. (Equation1
(1)
(1) ) are based on modelling the single dosage of drug flow via GI tract to tissue.
Solving Eq. (Equation1(1)
(1) ), we have
(2)
(2)
(3)
(3) Eq. (Equation2
(2)
(2) ) corresponds to the exponential decay of drug in terms of absorption. Hence, the long term behaviour of drug concentration in GI tract will diminish to level zero.
The dosage of medicine varies from patient to patient depending upon the condition and severity of the disease. Thus, the amount of drug administration is not uniform. The oral drug medication is considered to be less efficient in some cases due to several reasons, including stomach sensitivity, liver dysfunction, delayed reaction, etc. Under such conditions, the effective and rapid drug dosage is mainly based on intravenous administration. Also in some emergency cases, medicine needed to be rapidly absorbed by the body tissues. Presence of some enzymes may break down certain delicate medications, so these medicines are directly injected into the blood stream.
2.2 Model-II
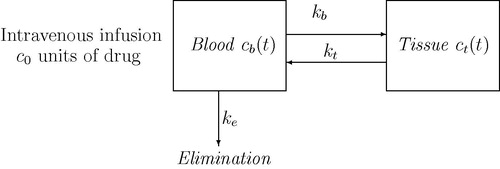
In order to formulate a mathematical model for the intravenous drug administration, two different models Model-II and Model-III were considered on the basis of reversible and irreversible rate constants. In this model, only two compartments viz. blood and tissue were identified as the main exchangers. The first one is the blood stream into which the drug is injected and the second one is the tissue where the drug has the therapeutic effect. We assume that blood takes a part of drug at the rate of onto tissue and a fraction of it gets eliminated from blood with the clearance rate of
.
Thus the mathematical formulation in this case is governed by the system of two ODEs, each equation describing the rate of change of drug concentration with respect to time in their respective compartments as shown in .
Let denote the concentration of drug in the compartments blood and tissue respectively and
be the initial concentration of drug injected through intravenous route the body. Then the mathematical form of a two compartment model describing the drug administration is
(4)
(4) Rewrite Eq. (Equation4
(4)
(4) ) in the matrix form as
(5)
(5) where
(6)
(6) with initial condition
.
Applying Laplace transformor,
(7)
(7) where
represents the Laplace transform, and
In order to solve the system of Eq. (Equation7
(7)
(7) ), we require
.
Now,(8)
(8) where
(9)
(9) Clearly
i.e., the eigenvalues
are real.
Making use of Eq. (Equation9(9)
(9) ) together with the
, it is clear that
, for all
.
Hence,(10)
(10) and
(11)
(11) To calculate the actual concentration of drug in the blood compartments and in tissue, we invoke the inverse Laplace transform of Eqs. Equation10
(10)
(10) Equation11
(11)
(11) and make use of Heaviside’s theorem. Therefore, we have
(12)
(12)
(13)
(13) where
and
are the roots of the equation
For
, it follows from Eq. (Equation12
(12)
(12) ) that
and from Eq. (Equation13
(13)
(13) ) it is clear
, which is not possible.
2.3 Model-III
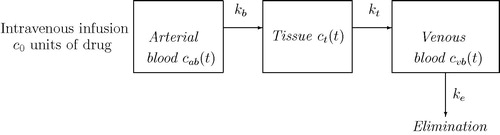
Since the blood flow in cardiovascular system is one directional, therefore the drug administration through venous blood admits the pattern shown in . It is evident that the drug carried out by the venous blood hits the target site through the capillary bed and the residual drug either gets eliminated or taken back by the arterial blood to the circulatory system. Assume that the consumption of drug by arterial blood towards tissue flows at the rate of and from tissue compartment to the venous blood with the rate of
. Because the level of drug in the venous blood increases with time and ultimately reaches to zero when the kidneys and liver excrete the drug from the body organs. Let
be the clearance rate of drug from the blood.
Figure 3 Drug administration through arterial blood and tissue and venous blood with initial drug intake .
Let denote the concentration of drug in the arterial blood, tissue and venous blood compartment respectively with
as initial drug dosage. The mathematical formulation for the drug concentration with respect to these compartments is based on the following system of initial value problems:
(14)
(14) On solving Eq. (Equation14
(14)
(14) ), we have
(15)
(15)
(16)
(16)
(17)
(17) Since
, it follows that
are all positive.
3 Discussion
The paper describes three compartment models for the drug distribution through oral and intravenous modes. Depending upon the condition of the patient and severity of the disease, an oral or intravenous drug administration can be followed. The formulation of these models is based on the Fick’s perfusion principle, first order kinetics and balance law. These models were solved by using eigenvalue method and the results were plotted using MATLAB software. Laplace transform method was additionally used to solve Model-II in order to avoid the complication raised while finding the eigenvector in eigenvalue method.
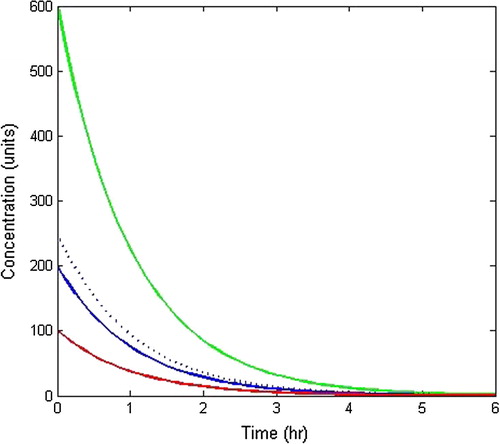
In Model-I, two different situations were discussed - first when whole body is treated as a single compartment and second when the body is composed of two compartments. The behaviour of drug concentration within the body as a single compartment has been analysed by plotting graphs between drug concentration and time as shown in with an initial dosage of drug as 500 units at different rate constants. It is clear from the graphs that drug concentration gradually decreases with respect to time. From the curves of , it is depicted that more the rate constants, faster will be the absorption of drug within the body while as at lesser value of rate constant, a portion of drug will be retained within the body as a residue, thereby causing side effects. In order to avoid this process, two compartment model has been taken in which elimination also plays a vital role. Also, shows the drug concentration pattern within the body at different initial concentrations of units with
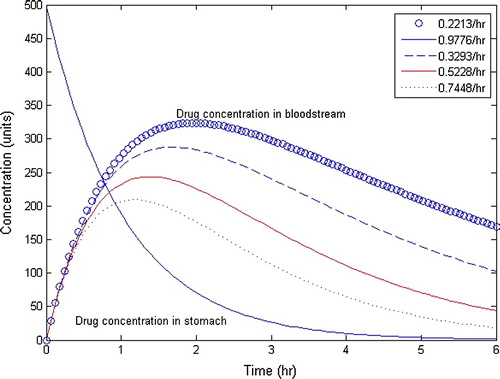
/hr. The drug dosage varies at different situations depending upon the concentration of the patient. For a prolonged dosage of amount of drug, the amount of drug infusion varies with different time intervals. Also, the efficacy of drug can be determined on the basis of drug dosage. In order to have the maximum therapeutic effect rate constants can be adjusted accordingly. shows the behaviour of drug concentration in the stomach and the bloodstream. It is evident from the graphs that the drug level in the GI tract decreases with the passage of time. Also, it is clear that the drug concentration in the blood increases from zero and reaches maximum level and then decreases.
Figure 4 Drug concentration pattern within the body as single compartment with different rate constants.
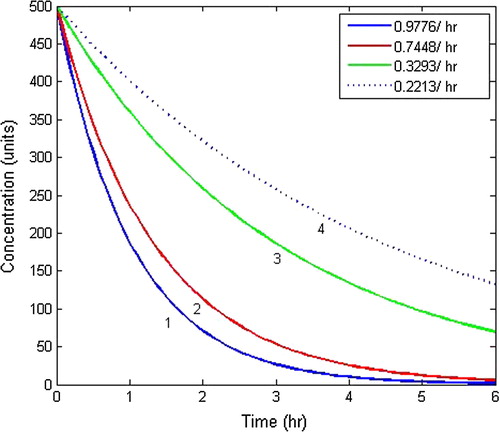
Figure 6 Drug distribution pattern in oral administration with /hr and 500 units of initial drug dosage for Model-I.
To see the behaviour of drug concentration with respect to Model-II and III, two different set of rate constants were taken as follows: In case I, while as in case II,
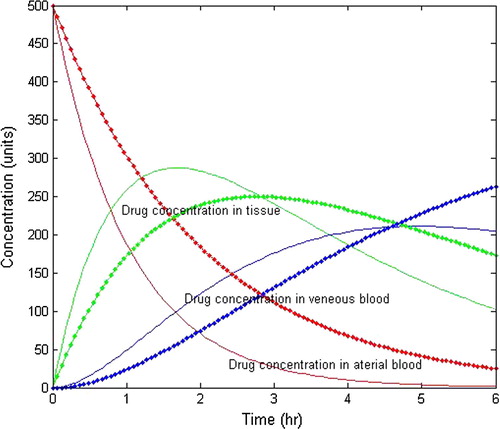
(/hr). The pattern of graph can be improved by incorporating the proper transport system of the drug. In order to obtain better results, Model-III has been formulated with a part of drug after absorption transferred from blood into tissue via arteries and back from tissues a fraction of drug is taken into blood via veins. The effect of blood flow is one of the pivotal differences between Models-II and III. shows the variation of drug concentration in arterial blood and venous blood. From graphs, it is clear that drug concentration in the arterial blood stream decreases rapidly and in the meantime, it increases in the venous blood.
Figure 7 Drug distribution pattern in intravenous administration with 500 units of initial drug dosage for Model-III.
The models established in this study are applicable in three types of problems on drug diffusion in biological tissues. The Models-I, II and III were formulated based on the various limitations arising from the diffusion processes taking place across the compartments in the biological systems. The models can be extensively used for various drug diffusion problems arising in pharmaceutical studies. Moreover, the models generalize the properties of drug diffusion to flexibility of parameters and role of drug infusion through different routes. The study has a good number of applications in drug control, drug dosage and other related problems of pharmaceutical research.
Conflict of interest
None declared.
Acknowledgement
The authors wish to thank the DST, Govt. of India for providing financial support under DST- INSPIRE programme.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 26 July 2016
References
- Widmark EMP. Principles and applications of medicolegal alcohol determination. English translation of 1932, German edition, Davis Publications; 1981.
- M.S.FeizabadiC.VolkS.HirshbeckA two compartment model interacting with dynamic drugsAppl Math Lett22200912051209
- G.A.Koch-NobleDrugs in the classroom: using pharmacokinetics to introduce biomathematical modelingMath Model Nat Phen662011227244
- O.HrydziuszkoA.WronaJ.BalbusK.KubicaMathematical two-compartment model of human cholesterol transport in application to high blood cholesterol diagnosis and treatmentElec Notes Theo Comp Sci30620141930
- Y.CherruaultB.SarinV.A three compartment open model with two time lagsInt J Biomed Comput321993269277
- R.L.BorrelliC.S.ColemanDifferential equations, a modelling perspective2004John Wiley and Sons, Inc.
- A.W.El-KarehT.W.SecombA mathematical model for comparison of bolus injection, continuous infusion and liposomal delivery of doxorubicin to tumor cellsNeoplasia242000325338
- M.B.Abd-el-MalekM.M.KassemM.L.M.MekyGroup theoretic approach for solving the problem of diffusion of a drug through a thin membraneJ Comp Math142002111
- M.A.KhandayRafiqAasmaVariational finite element method to study the absorption rate of drug at various compartments through transdermal drug delivery systemAlexandria J Med5132015219223
- M.A.KhandayRafiqAasmaNumerical estimation of drug diffusion at dermal regions of human body in transdermal drug delivery systemJ Mech Med Bio16320161650022 9 pp. doi: http://doi:10.1142/S0219519416500226
- M.A.KhandayA.NajarMaclaurin’s series approach for the analytical solution of oxygen transport to the biological tissues through capillary bedJ Med Imag Health Informatics552015959963
- M.A.KhandayA.NajarMathematical model for the transport of oxygen in the living tissues through capillary bedJ Mech Med Bio1542015155005510.1142/S0219519415500554