Abstract
Background
MDR continues to be a major challenge to effective chemotherapeutic interventions against cancer. Defining major factor contributing to MDR and inhibiting their action may thus be used for reversing MDR.
Aim
This work aimed to evaluate the role played by MRP-1 and GST-Pi in MDR, and to explore the possible role of indomethacin as an inhibitor of chemotherapy resistance in patients with AML.
Subjects and methods
The study included 2 groups, one included 20 healthy volunteers and the second included 50 AML patients. All patients received one cycle of standard induction chemotherapy, then regrouped according to their response to either CR group or unremitted group. Unremitted patients received a second cycle of chemotherapy combined with indomethacin. From each subject a blood sample was drawn before and after the 1st cycle of chemotherapy and after the 2nd cycle. From blood, mononuclear cells were separated, mRNA was extracted, and RT-PCR was carried out to detect GST-Pi and MRP-1 gene expression.
Results
GST-Pi expression in CR group was 60% before therapy that significantly decreased to 30% after therapy. While in unremitted group, its expression significantly increased from 30% before to 80% after therapy. GST-Pi positive patients had a significantly lower overall and disease free survival time than GST-Pi negative patients (P = 0.000 and 0.039, respectively). While MRP-1 expression was so low (20%) and remained unchanged after therapy in both groups. MRP-1 expression did not affect overall or disease free survival. Taking indomethacin with 2nd cycle of chemotherapy in unremitted patients resulted in a significant inhibition of GST-Pi expression and a significantly longer overall survival time than those taking 2nd cycle chemotherapy alone (P = 0.034).
Conclusion
MRP-1 is not likely to contribute to MDR, while GST-Pi might have a role in MDR phenotype in AML patients. Furthermore, GST-Pi inhibition significantly reduced MDR in AML patients.
1 Introduction
Multidrug resistance (MDR) is a prime problem restricting the success of chemotherapy in patients with a variety of hematological and solid malignancies. MDR can be defined as the cross-resistance of cancer cells to a number of structurally or functionally unrelated anticancer drugs. Tumors are composed of heterogeneous populations of malignant cells. Some populations are chemo-sensitive while the remaining are chemo-resistant. Chemotherapy kills sensitive cells, leaving a high proportion of resistant cells that would begin to grow again, and chemotherapy may fail.Citation1
Various mechanisms were proposed that underlie MDR in malignant cells. One mechanism, involves molecular pumps present in membranes of tumor cells that actively eject drugs from the cell, which allows tumor cells to avoid the toxic effects of the drug. The pump that was found responsible for MDR in acute myeloid leukemia (AML) is the multidrug resistance–associated protein-1 (MRP-1). MRP1 is an ATP-binding cassette transporter protein which was identified as the gene that confers MDR in lung cancer cells. MRP1 acts in order to protect certain tissues from xenobiotics by mediating the efflux of many organic anions, including glutathione conjugates. MRP1 participates in cellular efflux of reduced and oxidized forms of glutathione and thus contributes to the many physiological and pathophysiological processes influencing glutathiones, especially oxidative stress.Citation2
Another important mechanism of MDR in cancer cells is by increasing cell detoxifying enzymes in tumor cells, most importantly Glutathione-S-Transferase-Pi (GST-Pi).Citation3 GST-Pi is a cytosolic phase II detoxification enzyme that promotes the conjugation of glutathione to an electrophilic center of endogenous and exogenous compounds, resulting in glutathione-conjugates formation. GST-Pi is thought to participate in the development of drug resistance in two ways, the first via direct detoxification of chemotherapy agents. The second, through direct glutathionylation of critical signaling molecules that may serve as a trigger for cellular events that are influenced by oxidative stress. More specifically, GST-Pi inhibits the MAPK pathway that promotes cell survival in response to oxidative stress.Citation4
Since its discovery, overcoming MDR has been a major concern in cancer therapy. This is most effectively achieved through inhibiting the action of key role players in the molecular mechanisms underlying MDR, thus reversing MDR.Citation5 Thus inhibiting MRP-1 and/or GST-Pi is expected to reverse MDR in patients expressing either of them. Indomethacin, 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic acid, is a non-steroidal anti-inflammatory drug that displays anti-inflammatory, analgesic, and antipyretic properties. It acts by non-selectively inhibiting both cyclooxygenase (COX)-1 and COX-2.Citation6 Studies have suggested that indomethacin can modulate both GST-Pi and MRP-1, as it is a potent non-competitive inhibitor of GST-Pi,Citation7 and by inhibiting the MRP-1 promoter activity, it inhibits MRP-1.Citation8
This work aimed to evaluate the role of MRP-1 and GST-Pi in multidrug resistance in AML patients, and to explore the possible role of indomethacin as an MDR inhibitor in leukemic patients.
2 Subjects and methods
2.1 Subjects
The study included 70 subjects assigned to 2 groups: group 1, Included 20 healthy volunteers (8 males and 12 females) clinically free from any disease, their mean age was 42.40 ± 4.47 y, and group 2, Included 50 patients with AML (26 males and 24 females), of matched age (47.72 ± 2.46 y) as group 1. Patients were recruited from the Haematology Department, Medical Research Institute, Alexandria University; during the period from March 2006 till December 2008. The study has been approved by the local ethics committee, and subjects enrolled in the study provided an informed consent each.
2.2 Methods
To all patients the following was done: full history recording, thorough clinical examination, routine laboratory investigations including complete blood picture, liver and kidney functions, Radiological investigations including chest X-ray, abdominal ultrasound and ECG, and bone marrow examination including morphological and cytochemical confirmation for FAB classification.
Exclusion criteria included preceding clonal hematological diseases, previous chemo- or radiotherapy for solid tumors, abnormal kidney and liver functions, clinical or ECG signs of heart failure.
All patients received one cycle of standard induction chemotherapy, consisting of daunorubicin 45 mg/m2 daily on days 1–3 and cytarabine arabinoside 200 mg/m2 daily on days 1–7 (3 + 7). Ten patients died during the induction cycle, and the remaining 40 patients were evaluated after receiving the induction cycle to determine whether they achieved complete remission (CR) or not. Twenty patients were considered to be in CR group as they met the established criteria, including blast cells <5%, platelets count ⩾100 × 109/l and no evidence of leukemia at other sites observed within six months. The unremitted (UR) group included 20 patients with blast cells >5%, or evidence of leukemia at other sites. The UR group, including patients showing no response to treatment, received a second induction cycle in combination with oral indomethacin (50 mg/8 h daily on days 1–7).
2.2.1 Blood sampling
A five ml venous blood sample was collected from each control subject, and patient (at presentation, after the 1st cycle of chemotherapy and after the 2nd cycle combined with indomethacin). Blood samples were collected into EDTA coated tubes and were processed using gradient centrifugation by Ficol-Paque Plus (Biochrom AG, Berlin, Germany) to obtain the buffy layer. Cells were washed by PBS twice, pelleted and then stored at -80 °C until they were used.
2.2.2 RNA extraction
Total RNA was extracted from patients’ samples using the SV Total RNA Isolation System (Promega Corporation, Madison, USA). According to the manufacturer’s instructions, cells were thawed in lysis buffer, diluted and centrifuged at 12,000–14,000g for 10 min to remove cell debris. RNA was precipitated from the cleared lysate by 95% ethanol then separated through spin columns. RNA was washed and treated by DNase and washed again before elution by 100 μl of nuclease-free water.
The concentration of the extracted RNA was assessed by the NanoDrop® ND-1000 UV–Vis Spectrophotometer that measures the optical density at 230, 260 and 280 nm. All samples included in the study had A260/A280 ratio ranging from 1.7 to 2.1.
2.2.3 RT-PCR for MRP-1 and GST-Pi
Reverse transcription was done by Reverse Transcription System (Promega, Madison, USA). The kit used AMV Reverse Transcriptase and oligo (dT)15 primer to synthesize single-stranded cDNA from total RNA.
PCR was carried out using Go Taq® Green Master Mix (Promega Corporation, Madison, USA). Each PCR mixture consisted of 25 μl PCR master mix; 1 μl of each amplification primer (100 pmol/μl) and 1 μg cDNA and the volume was brought to 50 μl by adding deionized water. The primers used for the amplifications are as follows:Thermal cycling started by a first denaturation step of 5 min at 94 °C, followed by 40 cycles of 95 °C for 60 s, 52 °C for 60 s and 72 °C for 90 s and a final extension for 10 min at 72 °C. PCR products were separated by electrophoresis on 2% agarose gel, then they were stained by ethidium bromide and finally visualized by UV .
2.3 Statistical analysis
Statistical analysis was performed using the SPSS (Statistical Package for the Social Sciences) software version 18.0 (SPSS Inc., Chicago, IL, USA).
MRP-1 and GST-Pi expression was converted to dichotomous variable either negative or positive by combining the several grades of positivity into positive. For variables’ description, the percent was used for qualitative variables and the mean with the standard deviation for quantitative normally distributed variables. The relative risk was used to assess the risk among those who are parameter positive relative to those who are parameter negative. Kaplan–Meier survival curves were used to demonstrate disease free and overall surviving through the follow-up duration, using the mean survival time and its 95% confidence interval as descriptive for the different survival times. Comparisons between the different survival distributions were done using the log rank test. All tests were 2 sided and alpha was set at 0.05.
3 Results
The study included 50 AML patients and 20 healthy individuals as a control group. After the first cycle of chemotherapy AML patients were divided into two groups, CR group (n = 20) and UR group (n = 20), the latter was treated with a 2nd cycle plus indomethacin. Ten patients died during the induction cycle of chemotherapy.
3.1 Hematological results
Mean ± standard error (S.E.) of all hematological parameters in control group, AML patients, complete remission group and UR group are presented in .
Table 1 Mean ± standard error (M ± SE) of all hematological parameters in control group, AML patients, complete remission group and UR group.
WBCs count and the blast cells percent followed the same pattern. The mean values of WBCs count and blast cells percent in AML patients were significantly higher than those in control group (32.15 ± 6.29 vs 5.59 ± 0.33 × 109/l and 55.20 ± 5.20 vs 0.0 ± 0.0; respectively). In CR group, at presentation they were significantly higher than in control group (33.44 ± 12.12 × 109/l and 62.6 ± 7.7; respectively), and significantly decreased after the 1st cycle of chemotherapy to the normal control level (4.6 ± 0.7 × 109/l and 0.9 ± 0.48; respectively), while in UR group, levels at presentation and after induction therapy were significantly higher than the control group and not significantly different from each other (23.03 ± 7.29 and 30.53 ± 11.69 × 109/l and 44.40 ± 7.44 and 52.70 ± 9.75; respectively). After the 2nd cycle of chemotherapy combined with indomethacin, WBCs and blast cells percent levels significantly decreased and became within the control level (5.74 ± 2.19 × 109/l and 1.5 ± 1.5; respectively).
The mean value of platelets count in patients with AML was significantly lower than that in control group (107.6 ± 17.1 vs 250.3 ± 11.3 × 109/l; respectively). In CR group at presentation and after the 1st cycle of chemotherapy mean values were significantly lower than those in the control group and not significantly different from each other (129.1 ± 33.0 and 118.7 ± 13.5 × 109/l; respectively). While in UR group, at presentation, at relapse and after the second cycle of chemotherapy combined with indomethacin mean levels were significantly lower than in control group and showed insignificant variations (108.7 ± 25.9, 85.1 ± 24.2 and 128.3 ± 24.1 × 109/l; respectively).
The mean values of RBCs count and hemoglobin concentration followed the same pattern. In patients with AML, they were significantly lower than in control group (2.98 ± 0.16 vs 4.38 ± 0.11 × 106/l and 8.57 ± 0.45 vs 12.02 ± 0.27 g/dl). In CR group, mean levels at presentation and after the 1st cycle of chemotherapy were nearly within the same range and were significantly lower than in control group (3.01 ± 0.25 and 3.41 ± 0.22 × 106/l and 8.74 ± 0.72 9.68 ± 0.68 g/dl; respectively). Also in UR group, the mean values at presentation, after induction cycle and after the 2nd cycle of chemotherapy combined with indomethacin showed insignificant variations and were significantly lower than in control group (3.02 ± 0.24, 2.89 ± 0.21 and 3.25 ± 0.09 × 106/l and 8.76 ± 0.52 8.36 ± 0.52 and 8.66 ± 0.37 g/dl; respectively).
3.2 GST-Pi expression in patients with AML
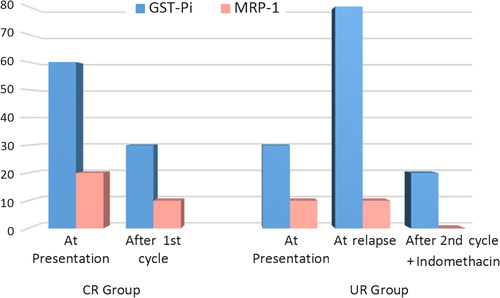
Number and percent of AML patients with positive GST-Pi and MRP-1 expression and comparisons of studied groups and subgroups are represented in . Half the control group subjects and 52% of AML patients had positive GST-Pi expression, with no significant difference between the two groups.
Table 2 Number and percent of AML patients with positive GST-Pi and MRP-1 expression and comparisons of groups and subgroups.
In CR group, at presentation 60% of patients were positive for GST-Pi, which decreased to 30% after the 1st cycle of chemotherapy. Neither percent was significantly different from the control group, but they were significantly different from one another. In UR group, at presentation 30% of patients expressed GST-Pi which was not significantly different from the control percent, while after induction cycle, the percent positivity increased to 80%, which was significantly higher than the control percent. After the 2nd cycle combined with indomethacin, the percent of patients with positive GST-Pi expression decreased to 20%, which was significantly lower than at relapse and control percent.
The percent of GST-Pi expression positivity after the 1st cycle of chemotherapy in CR group was significantly lower than the percentage after induction cycle in the UR group.
3.3 MRP-1 expression in patients with AML
In the control group, 20% of subjects had positive MRP-1 expression, while in AML patients only 12% were positive for MRP-1, with no significant difference between the two groups. In CR group, at presentation 20% of patients were positive for MRP-1, which decreased to 10% after the 1st cycle of chemotherapy. In UR group, at presentation and after induction cycle, only 10% of patients expressed MRP-1 while after the 2nd cycle combined with indomethacin, none of the patients was positive for MRP-1 expression. None of the percents was significantly different from the control group or from one another.
represents the percent positivity of both GST-Pi and MRP-1 in the two groups through all study points.
3.4 Disease free survival (DFS)
a represents the Kaplan–Meier DFS curve of AML patients classified according to GST-Pi expression. Patients with positive GST-Pi expression had shorter mean DFS time (13.09 months) than patients negative for GST-Pi expression (28.56 months). The difference was statistically significant (P = 0.039), with a log-rank value of 4.23.
Figure 3 Disease free survival according to (a) GST-Pi and (b) MRP-1 expression and overall survival according to (c) GST-Pi and (d) MRP-1 expression in AML patients.
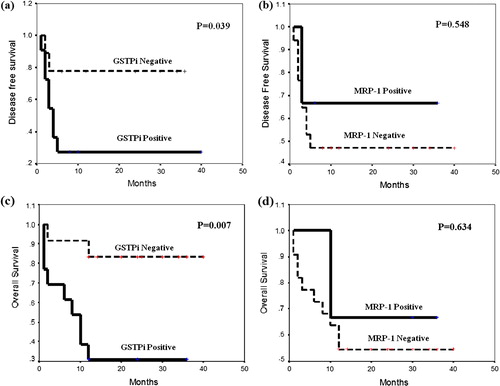
b represents the DSF curve of AML patients according to MRP-1 expression. There was no significant difference (P = 0.548) in mean DFS time between patients with positive (25.00 months) and negative (20.35 months) MRP-1 expression.
3.5 Overall survival (OS)
c represents OS curve of AML patients according to GST-Pi expression. Patients with positive GST-Pi expression had shorter mean OS time (15 months) than patients with negative GST-Pi expression (34.50 months). The difference was statistically significant (P = 0.007), with a log-rank value of 7.25.
d represents OS curve of AML patients according to MRP-1 expression. There was no significant difference (P = 0.634) in mean OS time between patients with positive (27.33 months) and negative (24.41 months) MRP-1 expression.
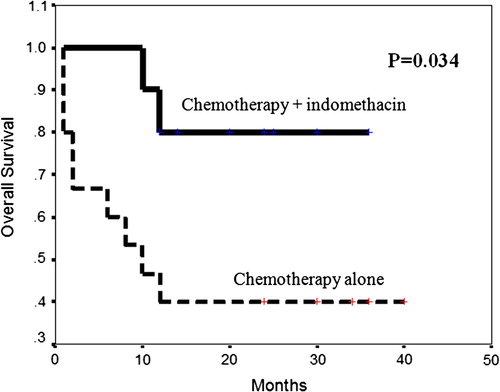
3.6 Survival when combining indomethacin with chemotherapy
represents the OS curve of patients treated by chemotherapy alone and patients treated by chemotherapy with indomethacin. Patients taking chemotherapy in combination with indomethacin had longer mean OS time (31 months) than patients taking chemotherapy alone (18.87 months). The difference was statistically significant (P = 0.034), with a log-rank value of 4.45.
3.7 Correlation with laboratory parameters
As presented in , in CR group there was a significant positive correlation between GST-Pi expression and both WBCs count and blast cells % at presentation (r = 0.583, 0.612 and P = 0.038, 0.030; respectively), while in UR group, GST-Pi expression was significantly correlated with WBCs count and blast cells % after induction cycle (r = 0.612, 0.667 and P = 0.030, 0.018; respectively) and after 2nd cycle + indomethacin (r = 0.667, 0.667 and P = 0.018, 0.018; respectively).
Table 3 Correlation of GST-Pi and MRP-1 with laboratory parameters in all studied groups.
As for MRP-1 expression, there was a significant positive correlation between MRP-1 expression and WBCs count in CR group at presentation (r = 0.612 and P = 0.030). There was no significant correlation between GST-Pi or MRP-1 and any of the other parameters in all studied groups.
4 Discussion
MDR continues to be a central problem in treatment with chemotherapy, especially in leukemia. New drugs and treatment protocols have enhanced disease prognosis among AML patients; nevertheless, initially responsive tumors ultimately relapse and develop resistance to drugs. Treatment of resistant leukemic cells is usually very difficult because the range of resistance generally extends even to drugs to which leukemic cells have never been exposed.Citation9
4.1 Expression of GST-Pi and MRP-1 with MDR
There are five different classes of GSTs in human cells that act as determinants for response to chemical insult.Citation10 GST-Pi isoenzyme inactivates chemotherapeutic drugs by conjugating them to glutathione. The involvement of GST-Pi in chemo-resistance is established for cultured cancer cell lines, but its role in vivo is still unclear. Increased GST-Pi expression detected as strong immunoreactivity has been documented to contribute to chemotherapy resistance in AML patients.Citation11
Our results showed that no significant difference in percent positivity of GST-Pi expression existed between treatment naïve AML patients and controls (52% and 50%; respectively), which indicates that GST-Pi is innately expressed in peripheral mononuclear cells. Our results were in agreement with those of Lohri et al.Citation12 who observed that mRNA levels of GST-Pi were equally expressed in healthy donors and in AML patients.
After the first cycle of chemotherapy, the percentage of GST-Pi expression in CR group significantly decreased from 60% before treatment to 30% after achieving CR; on the other hand, in UR group, it significantly increased from 30% at presentation to 80% after induction cycle. In addition, GST-Pi expression was found to be correlated to WBCs count and % blast cells at presentation in both CR and UR groups. Our results confirmed the finding of other studies that GST-Pi RNA levels in treated AML patients remained the same or fell after chemotherapy in responding patients, indicating that GST-Pi expression is not induced by chemotherapy in responding patients.Citation13,Citation14 In UR group, treatment may have induced rapid apoptosis of the sensitive cell fraction leaving resistant cells. Thus up regulation of GST-Pi expression after induction cycle is most probably due to clonal selection of leukemic cells expressing GST-Pi rather than up-regulation of GST-Pi expression. These findings suggested that GST-Pi biological action may contribute to tumor cell survival and to MDR phenotype and may be correlated with the clinical course of the disease. Moreover, we may speculate that patients in the CR group who have high GST-Pi expression are more likely to relapse.
GST-Pi may participate in MDR development by acting two distinct roles. Firstly, GST-Pi may cause enzyme-mediated detoxification of lipid peroxidation products. Since anthracyclines are likely to cause peroxidation of membrane lipids, increased GST-Pi levels could contribute to protection from toxicity caused by these agents.Citation15 Secondly, GST-Pi plays a key role in regulating the MAP kinase pathway via inhibiting jun N-terminal kinase (JNK). In cells that are not stressed, JNK activity is low due to its sequestration in a GST-Pi:JNK complex. However, suppression of JNK activity is reversed by conditions of oxidative stress resulting in the dissociation of the GST-Pi:JNK complex and induction of apoptosis. Elevated expression of GST-Pi can alter the balance of regulation of kinase pathways during drug treatment, protecting cells from death or apoptosis.Citation16
As for impact of GST-Pi expression on patients’ prognosis, our study demonstrated that patients with positive GST-Pi expression had a statistically significant shorter mean overall survival (OS) time (15.0 months) than those with negative GST-Pi expression (34.5 months), with a relative risk of death in GST-Pi positive patients being 7.25-fold that of GST-Pi negative patients. As for Disease-free survival (DFS), after treatment, patients with positive GST-Pi expression had statistically significant shorter mean DFS time (13.09 months) than those with negative GST-Pi expression (28.56 months), with a relative risk of relapse in GST-Pi positive patients being 4.23-fold that of GST-Pi negative patients. Thus, GST-Pi expression at diagnosis may be an important prognostic marker for patients’ survival. These results are in agreement with Gilbert et al.,Citation17 who found that increased GST-Pi expression is a predictor of early death in breast cancer patients.
MRP-1, a member of the ATP-binding cassette superfamily, acts as drug efflux pump whose over-expression is an important cause of treatment failure in AML.Citation18 While some studies confirm MRP-1’s lack of prognostic utility,Citation19,Citation20 others have shown that MRP-1 detection is predictive of outcome, and adds prognostic value to P-gp for both CR rates, relapse-free, and OS for patients expressing both phenotypes.Citation21 These inconsistencies may be due to different populations, population sizes or methodological differences.
Our results showed that the percent positivity of MRP-1 expression in AML patients before treatment was very low and not significantly different from that in the control group. We could attribute the low percent positivity to heterogeneity of MRP-1 expression among AML patients with different FAB subgroups and cytogenetic abnormalities, or due to its deletion in specific cytogenetic karyotypes.Citation22 Our results were consistent with other studies that found that mRNA levels of MRP-1 were equally expressed in healthy donors and AML patients.Citation12,Citation19,Citation20,Citation22
In CR group, MRP-1 expression at presentation was not significantly different from its expression after achieving CR. Our results were confirmed by studies of Schaich et al.Citation23 and Filipits et al.Citation20 who found that MRP-1 expression had no value in predicting response to induction chemotherapy and no effect on remission rate.
In UR group, MRP-1 expression at presentation was not significantly different from that after induction cycle, which confirms the findings of Van der Kolk et al.Citation22 who observed no consistent up-regulation of MRP-1 mRNA expression in relapsed versus de novo AML cells. On the contrary, Mahjoubi et al.,Citation21 found that high MRP-1 expression was associated with poor clinical outcome.
As for survival, no statistically significant difference in mean DFS or OS time was found between patients with positive and negative MRP-1 expression. That was consistent with Filipits et al.Citation19 and Lohri et al.Citation12, who reported that MRP-1 expression had no impact on OS or DFS and had no prognostic value.
In CR group, there was a significant positive correlation between MRP-1 expression and WBCs count at presentation which could be attributed to its production by leukocytes. These results were in accordance with Schaich et al.Citation23 who found an association between higher MRP-1 expression and WBC counts >100 × 109/l.
Our results also showed that there was no significant correlation between GST-Pi and MRP-1. This may be due to low level of MRP-1 expression. Do et al.Citation24 and Moureau-Zabotto et al.Citation25 also observed no significant correlation between mRNA expression of GST-Pi and MRP-1, which agrees with the present study.
4.2 Modulation of multidrug resistance
MDR renders chemotherapy ineffective, and if high doses of drugs are used to overcome resistance, toxic effects appear. MDR could be modulated through modulation of agents implicated in resistance. Modulation can be done by using a relatively non-toxic agent, in combination with an anti-cancer drug, in order to improve therapeutic efficacy by disturbing drug resistance mechanisms.Citation26
The data fore mentioned suggest that GST-Pi may play a role in MDR in AML patients, while MRP-1 is not expected to have a significant role in the process. Thus, to reverse MDR, indomethacin, an inhibitor of GST-Pi isoenzyme, was used along with the chemotherapy regimen for a 2nd cycle in UR patients.Citation27
After the 2nd cycle in combination with indomethacin the GST-Pi percent positivity significantly decreased (from 80% to 20%), probably due to the inhibitory effect of indomethacin on GST-Pi expression. The decrease in GST-Pi expression is expected to enhance cell death or apoptosis. Our results confirmed those of Draper et al.,Citation28 and Song et al.,Citation27 who found that the drug-sensitizing effect of indomethacin was dependent on direct inhibition of GST and activation of a chemotherapeutic drug-induced apoptotic pathway.
The present study showed that, patients taking 2nd cycle chemotherapy in combination with indomethacin had significantly longer mean OS time (31.0 months) than patients taking chemotherapy alone (18.87 months). The relative risk of death in patients taking chemotherapy alone was 4.25-fold that of patients treated by chemotherapy combined with indomethacin. That indicates that combination of indomethacin with chemotherapy could improve survival and reverse MDR phenotype in AML patients.
As for MRP-1, the change in percent positivity of MRP-1 after the 2nd cycle combined with indomethacin was not significant. Matsunaga et al.Citation29 findings were contradictory to our results, as he found that indomethacin was a sensitizer in doxorubicin resistant leukemia cells, which decreased expression of MRP-1 by inhibiting MRP-1 promoter activity. But our results did not support such inhibition due to the very low initial positivity of MRP-1 in our patients group.
In conclusion, GST-Pi may have a role in MDR phenotype in AML patients, but not MRP-1. Furthermore, GST-Pi inhibition by indomethacin significantly reduced MDR in AML patients and improved survival.
Conflict of interest
The authors declared that there are no conflict of interests.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 16 August 2016
References
- E.L.NieroB.Rocha-SalesC.LauandB.A.CortezM.M.de SouzaP.Rezende-TeixeiraThe multiple facets of drug resistance: one history, different approachesJ Exp Clin Cancer Res33201437
- S.P.ColeTargeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and futureAnnu Rev Pharmacol Toxicol54201495117
- J.P.GilletM.M.GottesmanMechanisms of multidrug resistance in cancerMethods Mol Biol59620104776
- S.SinghCytoprotective and regulatory functions of glutathione S-transferases in cancer cell proliferation and cell deathCancer Chemother Pharmacol752015115
- R.O’ConnorThe pharmacology of cancer resistanceAnticancer Res200712671272
- YC1GuoC.M.ChangW.L.HsuS.J.ChiuY.T.TsaiY.H.ChouIndomethacin inhibits cancer cell migration via attenuation of cellular calcium mobilizationMolecules18201365846596
- F.A.NichollsJ.T.AhokasInhibition of purified glutathione S-transferases by indomethacinBiochem Biophys Res Commun119198410341038
- D.J.A.de GrootM.van der DeenT.K.P.LeA.RegelingS.de JongE.G.E.de VriesIndomethacin induces apoptosis via a MRP1-dependent mechanism in doxorubicin-resistant small-cell lung cancer cells overexpressing MRP1Br J Cancer97200710771083
- J.H.WeisburgMultidrug resistance in acute myeloid leukemia: potential new therapeuticsJ Nucl Med49200814051407
- P.G.BoardD.MenonGlutathione transferases, regulators of cellular metabolism and physiologyBiochim Biophys Acta1830201332673288
- B.T.HuangZ.XiaoY.T.ShiS.HaW.H.ZhaoD.GaoExpressions of LRP, GST-pi and MRP1 in acute leukemia patients and its clinical significanceZhongguo Shi Yan Xue Ye Xue Za Zhi152007262266
- A.LohriB.V.HilleM.BacchiM.FoppF.JoncourtJ.ReuterFive putative drug resistance parameters (MDR1/P-glycoprotein, MDR-associated protein, glutathione-S-transferase, bcl-2 and topoisomerase IIα) in 57 newly diagnosed acute myeloid leukaemiasEur J Haematol592009206215
- S.GalimbertiR.TestiF.GuerriniR.FazziM.PetriniThe clinical relevance of the expression of several multidrug-resistant-related genes in patients with primary acute myeloid leukemiaJ Chemother12003374379
- H.ShiD.LuY.ShuW.ShiS.LuK.WangExpression of multidrug-resistance-related proteins P-glycoprotein, glutathione-S-transferases, topoisomerase-II and lung resistance protein in primary gastric cardiac adenocarcinomaCancer Invest262008344351
- V.J.FindlayD.M.TownsendK.D.TewGlutathione and Glutathione S-Transferases in drug resistanceB.A.TeicherCancer drug resistance2006Humana Press Inc.New Jersey (USA)213222
- A.De LucaL.FedericiM.De CanioL.StellaA.M.CaccuriNew insights into the mechanism of JNK1 inhibition by glutathione transferase P1-1Biochemistry51201273047312
- L.GilbertL.J.ElwoodM.MerinoS.MasoodR.BarnesA pilot study of the pi class Glutathione-S-Transferase expression in breast cancer: correlation with estrogen receptor expression and prognosis in node-negative breast cancerJ Clin Oncol1119934958
- G.A.AltenbergStructure of multidrug-resistance proteins of the ATP-binding cassette (ABC) superfamillyCurr Med Chem Anticancer Agents420045362
- M.FilipitsR.W.SuchomelS.ZöchbauerR.BrunnerK.LechnerR.PirkerMultidrug resistance-associated protein in acute myeloid leukemia: no impact on treatment outcomeClin Cancer Res3199714191425
- M.FilipitsT.StranzlG.PohlH.HeinzlU.JagerK.GeisslerDrug resistance factors in acute myeloid leukemia: a comparative analysisLeukemia1420006876
- F.MahjoubiM.GolalipourA.GhavamzadehK.AlimoghaddamExpression of MRP1 gene in acute leukemiaSao Paulo Med J1262008172179
- D.M.Van der KolkE.G.de VriesL.NoordhoekActivity and expression of the multidrug resistance proteins P-glycoprotein, MRP1, MRP2, MRP3 and MRP5 in de novo and relapsed acute myeloid leukemiaLeukemia15200115441553
- M.SchaichS.SoucekC.ThiedeG.EhningerT.IllmerSHG AML96 Study Group: MDR1 and MRP1 gene expression are independent predictors for treatment outcome in adult acute myeloid leukaemiaBr J Haematol1282005324332
- J.H.DoS.H.OhE.J.SongJ.S.ChungC.D.KangE.Y.LeeTreatment outcome of multidrug resistance related mRNA expression and c-jun-N-terminal kinase activity in patients with acute myeloid leukemiaKorean J Lab Med272007229236
- L.Moureau-ZabottoS.RicciJ.P.LefrancF.CouletC.GenestieM.AntoinePrognostic impact of multidrug resistance gene expression on the management of breast cancer in the context of adjuvant therapy based on a series of 171 patientsBr J Cancer942006473480
- E.TurriniL.FerruzziC.FimognariNatural compounds to overcome cancer chemoresistance: toxicological and clinical issuesExpert Opin Drug Metab Toxicol10201416771690
- J.H.SongS.H.KimH.-J.KimS.Y.HwangT.S.Sung KimAlleviation of the drug-resistant phenotype in idarubicin and cytosine arabinoside double-resistant acute myeloid leukemia cells by indomethacinInt J Oncol322008931936
- M.P.DraperR.L.MartellS.B.LevyIndomethacin mediated reversal of multidrug resistance and drug efflux in human and murine cell lines overexpressing MRP, but not P-glycoproteinBr J Cancer751997810815
- S.MatsunagaT.AsanoA.Tsutsuda-AsanoY.FukunagaIndomethacin overcomes doxorubicin resistance with inhibiting multi-drug resistance protein 1 (MRP1)Cancer Chemother Pharmacol582006348353