Abstract
Mesenchymal stem cells (MSCs) were isolated by gradient density centrifugation from umbilical cord blood. Spindle-shaped adherent cells were permitted to grow to 70% confluence in primary culture media which was reached by day 12. Induction of differentiation started by culturing cells with differentiation medium containing FGF-4 and HGF. Under hepatogenic conditions few cuboidal cells appeared in culture on day 7. From day 21 to day 28, most of cells became small and round. The control negative cells cultured in serum free media showed fibroblast-like morphology. Urea production and protein secretion by the differentiated hepatocyte-like cells were detected on day 21 and increased on day 28. Protein was significantly increased in comparison with control by day 28. The cells became positive for AFP at day 7 and positive cells could still be detected at days 21 and 28. The cells in the control group were stained negative for AFP. The cells expressed albumin gene at the 14th day that became markedly increased at the 28th day of culture with HGF and FGF-4. No albumin expression was observed in the 7th day sample and the control. This study demonstrated that UCB-derived MSCs had the ability to differentiate into functioning hepatocyte-like cells starting from the 7th day after culturing under hepatogenic conditions and became well functioning at days 21 and 28. These data indicated that UCB-derived MSCs can be a promising source of cell therapy for intractable liver diseases.
1 Introduction
Liver is dedicated to perform an extensive range of tissue specific functions including intermediary metabolism, detoxification of drugs or other xenobiotics as well as endogenous substrates, synthesis of plasma proteins and bile formation.Citation1 The principal cells of mature liver that carry out these functions are parenchymal hepatocytes which represent approximately 80% of total hepatic volume.Citation2
Liver dysfunction is a major health problem in the world, and liver transplantation is the only conventional thriving treatment of end-stage liver disease.Citation3 Unfortunately, human donor livers are very limited and are unavailable to most people worldwide who necessitate whole organ transplantation. Hepatocytes transplantation and the use of hepatocytes with extracorporeal bioartificial liver supports are other alternatives.Citation4 However, the shortage of cell supplies and the long-term efficiency remains unclear which limits this strategy.Citation3
There are requires for efficient in vitro models of hepatocytes developed from other sources with full metabolic function. Some cell types exhibit the potential to develop into viable hepatocytes such as embryonic stem cells (ESCs) and adult stem cells (ASCs).Citation5 Studies on ESCs were limited in clinical therapeutic applications as a result of ethical controversies. On the other hand, adult SCs (like bone marrow stem cells) can be propagated in large quantities and remain capable to differentiate into various tissue typesCitation6 including hepatocyte lineage.Citation7
Recently, human umbilical cord blood (HUCB) has been used as a rich, easily available and ethically acceptable source of stem cells (SCs). UCB can be collected from donors without invasive or painful procedure and is rarely infectious.Citation8 UCB-derived cells are also considered more primitive than BM-derived cells so they represent a more appropriate source for cellular therapies.Citation9
There are 2 types of stem cells in HUCB: mesenchymal (MSCs) and hematopoietic. Lately it has been reported that UCB mesenchymal progenitor cells are capable of differentiating into hepatocytes.Citation10 Isolated MSCs from HUCB were induced to differentiate into hepatocyte-like cells under the effect of pro-hepatogenic conditions of different cytokines and growth factors including fibroblast growth factor (FGF) and hepatocyte growth factor (HGF).Citation10–Citation13 Transformation of the fibroblast-like cells into round epithelioid cells expressing mRNAs of albumin (ALB), and alpha-fetoprotein took place under these hepatogenic conditions Citation10,Citation11,Citation13. The functional capacity of the hepatocyte-like cells was assessed by albumin and urea secretionCitation10,Citation12 and the ability to integrate DiI-acetylated low-density lipoprotein.Citation10,Citation11
The aim of this work was to study the functional activity of hepatocyte-like cells derived from HUCB-MSCs at different time points by reverse transcription–polymerase chain reaction (RT-PCR) for albumin gene expression level, immunocytochemical stain for detection of alpha-fetoprotein in the cell cytoplasm and biochemical assay of protein and urea levels secreted in the culture media.
2 Materials and methods
The study protocol was approved by the ethics committee of the Mansoura University.
2.1 Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), recombinant human fibroblast growth factor (FGF)-4, penicillin/streptomycin/l-glutamine (100×), recombinant human hepatocyte growth factor (HGF), Ficoll-Paque (1077), all materials were purchased from Sigma, Egypt.
2.2 Collection of umbilical cord blood
UCB samples (No = 22) were obtained from Department of Obstetrics and gynecology, Mansoura University Hospital. Samples were collected after informed consent was given from full-term elective cesarean sections before placental separation. Family history of gene disorders and maternal fever was excluded. The umbilical cord was double clamped two inches apart from infant’s abdomen, wiped with 70% alcohol followed by Betadine at needle insertion site to insure sterility. The needle insertion site was just above the clamp. The blood samples were obtained in special cord blood collection bags.Citation14
2.3 Mesenchymal stem cell isolation and culture
The collected blood processed within 6 h of collection and the samples were transported in sterile tubes and diluted with phosphate buffer saline (PBS) in concentration 1:1. Mesenchymal stem cells were isolated using Ficoll gradient density centrifugation. Ficoll was added to the sample in concentration 3:1. Ficoll-Paque was normally placed at the bottom of a conical tube, and the blood was then slowly layered above it. Centrifugation of the tubes was done at 400 RCF for 30 min. The supernatant was discarded and the puffy coat layer which contained mononuclear cells was aspirated and transferred into other tube. PBS was added for washing in concentration 1:1 and centrifuged at 2000 RPM for 5 min. The supernatant was discarded.
The suspended precipitated cell pellet was cultured in primary complete culture media (containing 20 ml DMEM – 1% l-glutamine- 10% fetal bovine serum – 1 ml antibiotic) and plated in tissue culture flasks (25 cm2). The samples were incubated in incubator (37 °C) with 5% CO2 level and were nourished every 3 days. Feeding step was performed by discarding the old media and adding 20 ml new complete media as mentioned before.
After culturing cells for 12 days in primary culture media, cells reached 70–80% confluence. The old media was discarded and 10 ml trypsin was added to each flask for 5 min. The flasks were shaken to ensure that the cells were completely detached from the flask wall, then the content of the flasks was put in tubes to be centrifuged for 5 min at 2000 RBM. The cell pellets appeared on the wall of the tube, the supernatant was discarded, finally the cells in the media were transferred into new flasks (25 cm2) for subculture.Citation15
2.4 Hepatogenic differentiation
To start induction of cell differentiation into hepatocytes, second- to third-passage cells, at approximately 70% confluence were treated with differentiation medium consisting of serum free DMEM supplied with 20 ng/mL HGF, 10 ng/ml FGF4 and 1% antibiotic penicillin and streptomycin 12. Feeding was carried out two times per week and hepatogenic differentiation was investigated at different time points (7th -14th -21st -28th day) after treatment with differentiation medium.
2.5 Reverse transcription–polymerase chain reaction (RT-PCR) for albumin gene
Extraction of RNA was done from 1 × 106 MSCs differentiated cells using RNEasy (Sigma) as per the manufacturer’s instructions. The reverse transcription of mRNA to complementary DNA was done using Advantage RT-for-PCR (Sigma) as per the manufacturer’s instructions.
The resulting cDNA was amplified by the use of ABI GeneAmp PCR System 2400 (Sigma) under the following conditions: For human albumin gene (50-CTTGAATGTGCTGATGACAGG, 50 –GC AA GT CA GC AGGCATCTCATC) amplification: initial denaturation (94 °C for 5 min), annealing (35 cycles at 94 °C for 90 s), extension (57 °C for 2 min) and final extension (72 °C for 3 min). For GAPDH gene (50-GT CTTCTCCACCATGGAGAAGGCT, 50-CA TG CC AG TGAGCT TC CCGTTCA) amplification: denaturation at 95 °C for 300 s, annealing (30 cycles at 94 °C for 30 s), extension (55 °C for 30 s) and final Extension (72 °C for 60 s).
Detection of amplification products: The products of PCR were subjected to agarose gel electrophoresis using 2% agarose gel containing 2 μL of 10 mg/1 ml ethidium bromide (Sigma), using 1× Tris–Borate-EDTA Buffer (TBE Buffer) for one hour at 70 V.
2.6 Assay of protein level and urea in the culture media at different time points
Samples were obtained from cell culture media under hepatogenic conditions at different time points (7th -14th -21st -28th day). Serum free medium was used as a negative control. Collection and investigations of samples were done for urea and protein levels.Citation16
2.7 Immunocytochemical study for alpha fetoprotein (AFP)
Cells were fixed with 95 mL/L ethanol for 10 min at 4 °C, and incubated with blocking solution for 10 min to block endogenous peroxidases. Incubation with the primary antibody (anti-AFP) was done for 30–60 min at room temperature. The slides were then incubated with enzyme conjugate followed by DAB (Power-Stain TM 1.0 Poly HRP DAB Kit, Genemed Biotechnologies, Inc.) according to the manufacturer’s instructions. The slides were then counterstained with hematoxylin, dehydrated and mounted. Positive reaction appeared as brown cytoplasmic stain.
2.8 Statistical analysis
The data of protein and urea assay were expressed as mean ± SD. The statistical software SPSS12.0 was used. The results were analyzed by t-test. P < 0.05 was considered statistically significant.
3 Results
3.1 Isolation and culture of MSCs from UCB
Mesenchymal stem cells (MSCs), obtained by gradient density centrifugation, were heterogeneous during the first few days. Spindle-shaped cells were observed on the 4th day adherent to the bottom of culture flasks while many round cells were floating in the medium. Changing the medium progressively decreased the floating cells till their disappearance.
3.2 Expansion and hepatogenic differentiation of HUCB-MSC
Human umbilical cord blood derived mesenchymal stem cells (HUCB-MSC) were permitted to grow to 70% confluence in primary culture media. After 4 days of culture in primary culture media, the adhered cells were fibroblast-like and few in number (a). By day 12, the primary cultured cells reached a 70–80% confluence and the cells extensively expanded and overlapped in some areas (b).
Figure 1 Human umbilical cord blood derived mesenchymal stem cells (UCB-MSC) in primary culture media on day 4 with fibroblast like morphology (black arrow) (1a). Cells in primary culture media at day 12 were extensively expanded and overlapped in some areas (1b). After treatment with FGF4 and HGF for 7 days, few rounded cells appeared (white arrow) (1c). The number of cuboidal or round cells (white arrow) increased after 14 days of culture under hepatogenic conditions (d). By day 21 to day 28 of culturing under hepatogenic conditions, many cells were rounded (white arrow) (e). The cells of the control negative group were fibroblast-like (black arrow) (f). Phase contrast microscope ×100.
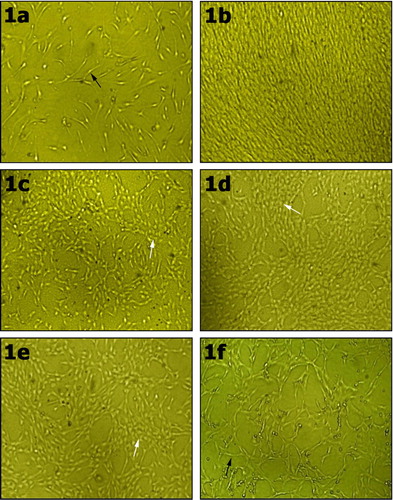
After that, cells were subcultured and differentiation was started by culturing cells with differentiation medium containing FGF-4 and HGF. Under hepatogenic conditions with FGF4 and HGF, cells did not expose any change compared with the control before the 7th day of treatment. On day 7, few cuboidal or round cells appeared in culture (c). After 14 days, the number of cuboidal cells increased in the culture (d). From day 21 to day 28, most of cells became cuboidal or round (e). The control negative cells cultured in serum free media containing DMEM, 1% l-glutamine, penicillin (100 U/mL) and streptomycin (100 U/mL), showed fibroblast-like morphology and no round shaped cells were found (f).
3.3 Urea and protein assay
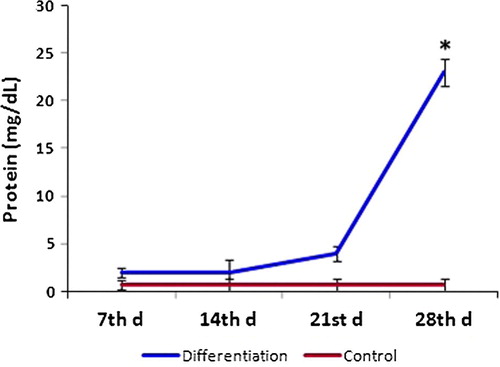
Urea production and protein secretion by the differentiated hepatocyte-like cells were detected at different time points during differentiation. Under hepatogenic conditions by treatment with FGF-4 and HGF, urea produced by MSCs was significantly higher compared to control on day 21 and increased on day 28 (). Protein was significantly increased in comparison with control by day 28 ().
3.4 Immunocytochemistry staining for AFP
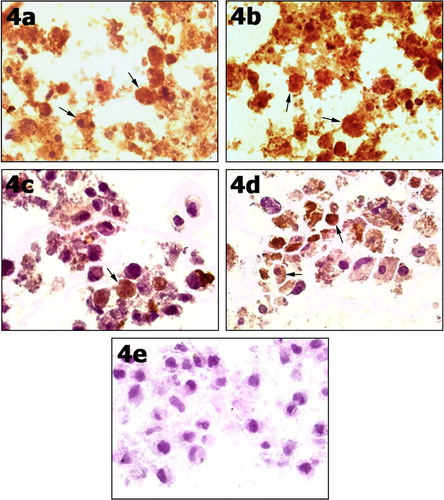
After culture with both HGF and FGF-4, the cells were positive for AFP at different time points 7, 14, 21 and 28. The results showed that the AFP appeared by day 7 and could still be detected by day 21 and day 28 (a–d). The cells in the control group were stained negative for AFP (e).
Figure 4 After 7 days of culturing UCB-MSC under hepatogenic conditions by treatment with FGF-4 and HGF, cells showed positive cytoplasmic reaction for AFP (arrows) (4a). At day 14 under hepatogenic conditions, almost all cells showed positively stained cytoplasm (arrows) (4b) and some cells were still positive by day 21 (4c) and day 28 (4d). Control negative cells cultured in serum free media showed undifferentiated cells stained negative for AFP (4e). Immunocytochemistry stain for AFP ×100.
3.5 Gene expression study using reverse transcription polymerase chain reaction (RT-PCR)
The expression of GAPDH as a control gene did not differ in between the control and other samples under hepatogenic conditions indicating valid mRNA in all specimens ().
Figure 5 A photograph showing reverse transcription polymerase chain reaction (RT-PCR) assays for albumin mRNA which was expressed by hepatocyte-like cells derived from MSC. The bands indicating the expression of albumin appeared at days 14, 21 and 28. No expression was detected at day 7 of differentiation, and the control sample. GAPDH mRNA, which was used as a control gene, was expressed in the 5 specimens (7, 14, 21, 28 days) under hepatogenic conditions and the control specimen.
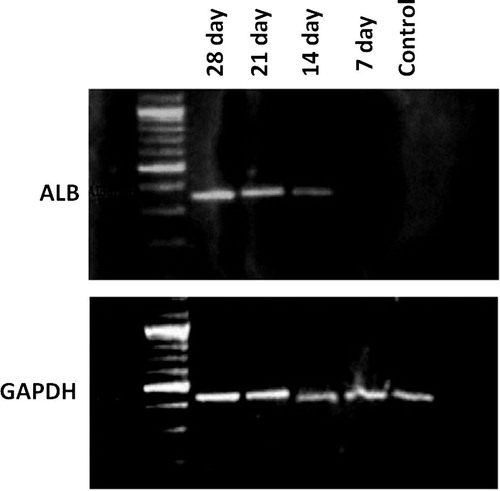
The albumin gene is known as a common hepatogenic marker. RT-PCR analysis of albumin gene expression showed that UCB-derived MSCs could express albumin gene in a time-dependent manner in the differentiating medium containing FGF-4 and HGF. The analysis revealed progressive increase in albumin expression from day 14 till day 28 of culture. No albumin expression was observed in the 7th day sample and the control specimen ().
4 Discussion
The characterization of stem cells present in adult BM and UCB has exposed that UCB-derived MSCs are similar to bone marrow-derived MSCs with respect to cellular properties and multi-lineage differentiation potential.Citation17–Citation19 However, UCB-derived MSCs have a low risk of immunoreactivity and can be isolated without any harm of the baby and no ethical concerns.Citation8
MSCs should be isolated from UCB sample immediately or within 6 h of collectionCitation20 because the activity and the quantity of MSCs in HUCB are low and decrease with time. In this study, MSCs were isolated from 3 out of 22 samples, which was in accordance with the previous study done by Kang et al. who isolated MSCs from 10 out of 39 samples.Citation12
In the present study, isolated MSCs from UCB were heterogeneous during the first few days. Cells adhering to the bottom of culture flasks were observed on day 4–5 and were fibroblast-like. The sub-cultured cells were much purer and fibroblast-like, with disappearance of other cells. According to set of standards proposed by The International Society for Cellular Therapy, a cell can be classified as a MSC when having plastic adherent properties under normal culture conditions and a fibroblast-like morphology. In fact, some claim that MSCs and fibroblasts are identical functionally.Citation21 In the present study the primary culture cells were allowed to grow to 70% confluence with a density of MSCs 1 × 106, prior to induction of differentiation by growth factors. Schwartz et al.Citation5, working on bone marrow derived progenitor cells, reported that a higher cell density favored hepatogenesis, whereas densities less than 12.5 × 103 failed to give rise to hepatocyte-like cells.
In the previous studies, differentiation of stem cells derived from UCB or matrix into hepatocytes was induced through addition of different mixtures of cytokines and growth factors like fibro-blast growth factor (FGF), hepatocyte growth factor (HGF), oncostatin M, epidermal growth factor, leukemia inhibitory factor, insulin-transferrin-selenium premix and others.Citation22,Citation10,Citation23–Citation26
Hepatocyte growth factor (HGF) was first recognized as a blood-derived mitogen for hepatocytes. HGF and its receptor c-Met are the key factors for liver growth and functionCitation27 and in helping the proliferation of hepatocytesCitation28 and liver regeneration.Citation27 HGF stimulates the proliferation of hepatic proginators to repair hepatic injury.Citation29 Adult human bone marrow MSCs were induced to differentiate into hepatocytes in vitro after being cultured with only HGF.Citation30
Fibroblast growth factor-4 (FGF-4) is mitogenic for fibroblasts and endothelial cells. Mouse embryonic stem cells could differentiate into cells expressing hepatocyte-specific genes and antigens when FGF-4 was added to the culture medium.Citation31 However, Schwartz et al. determined that the two essential growth factors for proper hepatocyte differentiation are FGF-4 and HGF.Citation5
This study showed that with the induction of HGF and FGF-4 the UCB-MSCs could expand in vitro and differentiate into hepatocyte-like cells. By day 7, some cells started to differentiate by acquiring a cuboidal or round form. At day 14, the number of differentiated cells increased and expressed albumin gene as detected by RT-PCR analysis. However, the secretion of the protein was significantly detected only by the 28th day. Urea secretion was significantly increased at the 21st day and elevated further by the 28th day. This indicates that MSCs started to differentiate as early as the 7th day but started to function by the 21st day of culture under hepatogenic conditions. These results are closely similar to those of Kang et al. who used the same hepatogenic induction technique by HGF and FGF-4.Citation12 However, they noticed earlier secretion of albumin (by day 16) detected by radioimmunoassay. They found also progressive increase in AFP level till day 28. In this study the AFP immuno-positive cells became less toward the 28th day. A similar observation has been found in hepatocytes derived from BM progenitor cells.Citation5 AFP is considered an early developmental marker of hepatoblasts formation. So, the decrease of its expression by some cells with time may indicate more maturation of hepatocyte-like cells.
5 Conclusion
This study demonstrated that UCB-derived MSCs had the ability to differentiate into hepatocyte-like cells starting from the 7th day after culturing under hepatogenic conditions and became well functioning at days 21 and 28. These data indicated that UCB-derived MSCs can be a promising source of cell therapy for intractable liver diseases.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 5 August 2016
References
- M.TakiguchiTheC/EBP family of transcription factors in the liver and other organsInt J Exp Pathol791998369391
- J.NeubergerLiver transplantationJ Hepatol322000198207
- S.KakinumaH.NakauchiM.WatanabeHepatic stem/progenitor cells and stem-cell transplantation for the treatment of liver diseaseJ Gastroenterol442009167172
- J.JensenJ.HyllnerP.BjörquistHuman embryonic stem cell technologies and drug discoveryJ Cell Physiol2192009513519
- R.E.SchwartzM.ReyesL.KoodieY.JiangM.BlackstadT.LundMultipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cellsJ Clin Invest10910200212911302
- I.KuehnleM.A.GoodellThe therapeutic potential of stem cells from adultsBr Med J3252002372376
- B.StiegerR.PetersM.A.SidlerP.J.MeierHepatocyte transplantation: potential of hepatocyte progenitor cells and bone marrow derived stem cellsSwiss Med Wkly1362006552556
- P.RubinsteinR.E.RosenfieldJ.W.AdamsonC.E.StevensStored placental blood for unrelated bone marrow reconstructionBlood81199316791690
- M.W.LeeI.K.JangK.H.YooK.W.SungH.H.KooStem and progenitor cells in human umbilical cord bloodInt J Hematol9220104551
- O.K.LeeT.K.KuoW.M.ChenK.D.LeeS.L.HsiehT.H.ChenIsolation of multipotent mesenchymal stem cells from umbilical cord bloodBlood103200416691675
- S.H.HongE.J.GangJ.A.JeongC.AhnS.H.HwangI.H.Yang2005 In vitro Differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cellsBiochem Biophys Res Commun330200511531161
- X.Q.KangW.J.ZangL.J.BaoD.L.LiT.S.SongX.L.XuFibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytesWorld J Gastroenterol114720057461
- Y.J.JungK.H.RyuK.A.ChoS.Y.WooJ.Y.SeohS.J.ChoIn vitro hepatic differentiation of human umbilical cord blood and bone marrow cellsPediatr Hematol Oncol252008481491
- J.JonesC.E.StevensP.RubinsteinR.R.RobertazziA.KerrM.F.CabbadObstetric predictors of placental/umbilical cord blood volume for transplantationAm J Obstet Gynecol18822003503509
- M.SeccoE.ZucconiN.M.VieiraL.L.FogaçaA.CerqueiraM.D.F.CarvalhoMultipotent stem cells from umbilical cord: cord is richer than blood!Stem Cells2612008146150
- F.DueñasV.BecerraY.CortesS.VidalL.SáenzJ.PalominoHepatogenic and neurogenic differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetusesBMC Vet Res102014154
- R.A.PanepucciJ.L.SiufiW.A.SilvaR.Proto-SiquieraL.NederM.OrellanaComparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cellsStem Cells22200412631278
- R.E.FeldmannK.BiebackM.H.MaurerA.KalenkaH.F.BürgersB.GrossStem cell proteomes: a profile of human mesenchymal stem cells derived from umbilical cord bloodElectrophoresis2614200527492758
- J.A.JeongS.H.HongE.J.GangC.H.AhnS.H.wangI.H.YangDifferential gene expression profiling of human umbilica lcord blood-derived mesenchymal stem cells by DNA microarrayStem Cells232005584593
- L.L.LuY.J.LiuS.G.YangQ.J.ZhaoX.WangW.GongIsolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentialsHaematologica91200610171026
- P.HemattiMesenchymal stromal cells and fibroblasts: a case of mistaken identity?Cyto Therapy1452012516521
- S.KakinumaY.TanakaR.ChinzeiM.WatanabeK.Shimizu-SaitoY.HaraHuman umbilical cord blood as a source of transplantable hepatic progenitor cellsStem Cells212003217227
- O.K.LeeT.K.KuoW.ChenK.LeeS.HsiehT.ChenIsolation of multipotent mesenchymal stem cells from umbilical cord bloodBlood1035200416691675
- D.CampardP.A.LysyM.NajimiE.M.SokalNative umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cellsGastroenterology13432008833848
- Y.J.MoonM.W.LeeH.H.YoonM.S.YangI.K.JangJ.E.LeeHepatic differentiation of cord blood-derived multipotent progenitor cells (MPCs) in vitroCell Biol Int3210200812931301
- S.SellamuthuR.ManikandanR.ThiagarajanG.BabuD.DineshD.PrabhuC.ArulvasuIn vitro trans-differentiation of human umbilical cord derived hematopoietic stem cells into hepatocyte like cells using combination of growth factors for cell based therapyCytotechnology632011259268
- K.MatsumotoT.NakamuraHepatocyte growth factor: molecular structure and implications for a central role in liver regenerationJ Gastroenterol Hepatol61991509519
- K.FujiwaraS.NagoshiA.OhnoK.HirataY.OhtaS.MochidaStimulation of liver growth by exogenous human hepatocyte growth factor in normal and partially hepatectomized ratsHepatology186199314431449
- S.HasuikeA.IdoH.UtoA.MoriuchiNumata M.TaharaHepatocyte growth factor accelerates the proliferation of hepatic oval cells and possibly promotes the differentiation in a acetyl amino fluorene/partial hepatectomy model in ratsJ Gastroenterol Hepatol20200517531761
- H.C.FiegelM.V.LioznovL.Cortes-DericksC.LangeD.KluthB.FehseLiver-specific gene expression in cultured human hematopoietic stem cellsStem Cells21200398104
- M.RuhnkeH.UngefrorenG.ZehleM.BaderB.KremerF.FändrichLong-term culture and differentiation of rat embryonic stem cell-like cells in to neuronal, glial, endothelial, and hepatic lineagesStem Cells212003428436