 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and aim
Previous studies have observed the association between inflammation and chronic kidney disease (CKD). The role played by Interleukin 8 (IL8) gene polymorphism has not been studied yet. Hence, the present study has been designed as the first attempt to identify the possible associations between polymorphism of the IL-8 gene and patients with diabetic CKD and on continuous ambulatory peritoneal dialysis (CAPD).
Materials and methods
A total of 150 participants were selected from a private nephrology outpatient clinic. The subjects were divided into three groups: healthy individuals without any renal complications (group 1, control, n = 50), patients with diabetic chronic kidney disease of stages 3 and 4 (group 2, n = 50) and CAPD (group 3, n = 50). Blood deoxyribo nucleic acid (DNA) isolated from the members of the study group, was confirmed by agarose gel electrophoresis and primers specific for IL8 gene were designed, using primer3 software tool.
Results
Restriction digestion of the amplicons with Escherichia coli restriction enzyme I (EcoRI) ended up in 203 base pairs (bp) band in control and 108 bp band in all diabetic and non-diabetic CKD. This indicated the presence of polymorphism in +781 Cytosine/Thymine (C/T) of IL-8 gene in diabetic CKD and CAPD patients. Statistical analysis of the distribution of frequencies of alleles C and T by chi square test confirmed the presence of polymorphism at +781 C/T of IL-8 gene in patient groups compared to control.
Conclusion
The polymorphism in +781 C/T of IL-8 gene studied in this work suggests its possible role as an inflammatory marker for both chronic kidney disease and CAPD.
Keywords:
1 Introduction
As indicated by world health organization (WHO) Worldwide disease burden task, infections of the kidney and urinary tract add to worldwide burden with around 850,000 passings consistently and 115,010,107 inability balanced life years.Citation1 CKD is the twelfth driving reason for death and seventeenth reason for disability. For quite a while, it has been assumed that about 100,000 new patients with ESRD in India require renal substitution treatment consistently in view of information from tertiary referral centers.Citation2,Citation3 The reported pervasiveness of CKD was 0.86% in the study population and 1.39% in the control district in Chennai when assessed amid counteractive action program began at group level.Citation4 The commonness of ESRD seems, by all accounts, to be 785 for every million population in India. Peritoneal dialysis (PD) is for some time built up as a noteworthy choice for renal replacement therapy in patients with end-stage renal infection or stage five of CKD. The essential intricacy of PD is infection due to the presence of a permanent tube in the abdomen. Continuous ambulatory peritoneal dialysis (CAPD) is one of the sorts of PD where the patient can do the exchanges himself three to four times each day. Inflammation contributes to the progression of CKD by inducing the release of cytokines and the increased production and activity of adhesion molecules, which together contribute to T cell adhesion and migration into the interstitium, subsequently attracting pro-fibrotic factors. Inflammation in CKD also causes mortality from cardiovascular disease by contributing to the development of vascular calcifications and endothelial dysfunction.Citation5
The inter individual variability in developing CKD might be because of polymorphisms of various genes encoding cytokines and other mediators of inflammation. Interleukin-8 (IL-8), a chemokine furthermore alluded to as neutrophil initiating peptide-1 and monocyte-inferred neutrophil chemotactic component, is combined as a 99-amino acid antecedent, discharged after cleavage of a single sequence of 20 residues, and handled by rehashed N-terminal cleavage yielding several biologically active variants.Citation6,Citation7,Citation41 Some IL-8 single nucleotide polymorphisms (SNPs) including +781C/T (rs2227306) were affirmed to be identified with the transcriptional level of IL-8Citation8 and might be connected with the event or advancement of an assortment of sicknesses.Citation9–Citation13
Past studies have noticed the relationship between inflammation and CKD,Citation14 but the relationship between these two has not been studied yet in point of interest as for IL-8; however, IL-1, IL-6 and IL-10 are understood as inflammatory markers connected with CKD.Citation15 Subsequently, the present case-control study was outlined as (to the best of our insight) the main endeavor to recognize conceivable relationship between polymorphism of the IL-8 gene and patients with CKD and on CAPD regimen in the number of inhabitants in Tiruchirappalli.
2 Materials and methods
2.1 Study subjects
A total of 150 participants were recruited from a private nephrology outpatient clinic and their clinical parameters are given in . The subjects were divided into three groups: patients with chronic kidney disease (group 1, n = 50) with various stages according to the National Kidney Foundation classificationCitation16: Stage I (GFR > 90 ml/min/1.73 m2) normal kidney function but urine findings or structural abnormalities or genetic trait points to kidney disease (n = 05), stage II (60–89 ml/min/1.73 m2) mildly reduced kidney function (n = 10), stage III (30–59 ml/min/1.73 m2) moderately reduced kidney function (n = 10), stage IV (15–29 ml/min/1.73 m2) severely reduced kidney function (n = 18) and stage V (<15 ml/min/1.73 m2) end stage kidney failure (n = 17); patients on dialysis (CAPD) (group 2, n = 50), healthy individuals without any renal complications (group 3, control, n = 50). The clinical characteristics of study population are given in . The following inclusion criteria were considered for the present study: (i) 18 years of age or older, (ii) estimated glomerular filtration rate (eGFR) between <15–89 mL/min/1.73 m2 for CKD group, (iii) adult CAPD patients ⩾18 years and at least 3 months experience on CAPD, (iv) willing and able to comply with clinic visits and study-related procedures and (v) provide signed informed consent and exclusion criteria were as follows: (i) recent infection or hospitalization (within one month), (ii) history of active or chronic hepatitis B, history of active or chronic hepatitis C, human immunodeficiency virus (HIV), (iii) history of tuberculosis (patient must be purified protein derivate negative), (iv) patients taking tumor necrosis factor (TNF) inhibitors, TNF blocker, interleukin-6 (IL-6) blockers or interleukin-1 (IL-1) blocking drugs, (v) having clinically significant chronic lymphopenia (low white blood cell count), and (vi) history of malignancy in the prior 5 years. Any history of melanoma or lymphoma was excluded from the study. Informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present study protocol has been approved by local hospital ethics committee.
Table 1 Clinical parameters for study population.
2.2 Genotyping
Blood samples (5.0 ml) were drawn from the peripheral vein of cases and controls into ethylene diamine tetra acetic acid (EDTA) tubes by a qualified laboratory technician. Genomic DNA extraction from the samples was performed by the standard salting out method (Medox Biotech Pvt. Ltd., Chennai, India Catalog. No: MX-1135-02).
About 4 μl of the DNA sample was mixed with 2996 μl of TE buffer and its absorbance was read at 260 nm by using UV–Visible spectrophotometer. 3 mL of TE buffer served as a blank. An optical density (OD) of one corresponds to approximately 50 μg of double standard DNA.Citation17 Based on the OD value, DNA sample concentration was quantified.
Primers specific for IL8 gene were designed using primer3 software tool and the details of forward primer and reverse primer 5′-CTCTAACTCTTTATATAGGAATT-3′ (length: 23 bp, Tm: 46 °C), 5′-GATTGATTTTATCAACAGGCA-3′ (length: 21 bp, Tm: 47 °C) respectively.
Genomic DNA isolated from blood was subjected to PCR using the primers mentioned above followed by restriction digestion with EcoRI. Polymerase chain reaction with restriction fragment length polymorphism (PCR–RFLP) was used to genotype selected polymorphisms within IL-8 + 781 C/T according to protocols described by Carrero et al.Citation18 PCR amplification of IL 8 gene was evaluated in a 20 μl reaction mixture containing 200 ng of the template DNA, 7.5 pmol/l of each primer, 0.2 mM of each dNTP, 1.5 mM MgCl2, 2.5 μL10X buffer and 1 U Taq polymerase. The cycling conditions for PCR were initial denaturation at 94 °C for 1 min 1 cycle, followed by 30 cycles of 94 °C for 1 min (melting), 58 °C for 30 s (annealing), 72 °C for 1 min (extension) and final extension at 72 °C for 8 min. The amplified PCR products were analyzed on 2% agarose gel (w/v) containing ethidium bromide. Agarose gel was visualized using gel documentation system (BioRad, USA). Strict aseptic conditions were followed and negative control was included. About 5 μl aliquot of products was loaded on 2% agarose in 1X TAE buffer and at 50 V for 45 min. 100 bp DNA ladder was used as the marker and the products were visualized in a UV transilluminator.
2.3 Statistical analysis
All statistical analyses were performed with SPSS software version 14.0 for Microsoft Windows. Data were summarized as mean ± SD, range or percentage. Allele and genotypic frequency was calculated by direct gene counting method. Comparison of the different genotypes was done by using Chi-square test. Odds ratios were calculated with a 95% confidence interval limit. P < 0.05 was considered statistically significant.
3 Results
Blood DNA isolated from the members of the study group was confirmed by agarose gel electrophoresis and the size was found to be 23 kb as compared with the Lamba DNA/Hind III digest used as marker (). IL-8 polymorphism was checked in study groups using PCR-RFLP as per prescribed protocols. PCR amplification of blood DNA of both controls and cases resulted in a band of length 203 bp which was confirmed by loading on 2% agarose gel (). Restriction digestion of the amplicons with EcoRI ended up in 203 bp band in controls but a band of 108 occurred in both CKD and CAPD groups (). This indicated the presence of polymorphism in +781 C/T of IL-8 gene in both CKD and CAPD groups. The genotype and allele distribution of IL-8 gene polymorphisms in three groups under study are given in . The IL 8 genotype was distributed as CT, 24 (48%), CC, 18 (36%); TT and 8 (46%), in control group. The CAPD patients represented CC, 13 (26%); TT, 12 (24%) and CT, 25 (50%). The CKD group has shown CC, 9 (18%); TT, 20 (40%) and CT, 21 (42%).
Figure 1 Genomic DNA isolation from blood of cases and controls. Lane 1 – marker (Lambda DNA/EcoRI digest), Lane 3 – blood genomic DNA (group I), Lane 4 – blood genomic DNA (group II), Lane 5 – blood genomic DNA (group III).
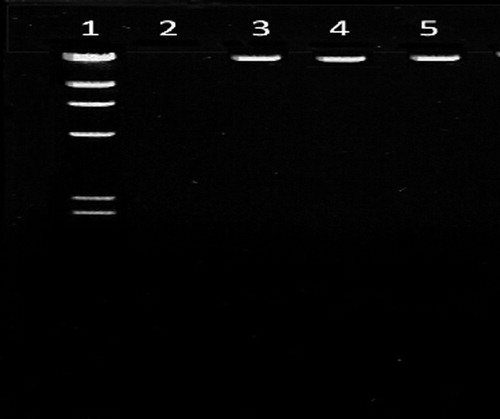
Figure 2 PCR amplification of IL-8 gene in cases and controls. Lane 1 – marker (100 bp ladder), Lane 3 – PCR product (group I), Lane 4 – PCR product (group II), Lane 5 – PCR product (group III).
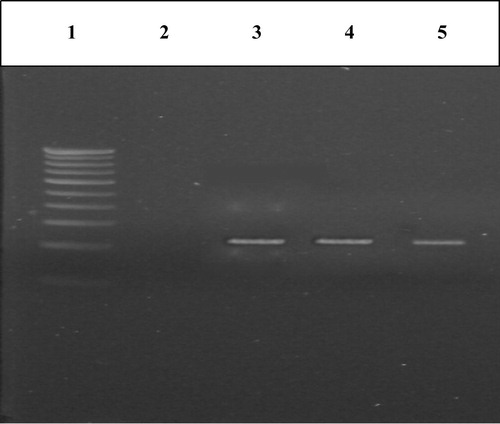
Figure 3 Restriction digestion of PCR products of IL-8 gene in cases and controls for detection of +781 C/T polymorphism. Lane 1 – marker (100 bp ladder), Lane 2 – EcoRI digested PCR product (group I), Lane 3 – EcoRI digested PCR product (group II), Lane 4 – EcoRI digested PCR product (group III).
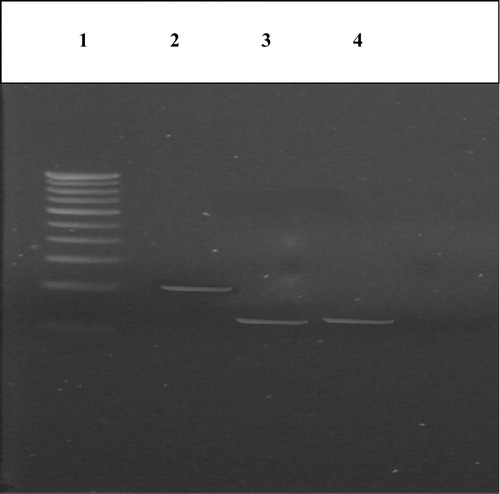
Table 2 Distribution of IL-8 genotypes and allele frequencies in study groups.
The genotypic (CC and TT) and allelic (C and T) frequency distributions in the study groups were also compared and are presented in . Significantly different CC and TT genotypes and C and T allele distributions were observed in CKD as compared to controls and also significant differences were observed between Control and CAPD patients ().
Table 3 Comparison of genotype and allelic frequency distribution in study groups.
Statistical analysis of the distribution of frequencies of alleles C and T was done by chi square test. Hence, the null hypothesis of equal distribution of the two alleles in the study population could be rejected with bias for T allele. This confirmed the presence of polymorphism at +781 C/T of IL-8 gene in cases compared to controls.
4 Discussion
Cytokines are vital modulators of inflammation, and the harmony among pro and anti-inflammatory cytokines decides the incendiary reaction and may intervene the progression of atherosclerosis and resulting CKD.Citation19,Citation20 Hereditary polymorphisms of these cytokines have been appeared to be connected with comorbidities, for example, cardiovascular sickness, in ESRD patientsCitation21–Citation23, or with ESRD defenselessnessCitation24; yet, there are questionable results that no polymorphisms of the IL6, IL10, and IL1 genes were connected with ESRD.Citation25 The confirmation for polymorphisms in cytokine genes influencing the risk of CKD itself is rare and this issue has not been completely illuminated, particularly in the all inclusive communities.
IL-8 concentration in dialysates from patients with dynamic Ulcerative colitis was altogether higher than in controls and connected with illness movement.Citation26 The raised cytokines may assume a part in the protection of the female urinary tract from certain renal illnesses, for example, pyelonephritis and other inflammatory and sclerotic kidney ailments.Citation26 Genotyping patients for IL-8 polymorphisms might be valuable in anticipating malady result and individualizing immunosuppressive treatment.Citation27 Prior studies did not give obvious proof of the potential commitment of the hemodialysis strategy to inflammation, as surveyed by markers of inflammation, for example, cytokine levels and acute phase protein generation. Diverse examples of pro-inflammatory and anti-inflammatory cytokine expression and initiation describe acute kidney injury (AKI), glomerulonephritis (GMN), and end-stage kidney disease (ESKD). In addition, plasma levels of specific cytokines and quality polymorphisms for specific cytokines may have prescient worth in these distinctive clinical situations.Citation28 Chronic kidney disease (CKD) is connected with a master provocative state and an overabundance of cardiovascular risk. The relationship of single nucleotide polymorphisms (SNPs) −251 T/A, +781 C/T, +1633 C/T and +2767 A/T in the IL-8 quality, +2608 G/C in the CXCR1 quality and +1208 C/T in the CXCR2 quality with weakness to Intense Polynephritis was reported in the Slovak population.Citation29
IL-8 – IL-8/CXCL8 was the primary chemoattractant cytokine found, a development in the history of immunology. IL-8/CXCL8 has a place with the CXC chemokine subfamily and is a prevalently neutrophil chemoattractant.Citation30,Citation31 The urinary discharge of β2 – microglobulin, IL-6 and IL-8/CXCL8 is connected to renal provocative movement in lupus nephritis.Citation32,Citation33 There is confirmation of expanded urinary discharge of IL-8/CXCL8 in patients with lupus nephritis or immunoglobulin An (IgA) nephropathy. Lessening of the urinary levels of IL-8/CXCL8 has been seen to match with reduction times of lupus nephritis.Citation34 Then again, Li et al. did not affirm this finding as, disregarding rise of the IL-8/CXCL8 levels in lupus patients, and no distinction was identified between patients with and without renal impairmentCitation35 Yokoyama et al. exhibited inclusion of IL-8/ CXCL8 in the intense period of IgA nephropathy, described by endocapillary proliferation.Citation36 Nonetheless, Huang et al. proposed IL-8/CXCL8 cooperation in the propelled periods of IgA nephropathy, as they demonstrated expanded urinary levels in correlation with prior phases of the illness and sound controls.Citation37 Expanded urinary IL-8/CXCL8 was additionally identified at the early phases of diabetic nephropathyCitation38. There is additionally clinical and test proof demonstrating that this chemokine impacts glomerular permeabilityCitation38,Citation39. Garin watched that IL-8/CXCL8 organization produces proteinuria in creatures, conceivably through expanded glomerular permeability. Cho et al. recognized expanded serum and urinary levels of IL-8/CXCL8 in pediatric patients with backsliding insignificant change nephrotic syndromeCitation40. Moreover, Souto et al. demonstrated a positive connection between the urinary discharges of IL-8/CXCL8 and protein in kids with essential nephrotic syndrome.Citation38 However, the previous studies fail to interpret IL8 gene polymorphism.
In conclusion, the polymorphism in +781 C/T of IL-8 gene studied in this work recommends its conceivable part of serving as an inflammatory marker for chronic kidney disease and CAPD. The present study has enlisted just a small number of patients and to affirm our outcomes, larger population study is exceptionally justified.
Conflict of interest
The authors declare that they have no conflict of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 9 July 2016
References
- S.H.TalibS.G.KulkarniKshitijaGadekarAjithKurienA study of various angioaccess in haemodialysis patientsOSR J Dental Med Sci1342014120131
- World Health Organisation – global burden of disease project (March 2006) available at: http://www.3.who_int/whosis/menu.cfm?path,.
- B.N.Vaishnavi AlamRaghavendraPrasadVidyasagarUpharGuptaStudy of etiology of chronic kidney disease in a teritiary care hospital in kolarEjpmr352016351354
- M.R.PrabaharV.ChandrasekaranP.SoundararajanEpidemic of chronic kidney disease in India -what can be done?Saudi J Kidney Disease Transpl952008847853
- D.M.SilversteinInflammation in chronic kidney disease: role in the progression of renal and cardiovascular diseasePediatr Nephrol248200914451452
- M.BaggioliniA.WalzS.L.KunkelNeutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophilsJ Clin Invest844198910451049
- M.BaggioliniI.Clark-LewisInterleukin-8, a chemotactic and inflammatory cytokineFEBS Lett3071199297101
- R.ColobranR.Pujol-BorrellM.P.ArmengolM.JuanThe chemokine network. II. On how polymorphisms and alternative splicing increase the number of molecular species and configure intricate patterns of disease susceptibilityClin Exp Immunol1502007112
- D.HackingJ.C.KnightK.RockettH.BrownJ.FramptonIncreased in vivo transcription of an IL-8 haplotype associated with respiratory syncytial virus disease-susceptibilityGenes Immun52004274282
- D.S.MichaudS.E.DaughertyS.I.BerndtE.A.PlatzM.YeagerGenetic polymorphisms of interleukin-1B (IL-1B), IL-6, IL-8, and IL-10 and risk of prostate cancerCancer Res66200645254530
- S.A.SavageL.HouJ.LissowskaW.H.ChowW.ZatonskiInterleukin-8 polymorphisms are not associated with gastric cancer risk in a Polish populationCancer Epidemiol Biomarkers Prev152006589591
- Z.D.JiangP.C.OkhuysenD.C.GuoR.HeT.M.KingGenetic susceptibility to enteroaggregative Escherichia coli diarrhea: polymorphism in the interleukin-8 promotor regionJ Infect Dis1882003506511
- Y.Y.TsaiJ.M.LinL.WanH.J.LinY.TsaiInterleukin gene polymorphisms in age- related macular degenerationInvest Ophthalmol Vis Sci492008693698
- Juan F.Navarro-GonzálezCarmenMora-FernándezMercedesMurosHaridianHerreraJavierGarcíaMineral metabolism and inflammation in chronic kidney disease patients: a cross-sectional studyClin J Am Soc Nephrol410200916461654
- PeterStenvinkelMarkusKettelerRichard J.JohnsonBengtLindholmRobertoPecoits-FilhoMiguelRiellaIl-10, Il-6, and TNF-α: central factors in the altered cytokine network of uremia—the good, the bad, and the uglyKidney Int67200512161233
- National Kidney FoundationK/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratificationAm J Kidney Dis392002S1S266
- J.SambrookE.F.FritschiT.ManiatisMolecular cloning: a laboratory manual1989Cold Spring Harbor Laboratory PressNew York
- J.J.CarreroS.H.ParkJ.AxelssonB.LindholmP.StenvinkelCytokines, atherogenesis, and hypercatabolism in chronic kidney disease: a dreadful triadSemin Dial222009381386
- A.HeinzmannI.AhlertT.KurzR.BernerK.A.DeichmannAssociation study suggests opposite effects of polymorphisms within IL8 on bronchial asthma and respiratory syncytial virus bronchiolitisJ Allergy Clin Immunol11432004671676
- D.M.SilversteinInflammation in chronic kidney disease: role in the progression of renal and cardiovascular diseasePediatr Nephrol24200914451452
- K.LuttroppB.LindholmJ.J.CarreroG.GlorieuxE.SchepersR.VanholderGenetics/genomics in chronic kidney disease–towards personalized medicine?Semin Dial222009417422
- M.RaoC.WongP.KanetskyM.GirndtP.StenvinkelM.ReillyCytokine gene polymorphism and progression of renal and cardiovascular diseasesKidney Int722007549556
- V.S.BalakrishnanD.GuoM.RaoB.L.JaberH.TighiouartR.L.FreemanHEMO study group. Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albuminKidney Int65200414491460
- A.KeshavarzianE.W.HolmesM.PatelF.IberJ.Z.FieldsS.PethkarLeaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damageAm J Gastroenterol941999200207
- A.BraunerM.SoderhallS.H.JacobsonJ.LundahlU.AnderssonJ.AnderssonEscherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cellsClin Exp Immunol1242001423428
- Brad H.RovinLu.LingXiaolan.ZhangA novel interleukin-8 polymorphism is associated with severe systemic lupus erythematosus nephritisKidney Int622002261265
- Luis M.OrtegaAlessiaFornoniRole of cytokines in the pathogenesis of acute and chronic kidney disease, glomerulonephritis, and end-stage kidney diseaseInt J Interferon, Cytokine Mediat Res220104962
- A.HeinzmannI.AhlertT.KurzR.BernerK.A.DeichmannAssociation study suggests opposite effects of polymorphisms within IL8 on bronchial asthma and respiratory syncytial virus bronchiolitisJ Allergy Clin Immunol1142004671676
- J.JavorM.BucovaO.CervenovaK.KralinskyE.SadovaM.SuchankovaGenetic variations of interleukin-8, CXCR1 and CXCR2 genes and risk of acute pyelonephritis in childrenInt J Immunogenet392012338345
- C.GerardB.J.RollinsChemokines and diseaseNat Immunol22001108115
- C.R.MackayChemokines: immunology’s high impact factorsNat Immunol2200195101
- T.WadaH.YokoyamaN.TomosugiY.HisadaS.OhtaT.NaitoDetection of urinary interleukin-8 in glomerular diseasesKidney Int461994455460
- C.Y.TsaiT.H.WuC.L.YuJ.Y.LuY.Y.TsaiIncreased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritisNephron852000207214
- Y.LiM.TucciS.NarainE.V.BarnesE.S.SobelM.S.SegalUrinary biomarkers in lupus nephritisAutoimmun Rev52006383388
- H.YokoyamaT.WadaK.FuruichiC.SegawaM.ShimizuK.KobayashiUrinary levels of chemokines (MCAF/MCP-1, IL-8) reflect distinct disease activities and phases of human IgA nephropathyJ Leukoc Biol631998493499
- F.HuangS.HorikoshiA.KurusuT.ShibataS.SuzukiK.FunabikiUrinary levels of interleukin-8 (IL-8) and disease activity in patients with IgA nephropathyJ Clin Lab Anal1520013034
- K.TashiroI.KoyanagiA.SaitohA.ShimizuT.ShikeC.IshiguroUrinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathyJ Clin Lab Anal16200214
- M.F.SoutoA.L.TeixeiraR.C.RussoM.G.PenidoK.D.SilveiraM.M.TeixeiraImmune mediators in idiopathic nephrotic syndrome: evidence for a relation between interleukin 8 and proteinuriaPediatr Res642008637642
- E.H.GarinCirculating mediators of proteinuria in idiopathic minimal lesion nephrotic syndromePediatr Nephrol142000872878
- M.H.ChoH.S.LeeB.H.ChoeS.H.KwonK.Y.ChungJ.H.KooInterleukin-8 and tumor necrosis factor-alpha are increased in minimal change disease but do not alter albumin permeabilityAm J Nephrol232003260266
- B.BagciG.BagciF.CandanO.OzdemirI.SezginThe protective effect of MCP-1 – 2518 A>G promoter polymorphism in Turkish chronic renal failure patients requiring long-term hemodialysisInt Urol Nephrol4732015551556
