Abstract
Background
Tuberculosis/Human Immunodeficiency Virus (TB/HIV) is a very common co-infection which carries a high mortality rate. Though World Health Organization recommends co-treatment of TB/HIV to improve its outcome, Rifampicin potentially induces metabolism and sub-therapeutic antiretroviral plasma levels of non nucleoside reverse transcriptase inhibitors and protease inhibitors which may cause inadequate virological suppression if corrections are not timely done. In Tanzania Therapeutic drug monitoring is not done; so the proportion of sub-therapeutic ARV plasma levels among TB/HIV patients co-treated with anti-tuberculous drugs is not known. The aim of this study was therefore to determine the magnitude and risk factors of sub-therapeutic ARV plasma levels among adult HIV patients co-treated with anti tuberculous Medications.
Materials and methods
A cross sectional hospital based study was conducted among adult HIV patients on ARV and TB co-treatment for at least one month. Patients were serially enrolled through routine HIV care and treatment services until the sample size was reached. The information about demographic, clinical and adherence level, Anti-TB duration, viral load, baseline and enrollment CD4 counts, Hepatitis B co-infection and ARV plasma levels was collected and analyzed using STATA 12 software.
Results
In total 118 patients were included in this study; of whom 26 (22%) had sub-therapeutic ARV plasma levels. The sub-therapeutic ARV levels were independently associated with adherence <95% (OR = 6.8, p = 0.001), female gender (OR = 3.4, p = 0.028) and virological failure (OR = 3.8, p = 0.016). NVP based regimen was associated with sub-therapeutic drug levels on univariate model (OR = 2.1, p = 0.010).
Conclusion
The magnitude of sub-therapeutic ARV plasma levels is high among adult HIV/TB co-infected patients on anti-TB co-treatment in Tanzania. These patients stand a high risk of inadequate virological suppression with a potential resistance development and a long term poor clinical outcome. Identifying at risk patients and adherence enhancement could potentially improve the overall outcome of this subgroup of patients in resource restricted setting like ours where TDM is not available.
1 Introduction
Tuberculosis/Human immunodeficiency virus (TB/HIV) co-infection has been a common phenomenon for decades, causing a substantially high morbidity and mortality with Tuberculosis ranking as the most common opportunistic infection and the most common cause of mortality among people living with HIV/AIDS (PLWHA) especially in resource restricted countries.Citation1,Citation2 In the year 2013 alone about 1.1 million new cases of TB were reported in HIV positive patients globally where Majority of them (up to 78%) occurred in Africa.Citation3 Tuberculosis occurs as the first manifestation of HIV/AIDS in more than 50% of HIV positive patients Citation4 and deaths that are linked to TB are significantly high especially in sub-Saharan Africa where in some countries this rate is reported to be in excess of 50%.Citation5
Early initiation of Antiretroviral therapy (ART) in the course of TB treatment has been shown to have a mortality benefit Citation6,Citation7 and WHO strongly recommends on co-treatment of HIV/TB co-infection,Citation8 with a rapid scaling up of Antiretroviral therapy programs especially in resource restricted countries, where tuberculosis is for the most part the widespread opportunistic disease.Citation9,Citation10 In these areas thus ART is regularly initiated when patients are being treated for tuberculosis,Citation11,Citation12 with a goal line being to provide an effective and safe Antiretroviral therapy and anti-tuberculosis management which is efficient enough to cure and prevent recurrence and resistance.Citation2,Citation13
Despite this overall success, HIV and TB co-treatment faces a number of important challenges including induction of sub-therapeutic levels of both Non Nucleotide Reverse Transcriptase Inhibitors (NNRTIs) and Protease Inhibitors (PIs). Rifampicin which is the most important component of anti tuberculous medications is remarkable for its induction effect on CYT P450 iso-enzymes which may adversely increase the metabolism and disposition of both NNRTIs and PIs which can potentially cause inadequate plasma levels of these drugs and severely limiting the treatment options for optimal Highly Active Antiretroviral Therapy (HAART) regimens Citation14–Citation19 especially in resource limited settings. Whereas it has been established from prior studies that Rifampicin may be a cause of significant suboptimal levels of both NNRTIs and PIs,Citation20,Citation21 sub-therapeutic ARV plasma levels as a consequence have been demonstrated to be associated with inadequate virological suppression which may subsequently lead into selection of resistant strains and a long term inadequate immune recovery and overall poor clinical outcome.Citation22,Citation23
In developed countries this challenge is overcome using therapeutic drug monitoring (TDM) that is readily available for routine practical use where the patients’ NNRTIs and PIs plasma levels are monitored for any adverse drug levels, and corrections of dosages are timely done to improve the therapeutic outcomes.Citation24,Citation25 TDM has been usefulness in a number of clinical settings including monitoring of ARV plasma levels in TB/HIV co-treatment. In this regard a better treatment outcome has been documented among patients whose treatment was TDM guided than those whose ARV plasma levels were not monitored.Citation25,Citation26 Even though TDM is not done in most of the resource limited countries, the available studies from these settings demonstrate that a significant proportion of HIV patients co-treated with anti Tuberculous drugs (Rifampicin) have sub-therapeutic NNRTIs and PIs plasma levels and some of the locations have reported even higher rates of sub-therapeutic ARV (NNRTIs and PIs) plasma levels than most of resource rich countries.Citation21,Citation27–Citation29
In Tanzania no study has ever reported ARV plasma levels in HIV positive patients who are co-treated with Rifampicin. The current study was therefore designed to determine the magnitude and the associated risk factors of sub-therapeutic NNRTIs (Efavirenz and Nevirapine) and PIs (Lopinavir) plasma levels among adult HIV positive patients who were co-treated with anti-TB in northwestern Tanzania. The results from this study will be useful to assist the overall optimization of management of patients on ARV/anti-TB co-treatment especially in resource limited settings. Also the results from this study will provide a base for further studies on the subject and add to the existing body of knowledge regarding ARV plasma levels especially in resource limited countries.
2 Materials and methods
2.1 Study design and setting
This was a cross-sectional hospital based study which was done between April 2012 and July 2013 at Bugando Medical Centre (BMC) at Care and Treatment Center (CTC) in Mwanza, Tanzania. BMC is a tertiary and teaching hospital for the North Western part of Tanzania. It has a capacity of 1000 beds, and it serves around 13 million people. At Bugando, CTC services started a way back 2004, and are routinely done as part and parcel of outpatient activities. Currently the center serves a total of more than 10,000 patients, whereby more than 4000 of them are active on ART. Tuberculosis screening is routinely done on daily bases and as of now about 300 patients are on TB/HIV co-treatment.
2.2 Study population
This study involved adult HIV and TB co-infected patients diagnosed to have either Pulmonary TB (smear positive and smear negative) or Extra Pulmonary Tuberculosis (EPTB) according to WHO TB diagnosis guideline 2010 Citation30 and put on anti-TB Treatment. The HIV diagnosis was as per WHO guidelines and all patients were treated with standard dose of Nevirapine 200 mg twice daily or Efavirenz 600 mg once daily or Lopinavir/r 400/100 mg twice daily and Rifampicin 600 mg or 450 mg once daily for patients weighing more than 45 kg and less than 45 kg respectively. All patients aging over 18 years and co-treated with ARV and anti-tuberculosis medications for at least one month were included in this study.
2.3 Sample size, patients’ enrollment and data collection
A minimum sample of 100 patients was estimated from cross sectional studies’ formula by Leslie Kish, assuming 30% of adult HIV positive patients co-treated with Rifampicin had subtherapeutic ARV levels Citation21,Citation28 at an allowable error of 0.09. After a written informed consent a structured questionnaire was used to collect information about demographic data, body mass index (BMI), date of HIV diagnosis, date of ART initiation, the ART regime, ART adherence level, baseline CD4 and on study CD4 count, Hepatitis B status, type of tuberculosis, time on anti tuberculous medication, viral load and plasma NNRTIs and PIs levels.
The ART adherence level in the last 30 days was assessed using pill counts.Citation31 The pill counts were performed by the study pharmacist, who counted the number of remaining pills at each drug refill visit. Pill count-based adherence was assessed using the formula [Adherence = (Number of pills dispensed -Number of pills returned 100)/(Number of pills prescribed daily × Number of days between pharmacy visits)]. Adequate adherence level was defined as a value ⩾95% pills whereas poor adherence was defined as a value ⩽95%. The patients were instructed to take their medication at night as prescribed and come the following morning for blood sample collection before their next ART dose. Two blood samples were drawn, one for viral load which was done at BMC main laboratory and the other sample was sent to Germany for TDM to determine the plasma concentrations of Efavirenz, Nevirapine and Lopinavir.
2.4 Sample collection, processing and analysis
For each patient, 5 ml of whole blood was collected in plasma EDTA bottles for TDM, approximately 8–12 h after the last dose of antiviral drugs and just before the next dose was done. The samples were immediately centrifuged at 3000 rpm for 3 min to obtain plasma that was transferred into cryovials. The cryovials were stored at −20 °C before shipment. The samples were packed and shipped to Germany in cold boxes with cooling packs maintaining a temperature of −30 °C. The plasma concentrations of NVP, EFV, and LPV were determined by a sensitive validated simultaneous assay using reverse-phase (Zorbax XD8-CI8; Agilent Technology) high performance liquid chromatography (HP 1100; Agilent Technology), coupled with tandem mass spectrometry (MS-MS) (API 2000; Applied Biosystems) as described previously.Citation32 An additional 5 ml of whole blood was collected in a tube supplemented with EDTA (BD Biosciences) for plasma preparation and sent to BMC main laboratory for viral load analysis using COBAS AmpliPrep/COBAS TaqMan (Roche Molecular Systems, USA) according to manufacturer’s guidelines as described previously.Citation33
2.5 Data management and analysis
Data were managed using Epi Data 3.1 (CDC Atlanta, USA) and analysis was done using STATA version 12 (College Station, Texas, USA). ARV drug concentrations were recorded as continuous variables. The therapeutic ranges in ng/ml were defined as 1000–4000 for EFV, and 3400–8000 and 3000–7000 for NVP and LPV respectively; thus three categories of ARV plasma drug concentrations were defined as used in other studies,Citation24 and any levels below the lower limit of the respective drug were coded as being sub-therapeutic ARV level for that drug and drug levels that were within therapeutic range were coded as therapeutic ARV levels, while those that were above the upper limit of therapeutic range were coded as supra-therapeutic ARV levels. Categorical variables were summarized as proportion and the significance of the difference in distribution within the categories of ARV plasma drug concentrations was assessed using Pearson’s Chi-square test or Fisher’s exact test where appropriate. We used probability plots and Shapiro-Wilk normality test to assess the normality of continuous variables. Parametric continuous data were summarized as mean with standard deviation and the significance of difference in means within categories of ARV plasma drug concentrations was assessed using Student’s t-test. Non-parametric continuous data were summarized as median with interquartile range and the difference in medians within the categories of ARV plasma drug concentrations was compared using Wilcoxon rank-sum test. The odds ratios (ORs) and 95% confidence intervals (CIs) of risk factors associated with sub-therapeutic ARV plasma levels were calculated using univariate logistic regression model followed by multivariate logistic regression model. All factors associated with sub-therapeutic ARV plasma levels in the univariate model with p-values less than 0.05 were considered for inclusion in the multivariate model. A stepwise approach was used to derive a Parsimonious model, and all associated factors in the final model were considered significant if the P-value was less than 0.05.
3 Ethical consideration
The permission to conduct this study and publish the results was found from The Catholic University of Health and Allied Sciences and Bugando Medical Centre (CUHAS/BMC) joint ethics review board. Written consent was obtained from all study participants. Patients’ identifiers were not included to maintain confidentiality.
4 Results
4.1 Baseline demographic, clinical and laboratory characteristics of 118 adult HIV positive patients cotreated with tuberculosis drugs at Bugando CTC
A total of 118 patients were included in this study, where more than 58% of these patients were females with a median age of 38 Citation32–Citation43years. The median time on anti-TB was 5 Citation2–Citation6 months, and on ARV 10 Citation3–Citation22 months with an adherence rate of >95% in more than 79% of the studied patients (). The most common regimen used was TDF + FTC + EFV 80 (67.8%), followed by AZT + 3TC + EFV 24 (20.3%) which shows that most patients 104 (88%) were on EFV based regimen as summarized in .
Figure 1 The combined Anti retroviral therapy regimens. ∗ABC: Abacavir; AZT: Zidovudine; d4T: Stavudine; ddI: Didanosine; EFV: Efavirenz; FTC: Emtricitabine; LPV: Lopinavir; NPV: Nevirapine, r: ritonavir; TDF: Tenofovir; 3TC: Lamivudine.
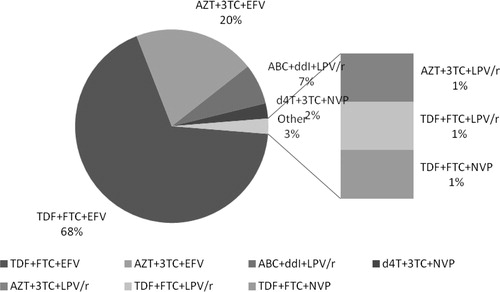
Table 1 The baseline demographic, clinical and laboratory characteristics of 118 adult HIV Positive patients attending CTC at Bugando with ARV and anti-TB Co-treatment.
4.2 Subtherapeutic ARV plasma levels and associated factors among 118 adult HIV positive patients co-treated with anti-tuberculosis drugs
In total 26 (22%) of the studied patients had sub-therapeutic ARV plasma levels (), and by regimen 19 (28.3%), 57 (54.8%), and 28 (26.9%) of those who were on EFV based regimen had sub-therapeutic, therapeutic and supra-therapeutic EFV plasma levels respectively, whereas 4 (40%), 5 (50%), and 10 (10%) had sub-therapeutic, therapeutic and supra-therapeutic LPV plasma levels respectively. Furthermore Sub-therapeutic and therapeutic NVP plasma levels were found in 3 (75%) and 1 (25%) respectively and there was no study participant who had supra-therapeutic NVP plasma levels (). summarizes the distribution of variables by plasma ARV levels. Several factors were tested for association with sub-therapeutic ARV plasma levels. On a univariate model the sub-therapeutic ARV plasma levels were strongly associated with a female gender (OR = 2.9, p = 0.031), adherence level of less than 95% (OR = 4.5, p < 0.002), virological failure (OR = 2.9, p = 0.027), and NVP based regimen (OR = 2.1, p = 0.010), whereas on a multivariate model only Female gender (OR = 3.4, p = 0.028) and adherence <95% (OR = 6.8, p = 0.001) remained independently associated with sub-therapeutic ARV plasma levels. Moreover the patients with sub-therapeutic ARV plasma levels were consequently more likely to have virological failure (OR = 3.8, p = 0.016) (). However there was no significant statistical association found between sub-therapeutic ARV plasma levels and age, geographical location, BMI, WHO clinical stage, CD4 levels, Hepatitis B co-infection, time on ART, time on anti-TB, and viral load of more than 400 copies/μl and TB category.
Figure 2 Distribution of ARV plasma levels as sub-therapeutic, Therapeutic or supra-therapeutic among 118 adult HIV patients cotreated with TB drugs. ∗ARV: Antiretroviral; EFV: Efavirenz; HIV: Human immunodeficiency virus; LPV: Lopinavir; NVP: Nevirapine; TB: Tuberculosis.
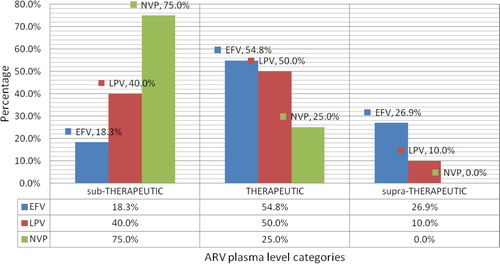
Figure 3 Distribution of variables by plasma ARV drug levels as sub-therapeutic or therapeutic. ∗AD < 95%: Adherence level of less than 95%; AD > 95%: Adherence level of more than 95%; ARV: Antiretroviral; HepB pos: Hepatitis B positive; HepB neg: Hepatitis B positive; VF: virological failure; WHO: world health organization; WHO 3: WHO clinical stage 3; WHO 4: WHO clinical stage 4.
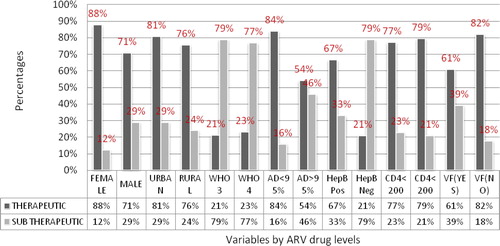
Table 2 Univariate and multivariate analysis for factors associated with sub-therapeutic ARV plasma levels among 118 adult HIV positive patients on TB co-treatment at Bugando.
5 Discussion
The objective of this study was to determine the proportion and risk factors of sub-therapeutic ARV plasma levels among adult HIV positive patients co-treated with anti-TB. In this study, 26 (22%) of the patients co-treated with anti-TB had subtherapeutic ARV plasma levels, and by regimen 19/104(18.3%) had subtherapeutic EFV levels, while 3/4 (75%) and 4/10 (40%) had subtherapeutic NVP and LPV levels respectively. The sub-therapeutic ARV plasma levels were independently associated with female gender and adherence level of less than 95% on a multivariate analysis; otherwise, NVP based regimen was only strongly in association with sub-therapeutic ARV plasma levels on univariate model alone.
Comparable ranges of sub-therapeutic ARV levels have been reported in different studies across the world assessing plasma concentration of NNRTIs or PIs. In previous studies sub-therapeutic EFV plasma levels were reported with a prevalence varying between 20% and 32%. For example in London Sathia et al. reported EFV sub-therapeutic plasma levels in 20% of patients who were co-treated with Rifampicin,Citation21 while in Kenya Citation34 this was reported in 32% of patients who were co-treated with Rifampicin. The magnitude of sub-therapeutic EFV levels reported from Kenya is comparatively higher than our finding, probably because this study was done among patients with a number of other co-morbidities requiring several other co-medications which could have considerably contributed to this higher report of sub-therapeutic Efavirenz plasma levels.
Sub-therapeutic NVP level is also reported in a range of 34–39% among patients co-treated with Rifampicin in various other studies. In Kenya 34.5% of patients studied for clinically significant drug interaction had sub-therapeutic NVP.Citation34 In studies from UK Citation21 and South Africa,Citation28 the sub-therapeutic NVP plasma levels were reported in 36% and 39% of patients co-treated with Rifampicin respectively. These rates are on the other hand lower than our findings, but of note all these studies suggest that TB/HIV co-treated patients on NVP-based regimen were the most likely to have subtherapeutic ARV drug levels, as compared to EFV based regimen even though the number of patients who were on NVP based regimen in our study was much smaller.
Rifampicin is remarkable for its CYP45O induction effect, which subsequently increases the metabolism of NNRTIs and PIs.Citation17,Citation35,Citation36 This phenomenon affects NVP more extensively than EFV, and it is essentially because of the differences in biotransformation pathways which are largely influenced by CYP2B6 and NAT2 genetic polymorphism.Citation37–Citation39 Nevirapine metabolism occurs through a number of CYP450 isoenzymes inclusive of CYP2B6, CYP3A and CYP2C which are Rifampicin “inducible”,Citation40 an event that has also been suggested to affect the PIs substantially.Citation10,Citation36,Citation41 As a consequence this increases the clearance of NVP and PIs among those individuals who are co-treated with Rifampicin containing tuberculosis drugs. A number of studies have demonstrated the consequence of this interaction between NVP and Rifampicin including a recent study from Mozambique which indicated that NVP plasma concentrations were much lower while on anti tuberculosis drugs than after discontinuation of tuberculosis drugsCitation42 with a large proportion of NVP concentration being below therapeutic range for the period of cotreatment with tuberculosis drugs than thereafter.
Rifampicin is remarkable for its CYP45O induction effect, which subsequently increases the metabolism of NNRTIs and PIs.Citation17,Citation35,Citation36 This phenomenon affects NVP more extensively than EFV, and it is essentially because of the differences in biotransformation pathways which are largely influenced by CYP2B6 and NAT2 genetic polymorphism.Citation37–Citation39 Nevirapine metabolism occurs through a number of CYP450 isoenzymes inclusive of CYP2B6, CYP3A and CYP2C which are Rifampicin “inducible”,Citation40 an event that has also been suggested to affect the PIs substantially.Citation10,Citation36,Citation41 As a consequence this increases the clearance of NVP and PIs among those individuals who are co-treated with Rifampicin containing tuberculosis drugs. A number of studies have demonstrated the consequence of this interaction between NVP and Rifampicin including a recent study from Mozambique which indicated that NVP plasma concentrations were much lower while on anti tuberculosis drugs than after discontinuation of tuberculosis drugsCitation42 with a large proportion of NVP concentration being below therapeutic range for the period of cotreatment with tuberculosis drugs than thereafter.
Even though protease inhibitors including Lopinavir have a short half life requiring multiple dosages a day, LPV is still the most preferred PI when used in its co formulated form with ritonavir as a pharmacological booster (LPV400 mg/r100 mg), since it is well tolerated and has a better virological efficacyCitation43,Citation44 as compared to other PIs. However at this strength of LPV/r co formulation Rifampicin significantly increases the clearance of both drugs leading into failure of virological control. Based on this a number of studies have demonstrated a better virological control with super boosted LPV/r including combined strengths of 400/400 mg or 800/200 mg regimens,Citation45,Citation46 however with increased risk of toxicity which could negatively affect the patients’ adherence to medications.
On the contrary Efavirenz is mainly metabolized by CYP2B6.Citation47,Citation48 Though this enzyme can also be induced by Rifampicin it has been shown that Isoniazid is one of important components of the TB fixed dose combination, inhibits CYP2B6 especially among individuals who are slow metabolizers (with CYP2B6 loss of function), and decreases the EFV clearance with attendant increase in plasma EFV concentration.Citation49,Citation50 This phenomenon is reportedly more frequent among African than European descendants,Citation51 and it has been observed in about 40% of African individuals in some study settings.Citation52 Among patients who were on TB treatment in Mozambique their plasma EFV concentrations were higher with anti tuberculosis than after discontinuation of anti tuberculosis drugs.Citation42 In this study it was further shown that only a small proportion of patients had sub-therapeutic EFV plasma levels (9%) as compared to more than 37% who had supra-therapeutic levels of EFV while on anti tuberculosis.
These observations are similar to our findings that of the 104 patients who were on EFV based regimen, 28 (26.9%) had supra-therapeutic (>4000 ng/mL) EFV plasma concentrations () whereas only 1 (10%) participant on LPV and none of those who were on NVP based regimen had supra-therapeutic plasma levels. These findings suggest that patient on HIV/TB co-treatment should as well be monitored for potential ART drug toxicities.
One aim of this study was to determine the factors that will give clinical prediction of subtherapeutic ARV levels in adult patients who are co-treated with anti-TB. These factors which have also been reported by other studies, include adherence of <95%,Citation53,Citation54 NVP based regimeCitation55 and female gender.Citation34,Citation53 In the face of this association our results suggest that the occurrence of any of these risk factors independently augments the likelihood of sub-therapeutic ARV levels plasma among patients co-treated with Rifampicin.
Many of the findings of this study were consistent with previous studies and logical but one surprising finding is that of the female gender being a predictor of sub-therapeutic plasma ARV levels among adult HIV patients co-treated with anti-TB. The female gender is documented as one of the inter-individual differences which have been shown to alter the apparent oral clearance of ARVs.Citation56 This may be one of the explanations that the female sex stands as one of the predictors of sub-therapeutic ARV levels among patients co-treated with anti-TB. Additional factors which may alter oral clearance of ARV are inclusive of geographical location and Hepatitis B Virus (HBV) co-infection.Citation56,Citation57 However in our study these two additional factors did not show any significant statistical association to sub-therapeutic ARV levels.
Apart from the fact that both NNRTIs and PIs undergo their disposition through CYP450 iso enzymes,Citation10 on the other hand the plasma levels of these drugs are determined by adherence levels to medications especially among patients on EFV based regimens.Citation42 Prior studies had indicated that the sub-therapeutic EFV plasma levels on TB co-treatment are less frequent and are more common among those patients with inadequate adherence level.Citation42 Since isoniazid reduces clearance of EFV, improvement of adherence to ART medications may positively alter the plasma levels of EFV and possibly give patients a much positive virological outcome as compared to NVP and Lopinavir based regimens.
Therapeutic drug levels are a key to successful ART,Citation57,Citation58 and any low drug levels observed in patients on ART have been extrapolative of a failure to achieve an immediate virological containment and a longer term immunological failure.Citation53,Citation59 Findings of sub-therapeutic drug levels among patients co-treated with anti-TB in the current study are accompanied by a co-occurrence of both high levels of virological failure and substantially low CD4 counts on enrollment as a consequent. In this study virological failure was found in 39.1% of the patients with sub-therapeutic ARV levels and on enrollment CD4 count of <200 cells/μl was found in more than one-third, 9/26 (34.6%) of these patients with sub-therapeutic NNRTIs and PIs plasma levels.
On clinical grounds these findings suggest that these patients stand a high risk of a subsequent potential of developing and accumulating resistant viral strains,Citation23,Citation60,Citation61 when these drug levels are not corrected quickly. However this is a great challenge in Tanzania and other resource limited settings where TDM is not done. Our findings that female gender, poor adherence, and certain regimens are predictive of sub-therapeutic drug levels are helpful to clinicians in these settings who need to maintain a high index of suspicion for sub-therapeutic drug levels in such patients and to counsel patients on the importance of adherence. This study had a number of limitations including a small sample size especially of patients on NVP and LPV based regimen. Also this being a cross sectional and a single clinic based study, the results from this study may not necessarily be generalizable to the general population; therefore, a longitudinal study with a larger sample size is recommended.
6 Conclusion
The magnitude of sub-therapeutic ARV plasma levels is significantly high among adult HIV positive patients on ARV and anti-TB co-treatment attending HIV care and treatment centers in Tanzania. These patients are at a high risk of immediate inadequate virological suppression with a potential resistance development and a long term poor clinical outcome. Since TDM is not available in most resource limited settings to assist in identifying these patients early and making timely correction to improve their overall outcome on ARVS, Clinician in resource limited settings such as Tanzania should maintain a high index of suspicion and identify potential patients for a closer clinical follow-up and adherence augmentation.
Competing interest
Authors declare that they had no competing interest and all the authors approved the final manuscript before submission.
Authors’ contribution
CK, SEK, HK and DWG conceived the idea and designed the study; DWG and CK performed the experiments and acquired the data; DWG and BRK analyzed and interpreted the data; DWG, CK, and
BCM did literature search; DWG did draft the manuscript; DWG, BCM, CK, BRK, HK, GWK and
SEK critically reviewed and edited the manuscript for its intellectual content.
Acknowledgments
The authors are thankful and appreciate the technical support provided by Dr. Robert N. Peck and all laboratory members at Bugando Medical Centre and Institute of Virology and immunology of Würzburg University.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 1 November 2016
References
- W.ManosuthiStandard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicinHIV Med952008294299
- E.L.CorbettThe growing burden of tuberculosis: global trends and interactions with the HIV epidemicArch Intern Med1639200310091021
- WHO, Global Tuberculosis Report; 2014.
- WHO, Interim policy on collaborative TB/HIV activities; 2004.
- WHO, Global tuberculosis control 2011, WHO., Editor; 2011.
- M.E.TorokJ.J.FarrarWhen to start antiretroviral therapy in HIV-associated tuberculosisThe New Engl J Med36516201115381540
- S.D.LawnM.E.TorokR.WoodOptimum time to start antiretroviral therapy during HIV-associated opportunistic infectionsCurrent Opin Infect Diseases24120113442
- G.FriedlandAdministration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIVJ Antimicrob Chemother586200612991302
- M.W.BrinkhofTuberculosis after initiation of antiretroviral therapy in low-income and high-income countriesClin Infect Dis4511200715181521
- A.BoulleOutcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapyJAMA30052008530539
- S.D.LawnBurden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis controlAIDS2012200616051612
- S.D.LawnR.WoodTuberculosis control in South Africa–will HAART help?S Afr Med J9662006502504
- K.R.CollinsImpact of tuberculosis on HIV-1 replication, diversity, and disease progressionAIDS Rev432002165176
- C.V.FletcherConcentration-controlled compared with conventional antiretroviral therapy for HIV infectionAIDS1642002551560
- H.McIlleronComplications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndromeJ Infect Dis196Suppl 12007S63S75
- G.RamachandranIncreasing nevirapine dose can overcome reduced bioavailability due to rifampicin coadministrationJ Acquir Immune Defic Syndr42120063641
- J.M.RaeRifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arraysJ Pharmacol Exp Ther29932001849857
- D.J.PepperCombined therapy for tuberculosis and HIV-1: the challenge for drug discoveryDrug Discov Today1221–222007980989
- E.RiberaRifampin reduces concentrations of trimethoprim and sulfamethoxazole in serum in human immunodeficiency virus-infected patientsAntimicrob Agents Chemother4511200132383241
- D.B.Pedral-SampaioEfficacy and safety of Efavirenz in HIV patients on Rifampin for tuberculosisBraz J Infect Dis832004211216
- L.SathiaConcomitant use of nonnucleoside analogue reverse transcriptase inhibitors and rifampicin in TB/HIV type 1-coinfected patientsAIDS Res Hum Retroviruses2472008897901
- P.ClevenberghImproving HIV infection management using antiretroviral plasma drug levels monitoring: a clinician’s point of viewCurr HIV Res242004309321
- D.W.GundaPlasma concentrations of efavirenz and nevirapine among HIV-infected patients with immunological failure attending a tertiary hospital in North-western TanzaniaPLoS One892013e75118
- M.DuongUsefulness of therapeutic drug monitoring of antiretrovirals in routine clinical practiceHIV Clin Trials542004216223
- S.H.KhooPharmacologic optimization of protease inhibitors and nonnucleoside reverse transcriptase inhibitors (POPIN)–a randomized controlled trial of therapeutic drug monitoring and adherence supportJ Acquir Immune Defic Syndr4142006461467
- F.V.LethPharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacyAIDS Res Hum Retroviruses2232006232239
- Kigen G et al., Prevalence of potential drug-drug interactions involving antiretroviral drugs in a large Kenyan cohort. PLoS One. 6(2): p. e16800.
- D.ElsherbinyPopulation pharmacokinetics of nevirapine in combination with rifampicin-based short course chemotherapy in HIV- and tuberculosis-infected South African patientsEur J Clin Pharmacol65120097180
- J.J.van OosterhoutNevirapine-based antiretroviral therapy started early in the course of tuberculosis treatment in adult MalawiansAntivir Ther1242007515521
- WHO, Treatment of tuberculosis Guidelines. 2010(WHO/HTM/TB/2009.420): p. 147.
- R.GrossbergR.GrossUse of pharmacy refill data as a measure of antiretroviral adherenceCurr HIV/AIDS Rep442007187191
- P.LangmannHigh-performance liquid chromatographic method for the determination of HIV-1 non-nucleoside reverse transcriptase inhibitor efavirenz in plasma of patients during highly active antiretroviral therapyJ Chromatogr B Biomed Sci Appl7551–22001151156
- COBAS®, COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version2.0. Doc Rev. 5.0 ed., R.m. systems, Editor. 2010, Roche molecular systems USA. p. 1–30.
- G.KigenPrevalence of potential drug-drug interactions involving antiretroviral drugs in a large Kenyan cohortPLoS One622011e16800
- D.G.De RequenaI.Jimenez-NacherV.SorianoChanges in nevirapine plasma concentrations over time and its relationship with liver enzyme elevationsAIDS Res Hum Retroviruses2162005555559
- S.C.PiscitelliK.D.GallicanoInteractions among drugs for HIV and opportunistic infectionsN Engl J Med344132001984996
- J.BertrandDependence of efavirenz- and rifampicin-isoniazid-based antituberculosis treatment drug-drug interaction on CYP2B6 and NAT2 genetic polymorphisms: ANRS 12154 study in CambodiaJ Infect Dis20932014399408
- K.CohenEffect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G>T polymorphism on efavirenz concentrations in adults in South AfricaAntivir Ther1452009687695
- J.BertrandMultiple genetic variants predict steady-state nevirapine clearance in HIV-infected CambodiansPharmacogenet Genomics22122012868876
- P.S.RiskaBiotransformation of nevirapine, a non-nucleoside HIV-1 reverse transcriptase inhibitor, in mice, rats, rabbits, dogs, monkeys, and chimpanzeesDrug Metab Dispos2712199914341447
- A.PatelSafety and antiretroviral effectiveness of concomitant use of rifampicin and efavirenz for antiretroviral-naive patients in India who are coinfected with tuberculosis and HIV-1J Acquir Immune Defic Syndr371200411661169
- N.B.BhattNevirapine or efavirenz for tuberculosis and HIV coinfected patients: exposure and virological failure relationshipJ Antimicrob Chemother7012015225232
- R.S.CvetkovicK.L.GoaLopinavir/ritonavir: a review of its use in the management of HIV infectionDrugs6382003769802
- V.OldfieldG.L.PloskerLopinavir/ritonavir: a review of its use in the management of HIV infectionDrugs669200612751299
- R.A.MurphyCoadministration of lopinavir/ritonavir and rifampicin in HIV and tuberculosis co-infected adults in South AfricaPLoS One792012e44793
- H.SunpathDouble-dose lopinavir-ritonavir in combination with rifampicin-based anti-tuberculosis treatment in South AfricaInt J Tuberc Lung Dis1862014689693
- E.T.OgburnEfavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylationDrug Metab Dispos387201012181229
- P.RiskaDisposition and biotransformation of the antiretroviral drug nevirapine in humansDrug Metab Dispos2781999895901
- T.N.GengiahThe influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosisEur J Clin Pharmacol6852012689695
- A.F.LuetkemeyerRelationship between weight, efavirenz exposure, and virologic suppression in HIV-infected patients on rifampin-based tuberculosis treatment in the AIDS Clinical Trials Group A5221 STRIDE StudyClin Infect Dis5742013586593
- S.UttayamakulEffects of CYP2B6 G516T polymorphisms on plasma efavirenz and nevirapine levels when co-administered with rifampicin in HIV/TB co-infected Thai adultsAIDS Res Ther720108
- P.ArnaldoFrequencies of cytochrome P450 2B6 and 2C8 allelic variants in the mozambican populationMalays J Med Sci20420131323
- C.S.AlexanderAntiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced diseaseJ Infect Dis18842003541548
- L.AhouaRisk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern UgandaBMC Infect Dis9200981
- W.ManosuthiA randomized trial comparing plasma drug concentrations and efficacies between 2 nonnucleoside reverse-transcriptase inhibitor-based regimens in HIV-infected patients receiving rifampicin: the N2R StudyClin Infect Dis4812200917521759
- B.S.KappelhoffNevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN studyAntivir Ther1012005145155
- A.I.VeldkampHigh exposure to nevirapine in plasma is associated with an improved virological response in HIV-1-infected individualsAIDS159200110891095
- R.GieschkeRelationships between exposure to saquinavir monotherapy and antiviral response in HIV-positive patientsClin Pharmacokinet37119997586
- D.M.BurgerLow plasma concentrations of indinavir are related to virological treatment failure in HIV-1-infected patients on indinavir-containing triple therapyAntivir Ther341998215220
- K.C.SigaloffUnnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in AfricaJ Acquir Immune Defic Syndr58120112331
- A.JohannessenVirological efficacy and emergence of drug resistance in adults on antiretroviral treatment in rural TanzaniaBMC Infect Dis92009108
