Abstract
Background
The results of “light and electron microscopic study” of the peripheral arteries and nerves biopsies in diabetic neuropathy comparing with biopsies of normal arteries and nerves of traumatic amputation as a control group.
Aim of the work
To evaluate the “electron microscopic changes” in the peripheral small arteries and nerves in the diabetic ischemic lower limbs.
Patients and methods
From January 2015 to June 2016 a total number of 20 patients with diabetic ischemic lower limbs (Diabetic patients group) compared with 20 non diabetic non ischemic persons as (Control group) who undergone traumatic lower limb amputation. All cases were subjected to complete history taking, complete clinical examination, and routine laboratory investigations. “Light and electron microscopic studies” of biopsies from the peripheral small arteries and nerves e.g. digital or posterior tibial arteries and nerves during amputation of diabetic gangrene of the toes, below knee, above knee amputation and from traumatic amputation of the control group.
Results
The results of an “electron microscopic study” of diabetic peripheral arteries and nerves biopsies, 12 out of 20 cases showed thrombi in small vessels. In some small vessels, masses of fibrin were seen within the lumen. In other vessels, older thrombi were present. 17 out of 20 cases showed endothelial cells hyperplasia in some vessels. The degree of hyperplasia was sufficient to occlude the lumen of the vessels. Some vessels showed degenerate pericytes and endothelial cells which contained large lipid droplets. The peripheral nerves showed patchy demyelination, areas of degeneration and regeneration, areas of infarction and necrosis and collagen fibers deposition. Among the control group, no cases contained thrombi, degenerated vessels, degenerate pericytes or lipid droplets within the endothelial cells.
Conclusion
The present study found that the diabetic microangiopathy is the main cause of diabetic neuropathy and diabetic foot lesions.
1 Introduction
Diabetes mellitus is one of genetic metabolic disorders which clinically manifested by fasting hyperglycemia, atherosclerotic and micro-angiopathic disease of vessels and nerves.Citation1
Figure 10 Diabetic posterior tibial artery (EM x5000/5b). – Part of red blood cell appears in the lower left corner (rbc); Fat droplets (F), Elastic fiber (E), and fibrous tissue (Ft).
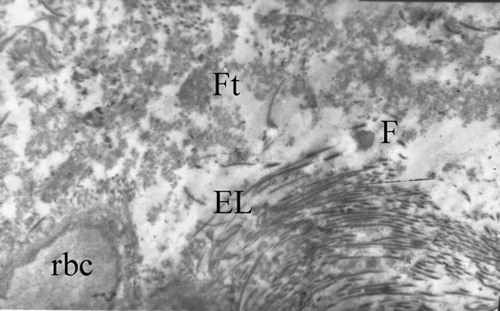
Figure 11 Diabetic posterior tibial artery (EM x40/8000/13). Media with 2 multinucleated giant cells (MGC), extensive collagen fibers (Cf).

Figure 15 Diabetic posterior tibial nerve (EM x 39/8000/4). – Endoneural vessel (EV) with large thrombus (T) and plasma protein (P) in the lumen (L); Nerve fibers are demyelinated all around (Dnf).
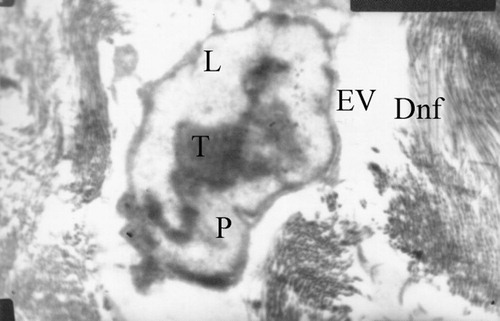
Figure 16 Diabetic posterior tibial nerve (EM x 42/20000/14). – Endoneural vessel (EV) with large thrombus (T) occluding the lumen (L); Collagen fibers deposition all around (C) thickened basement membrane (BM).

Absolute or relative deficiency of insulin – has a bad effect on the metabolism of carbohydrate, protein, fat, water and electrolytes, sometimes with grave consequences.Citation2,Citation3
The therapeutic use of insulin in diabetes mellitus has changed the behavior of this disease.Citation4,Citation5 Diabetic foot lesions may be aggressive to lead to loss of a leg or a foot.Citation6
Microvascular and macrovascular complications of diabetes are common. The most common causes of morbidity and mortality in diabetics are macrovascular changes. Also, it can lead to stroke and myocardial infarction.Citation7–Citation10
Vascular calcification and arterial occlusive disease are very common in diabetics.Citation11 Functional and structural abnormalities in diabetic vessels often occurred such as endothelial dysfunction, decreased vascular compliance, and atherosclerosis.Citation12,Citation13
Vascular abnormalities in biopsies of sural nerve from diabetics were first described by Fagerberg.Citation14 He stated that vascular changes: hyalinisation, narrowing of the lumen and thickening of the wall were more common in diabetics. He mentioned that the structural damage to the myelin sheath was due to progressive degenerative changes.
Timperley et al.Citation15 demonstrated that fibrin plugged the vessels and infiltrated its walls, organising thrombus occluded the vessels and necrosis in nerve bundles adjacent to diseased vessels.
Timperley and his colleaguesCitation15 presented the result of an “electron microscopic changes” in sural nerve biopsies from diabetic patients and attempted to clarify some of the mechanisms by which abnormalities of the coagulation pathways affect nerve function in diabetics.
2 Aim of the work
The aim of this study was to evaluate the “electron microscopic changes” in the peripheral small arteries and nerves in the diabetic ischemic lower extremities.
3 Patients & methods
3.1 Patients
Twenty patients of type 2 diabetes with lower limbs ischaemia who were subjected to minor or major amputations (Diabetic patients group).
Twenty non diabetic, non ischemic persons who were subjected to traumatic lower limb amputations (Control group).
Both groups were presented to the “Department of Surgery, Faculty of Medicine and Department of Experimental and Clinical Surgery, Medical Research Institute, Alexandria University, Egypt” from January 2015 to June 2016.
3.2 Methods
After local ethical committee of the “Faculty of Medicine, Alexandria University” approval and obtaining fully informed patients consent, the current study was conducted on all patients who were subjected to the following:
| 1. | Complete history taking. | ||||
| 2. | Through clinical examination. | ||||
| 3. | Routine laboratory investigations. | ||||
| 4. | Method of extraction of nerve.Citation13 | ||||
| 5. | “Light and electron microscopic studies” of biopsies from the peripheral small arteries and nerves e.g. digital or dorsalis pedis or posterior tibial arteries and nerves.Citation13 | ||||
4 Results
The mean age of the diabetic patient’s group was 61 years and 70% of them were males. The mean age of the control group was 48 years and 80% of them were males ().
Table 1 Age and sex distribution of the diabetic patient’s group.
The average duration of diabetes for the patients was 10.5 years and 60% of them were non-insulin dependent diabetics and 40% were insulin-dependent diabetics. Seventy percent of the patients were smokers, 40% were obese, 50% were hypertensive, 60% had hypercholesterolemia, 50% had hypertriglyceridemia and 100% had poor diabetic foot care ().
Table 2 Risk factors associated with D.M. in diabetic patient’s group.
Clinically, 100% of the diabetic patients had history of intermittent claudication, 80% had no dorsalis pedis pulse, 60% had no posterior tibial pulse, 100% had popliteal and femoral pulses, 90% had peripheral neuropathy, and 20% had gangrene of big toe, 80% had gangrene of one foot (40% of right foot and 40% of the left foot), and 20% had undergone big toe amputation, 50% had undergone below knee amputation of one leg and 30% had undergone above knee amputation of one leg.
4.1 Surgical treatment of lower limb ischemia in diabetic patients
Big toe amputation was done in 4 cases (20%), below knee amputation in 10 cases (50%) and above knee amputation in 6 cases (30%).
Biopsies were taken from the normal posterior tibial arteries and nerves during traumatic amputation (Control group). Light microscopic study of the normal arteries, and , electron microscopic study of the normal artery, . Light microscopic study of the normal nerves, and , electron microscopic study of the normal nerve, .
Figure 1 Normal posterior tibial artery (H&E x10). – Continuous endothelial cells. – Internal elastic lamina. – Smooth muscle cells of the media with collagen fibers in between. E.C.: Endothelial cell, L: Lumen, C: Collagen. – SMC: Smooth muscle cell, EL: Elastic lamina.
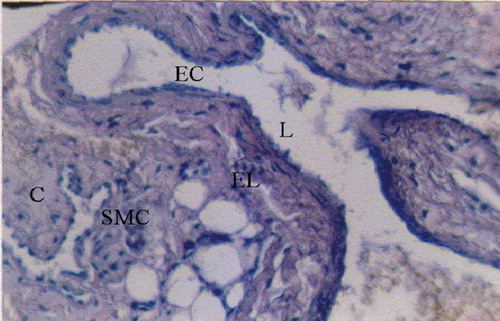
Figure 2 Normal posterior tibial artery (H&E x40). – Continuous endothelial cells. Narrow layer of the intima. – Internal elastic lamina. – Media with smooth muscle cells and collagen fibers; I: Intima.

Figure 3 Normal posterior tibial artery (EM x13000). – Normal smooth muscles cells. – Loose connective tissue. SMC: Smooth muscles cell, CT: Connective tissue.

Figure 4 Normal posterior tibial nerve (H&E x10). – Normal endoneural vessel in the center. – Wrapped in myelin sheath; EBV: Endoneural blood vessel, A: Axon, M: Myelin.

Figure 5 Normal posterior tibial nerve (H&E x40). – Nerve axons wrapped in myelin. – Endoneural vessel in the left upper corner; VN: Vasa nervosum, L: Lumen.
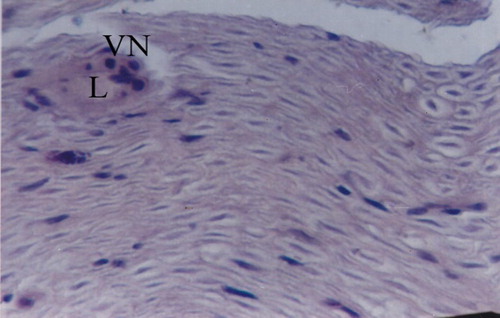
Figure 6 Normal posterior tibial nerve (EM x5000). Nerve bundles N between loose connective tissue CT.

Biopsies were taken from artery and nerve of the big toe and posterior tibial arteries, dorsalis pedis arteries and corresponding nerves (Diabetic patients group). “Light and electron microscopic studies” of diabetic peripheral arteries and , and light microscopic study & and – electron microscopic study. “Light and electron microscopic studies” of diabetic peripheral nerves and , light microscopic study & – electron microscopic study.
Figure 7 Diabetic dorsalis pedis artery (H&E x10). – Smooth muscle cell proliferation. – Cellular infiltration. – Collagen fibers. – Fat droplets; SMC: Smooth muscles cell, Ci: Cellular infiltration.

Figure 8 Diabetic posterior tibial artery (H&E x40). – Thickened intima with increased collagen fibers deposition. – Proliferation of smooth muscle cells. – Areas of hemorrhage and calcification. L, lumen, I: Intima, M: Media, Cf: Collagen fiber, H: Hemorrhage, C: Calcification, EL: Elastic laminae, SMC: Smooth muscles cell.

Figure 12 Diabetic posterior tibial artery (EM x41/8000/3). – 2 fibrocytes (F) with signs of adhesion; Dense collagen bundles (C).

Figure 13 Diabetic posterior tibial nerve (H&E x 40). – Patchy demyelination, collagen fibers deposition, areas of degeneration. – 2 endoneural vessels with narrow lumen and collagen deposition all around. – Decreased occupancy and density of nerve fibers; VN: vasa nervosum, De: demyelination, Dg: degeneration.

Figure 14 Diabetic posterior tibial nerve (EM x 99/8000/4). Area of old infarction with extensive fibrosis (F) and collagen deposition (C).

Figure 17 Diabetic micro vessel of posterior tibial nerve (EM x 826/5000/16). – Hyperplastic endothelial cells (E) show swelling, cytoplasmic vacuoles (V) bleb formation (B); Degranulated platelets (P).
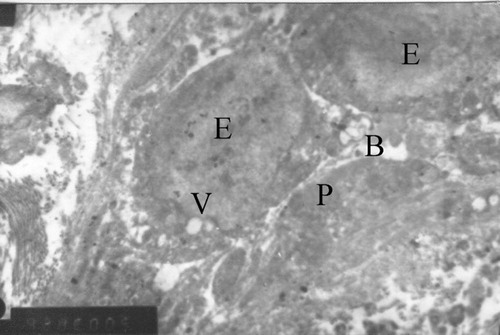
Table 3 Light microscapic (LM) changes in diabetic peripheral arteries.
Table 4 Electron microscopic (EM) changes in diabetic peripheral arteries.
Table 5 Light microscapic (LM) changes in diabetic peripheral nerves.
Table 6 Electron microscopic (EM) changes in diabetic peripheral nerves.
5 Discussion
It has been expected that 25% of diabetics will develop foot or a leg problems.Citation16 The diabetic foot lesions may be aggressive to lead to loss of a leg or foot.Citation17 The diabetic foot lesions are considered to be the consequence of peripheral vascular disease and peripheral neuropathy complicated by infection.Citation6
Many studies described the light microscopic changes in the diabetic peripheral arteries and arterioles as endothelial cell hyperplasia, areas of ulceration and desquamation of the endothelial surface with platelets were seen to be adhered and aggregated to the area of endothelial damage, thrombosis of the lumen was seen in some sections. The intima showed fibrosis with extensive collagen fibers deposition, smooth muscle cell proliferation, lipid droplets were seen deposited intra and extra-cellularly. The elastic laminae were fragmented with fibrous tissue deposition. The media showed proliferation of smooth muscle cell and migration into intima, areas of hemorrhage, red blood cells free in the wall of the artery, areas of calcification, cellular infiltration of plasma cells and multinucleated giant cells. Some authors reported complete loss of the vessel architecture in some sections. The vasa vasorum changes are: occluded vessel lumen with fibrin deposition and thrombosis, thickening of the wall with collagen fiber deposition and endothelial hyperplasia.Citation18–Citation21
The results of the present study confirmed the previous studies by using light microscope as it showed endothelial ulceration, collagen fibers deposition extensively in all layers, fibrous tissue deposition, cellular infiltration, fragmented elastic laminae, smooth muscle cell proliferation, interstitial hemorrhage, calcification and vasa vasorum changes.
The results of many studies by using electron microscopy were similar to light microscopic results in peripheral diabetic arteries and arterioles with some ultrastructural changes in the cells as the endothelial cells showed hyperplasia and swollen cells. Swelling of the endoplasmic reticulum, cytoplasmic vacuoles, the platelets adhered to the endothelial surface were degranulated, the smooth muscle cells were proliferated with dense cytoplasm and contractile myofilaments.Citation18,Citation20,Citation22
The electron microscopic results of the present study confirmed the results of the previous studies and the remarkable result is the extensive deposition of the collagen fibers in all layers.
Many studies of the diabetic peripheral neuropathy described the changes in the peripheral nerves by using the light microscopy as patchy demyelination, areas of degeneration, and regeneration, decreased nerve fibers density and occupancy, areas of infarction, areas of necrosis, areas of scarring with collagen fibers deposition and cellular infiltration of plasma cells and macrophages and the vasa nervosum changes.Citation23
The present study confirmed the results of the previous studies by using light microscopy.
By using electron microscopy, many studies descried changes in the diabetic peripheral nerves as patchy demyelination, diffuse loss of myelinated and unmyelinated nerve axons, areas of degeneration and regeneration of nerve fibers, the patchy degeneration affecting myelin more than the axons, and distal more than proximal, with focal areas of necrosis and scarring, loss of distal portions of small and large myelinated fibers, decreased myelinated fiber density and occupancy, areas of infarction, areas of necrosis, and collagen fiber deposition, cellular infiltration of plasma cells and macrophages, para-nodal swelling, thickened perineural cell basement membrane, loss of axoglial junctions and vasa nervosum changes.Citation22,Citation24,Citation25
The present study confirmed the previous studies by using electron microscopy with remarkable scar tissue deposition of collagen fibers and fibrous tissue.
Many studies described the microscopic findings of the vasa nervosum and vasa vasorum as endothelial cell hyperplasia, platelet adhesions and aggregation to the endothelial surface, narrow lumen with deposition of fibrin, thrombus formation and some found degenerated cells with pyknotic nuclei in the lumen. The endothelial cell showed swelling, cytoplasmic vacuoles, bleb formation and loosening of the junctions between endothelial cells, collagen fiber deposition in the subendothelium and in between the cells with thickening of the basement membrane.Citation5,Citation19,Citation21,Citation26,Citation27
The present study confirmed the results of the previous “light and electron microscopic studies”.
Many studies stated that the diabetic microangiopathy has a good role to play in the etiology of diabetic neuropathy and they stated that endoneural vascular changes are primary to neuropathy.Citation5,Citation13,Citation18,Citation28
Another studies stated that diabetics have no occlusive microvascular disease as it was not confirmed microscopically and the origin of the diabetic neuropathy is metabolic in nature.Citation12 Also, Foster 1988Citation3, Shimamout et al. 1973Citation22 added that diabetic neuropathy rather than the vascular disease is the primary etiology of diabetic foot lesions.
The results of the present study confirmed Timperely et al.Citation15 and many other studies and suggest that diabetic neuropathy is vascular in origin. This is because the results of the present study showed that 100% of the endoneural vessels showed changes and lumen occlusion. So, the severity of the vessel and fiber pathology is statistically associated and such vessel alteration have not been described as a secondary to neuropathy. The patchy fashion of demyelination and remylination represent areas of regional ischaemia and also areas of infarction and necrosis were detected in the sections, lastly the remarkable collagen fibers deposition represents scar tissue due to chronic ischemia.
Also, Timperley et al.Citation15 stated that “The neural changes proportioned with the degree of arterial narrowing or occlusion. A major objection to vascular theory of diabetic nerve damage is based on the fact that clinical manifestations of diabetic neuropathy are frequently variable and recoverable. This is particularly true of acute onset situation where severe motor neuropathy may recover relatively quickly, although less true in the more chronic, sensory type of neuropathy”.
In this study, we found that the diabetic microangiopathy is the main cause for diabetic neuropathy and diabetic foot lesions.
6 Conclusion
Extensive collagen fibers and fibrous tissue deposition is a remarkable character by “light and electron microscopy” in all layers of peripheral diabetic arteries and arterioles and they are considered as a scar tissue.
Endothelial cells of the peripheral diabetic arteries and arterioles show hyperplasia, cytoplasmic vacuoles and bleb formation.
Patchy demyelination is a remarkable feature in all sections of peripheral diabetic nerves by “light and electron microscopy” associated with collagen fibers and fibrous tissue depositions.
Areas of infarction and areas of necrosis were seen in sections of diabetic peripheral nerves.
Vasa vasorum and vasa nervosum changes were seen in almost 100% of the cases.
The present study found that the diabetic microangiopathy is the main cause of diabetic neuropathy and diabetic foot lesions.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 30 January 2017
References
- D.W.ZochodneG.A.KlineE.E.SmithM.D.HillDiabetic Neurology2016Informa HealthcareNew York, London
- American Diabetes AssociationDiagnosis and classification of diabetes mellitusDiab Care362013S67S7410.2337/dc13-S067
- R.T.AckermannY.J.ChengD.F.WilliamsonE.W.GreggIdentifying adults at high risk for diabetes and cardiovascular disease using hemoglobin A1c National Health and Nutrition Examination Survey 2005–2006Am J Prev Med402011111710.1016/j.amepre.2010.09.022
- K.H.GabbayHyperglycamia, polyol metabolism, and complications of diabetes mellitusAnnu Rev Med261975521536
- J.A.ColwellP.V.HalushkaK.E.SarjiM.F.Lopes-VirellaJ.SagelVascular disease in diabetes: pathophysiological mechanisms and therapyArch Int Med1391979225230
- A.J.BoultonThe diabetic footMed Clin North Am72198815131530
- X.GuoY.ShiX.HuangM.YeG.XueJ.ZhangFeatures analysis of lower extremity arterial lesions in 162 diabetes patientsJ Diab Res20132013781360
- S.RahmanT.RahmanA.IsmailA.RashidDiabetes-associated macrovasculopathy: pathophysiology and pathogenesisDiab Obes Metabol92007767780
- American Diabetes AssociationPeripheral arterial disease in people with diabetesDiab Care26200333333341
- M.BosevskiPeripheral arterial disease and diabetesPrilozi3320126578
- S.D.FunkA.YurdagulJrA.W.OrrHyperglycemia and endothelial dysfunction in atherosclerosis: lessons from type 1 diabetesInt J Vasc Med201220121910.1155/2012/569654
- H.ZhangK.C.DellspergerC.ZhangThe link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: an updateBasic Res Cardiol1072012111
- E.WilliamsW.R.TimperleyJ.D.WardT.DuckworthElectron microscopical studies of vessels in diabetic peripheral neuropathyJ Clin Pathol331980462470
- S.E.FagerbergDiabetic neuropathy, a clinical and histological study on the significance of vascular affectionsActa Med Scand Suppl3451959197
- W.R.TimperleyJ.D.WardF.E.PrestonT.DuckworthB.C.O’MalleyClinical and histological studies in diabetic neuropathy. A reassessment of vascular factors in relation to intravascular coagulationDiabetologia121976237243
- B.InanU.AydinM.UgurlucanC.AydinM.ElifTekerSurgical treatment of lower limb ischemia in diabetic patients – long-term resultsArch Med Sci9201310781082
- S.WildG.RoglicA.GreenR.SicreeH.KingGlobal prevalence of diabetes: estimates for the year 2000 and projections for 2030Diab Care27200410471053
- W.R.RobertessonJ.P.StrongAtherosclerosis in persons with hypertension and diabetes mellitusLab Invest181968538551
- A.N.BessmanDiabetic neuropathyThe Lancet333198911131114
- J.Vas P RM.E.EdmondsEarly recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessmentLancet Diab Endocrinol201610.1016/S2213-8587(16)30063-8
- J.R.WilliamsonC.H.KiloCapillary basement membranes in diabetesDiabetes32198396100
- T.ShimamotoT.SunagaThe contraction of the blebbing of the endothelial cells by acute infiltration of plasma substance in the vessel wall and their preventionT.ShimamotoF.NumanoG.M.AddisonAtherogenesis II. Excerpta Medica19733
- S.YagihashiH.MizukamiK.SugimotoMechanism of diabetic neuropathy: where are we now and where to go?J Diab Invest220111832
- M.C.RaffV.SangalangA.K.AsburyIschaemic mononeuropathy multiplex associated with diabetes mellitusArch Neurol181968487499
- J.DitzelE.StandelThe problem of tissue oxygenation in diabetes mellitusActa Med Scand Suppl57819755968
- L.O.AlmerG.SundkuistB.O.LiljaFibrinolytic activity, autonomic neuropathy and circulation in diabetes mellitusDiabetes3219831124
- R.J.JarrettH.KeenR.ChakrabartiDiabetes, hyperglycemia and arterial diseaseH.KeenR.J.JarrettComplications in Diabetes2nd ed.1982Edward ArnoldLondon179203
- H.E.BaysA.M.PfeiferPeripheral diabetic neuropathyMed Clin North Am72198814391464

