Abstract
Background
Variceal bleeding (VB), the most common lethal complication of cirrhosis, associated with high mortality. Timely prediction of esophageal varices (EV) represents a real challenge for the medical team. This study evaluated the level of plasma soluble CD 163 as a marker of the presence of EVs and to compare it with other noninvasive clinical, laboratory and ultrasonographic parameters as well as endoscopy.
Methods
This prospective controlled study was conducted on 80 adults. Gp I had no oesophageal varices, gp II had small varices, gp IIIa had large varices, gp IIIb are the same patients of gp IIIa but after eradication of varices and gp IV as healthy controls. Serum samples were assayed for soluble CD 163.
Results
soluble CD163 was statistically significant different between controls and all liver cirrhosis. it showed a statistically significant difference between group I and II (p = 0.009) and between group I and IIIa (p < 0.001) and between group II and IIIa (p < 0.001) but, no difference between group IIIa and IIIb (p = 0.179).
Conclusion
Serum soluble CD163 is a good noninvasive predictor for the presence of EVs and it may be used for grading of EVs. Its level does not change after esophageal varices eradication.
Trial registration: IRB No: 00007589 FWA No: 00015712 The Ethics Committee of the faculty of medicine Alexandria University.
1 Background
Cirrhosis is the end stage of every chronic liver disease, resulting in formation of fibrous tissue, dis-organization of liver architecture, and nodule formation, which interferes with liver function and results in portal hypertension. Portal hypertension is associated with development of a hyperdynamic circulation and complications such as ascites, hepatic encephalopathy, and oesophago-gastric varices.Citation1 Oesophago-gastric varices are the most relevant porto-systemic collaterals because their rupture results in variceal bleeding (VB), the most common lethal complication of cirrhosis, associated with a mortality of at least 20% at 6 weeks.Citation2
Timely prediction of esophageal varices (EV) represents a real challenge for the medical team. Nowadays, complete diagnosis of portal hypertension (PHT) requires measurement of the porto-systemic gradient, the parameter that gives the most accurate information about the development of (EV). Although it is safe but it still remains an invasive procedure.Citation3 The gold standard examination to establish the diagnosis of (EV) is endoscopy but, the available method in rural areas is laboratory examination and ultrasonography. Several studies have been performed to identify predictive factors for esophageal varices.Citation5
Current guidelines, recommend that all cirrhotic patients should be screened for varices at diagnosis, with follow up every 2–3 years for patients without varices and 1–2 years for patients with small varices. This guideline causes a significant burden and cost to endoscopy units.Citation4 The cost and invasive nature of endoscopic screening mean that there is interest in developing noninvasive predictors for EV.Citation5 Noninvasive predictive variables as platelet count, splenomegaly, Child Pugh, size of right liver lobe, albumin level and portal vein diameter are based on regular laboratory parameters and clinical signs relevant fibrosis, portal hypertension and hypersplenism.Citation6
CD (163) is a macrophage lineage-specific hemoglobin haptoglobin scavenger receptor and a specific marker for macrophage activation.Citation7,Citation8 CD (163) is shed into the circulation in a soluble form sCD (163) after Toll-like receptor activation by a similar mechanism as TNF-α.Citation9 Serum concentrations of sCD (163) are accordingly elevated during conditions of macrophage activation and proliferation.Citation10 Elevated circulating sCD (163) has been demonstrated in viral hepatitis, acute liver failure and cirrhosis.Citation11–Citation13 Hepatic kupffer cells are activated in cirrhotic patients in parallel with their portal hypertension. sCD (163) is a sensitive marker of macrophages activation that positively correlated with the degree of portal hypertension in cirrhotic patients.Citation14 In a recent study of highly selected cirrhotic patients setup for treatment with transjugular intrahepatic porto-systemic shunt (TIPS), sCD163 was released from the liver, confirming the activation of Kupffer cells, and its level rose with the portal pressure.Citation14 This study evaluated the level of plasma soluble CD 163 as a marker of the presence of esophageal varices (EVs) in cirrhotic patients and to compare it with other noninvasive clinical, laboratory and ultrasonographic parameters as well as upper gastrointestinal endoscopic findings.
2 Methods
After ethical approval for this clinical trial from the local committee of ethics in the faculty of medicine of Alexandria university and the department of tropical medicine, Informed consent was taken from healthy volunteers and the next of kin. This prospective controlled study was conducted on 80 adult subjects (n = 80), they were divided into 5 groups. Group I Contains 20 patients with liver cirrhosis without oesophageal varices. Group II contains 20 patients with liver cirrhosis with small oesophageal varices grade (I, II). Group IIIa contains 20 patients with liver cirrhosis with large oesophageal varices grade (III, IV). Group IIIb are the same 20 patients of group IIIa but after eradication of oesophageal varices. Group IV contains 20 healthy subjects as normal controls.
We classified all patients using upper endoscopy and The varices was classified according to Comar and Sanyal into small or large oesophageal varices. Also, portal hypertensive gastropathy was graded according to McCormack's classification into no, mild (snake skin appearance) and severe (submucosal hemorrhagic spots). Eradication of oesophageal varices was done for group IIIa patients by endoscopic variceal band ligation immediately at time of diagnosis and then other sessions 3 weeks, 6 weeks and 8 weeks later on (one or more sessions was needed with one-month interval until eradication of oesophageal varices was achieved), Banding was done by capturing the targeted varix till complete red out occurs.
We considered all hepatic patients admitted for enrollment in this study but we excluded patients on β-blockers or nitrates or any other pharmacological agents which reduce portal hypertension, patients with hepatocellular carcinoma or acute liver failure, patients with any infectious or inflammatory diseases (such as sepsis, tuberculosis, Rheumatoid arthritis etc.) and patients with diabetes mellitus, hypertension or renal impairment or other comorbid conditions which hinder doing the endoscopic procedure.
All enrolled persons included in this study were subjected to complete history taking including demographic data and clinical data such as abdominal distension, dyspepsia, jaundice, bleeding tendency, weight loss, anemia manifestation, hematemesis and melena. They were clinically examined for liver, spleen, detection of ascites and manifestations of hepatocellular failure. They were subjected to laboratory investigations as complete blood picture (CBC), prothrombin time (PT) and activity, Serum alanine aminotransferase (ALT), Serum aspartate aminotransferase (AST), Serum alkaline phosphatase (ALP), Total and direct serum bilirubin, Blood urea nitrogen (BUN), Serum Creatinine, fasting blood glucose and fasting insulin level. Insulin resistance index “HOMA IR” was calculated as “Blood glucose level mg/dl × insulin/405”.
Serum samples from all persons were assayed for our main study marker “Soluble CD 163”. Also, it was evaluated at time of eradicated varices for patients of group IIIb. We calculated Child Pugh score and classified all participating patients according to presence of hepatitis C virus antibodies (ELISA), hepatitis B surface antigen (ELISA) and Antischistosomal antibodies (IHAT).
Regarding ultrasonic parameters, we evaluated all enrolled patients using ultrasound assessment of liver and ascites to detect the presence of cirrhosis and/or bilharsial hepatic fibrosis. They were assessed using ultrasound measurement of right liver lobe diameter, splenic bipolar diameter and ultrasound Doppler measurement of the portal vein. We considered all noninvasive clinical, laboratory parameters and predictive Scores such as AST to platelets ratio index(APRI)Citation15 calculated as [(AST/ULN) × 100]/platelet count 109/L (ULN = the upper limit of normal), Index for liver fibrosis FIB4Citation16 calculated as [age (years) × AST (IU/L)]/[platelet count(109/L) × ALT (IU/L)1/2], Lok scoreCitation17 calculated as log odds = −5.56 − 0.0089 × platelet count(103/mm3) + 1.26 × (AST/ALT) + 5.27 × INR; Lok = [exp (log odds)]/[1 + exp(logodds)], Platelet Count 109/Spleen Diameter Ratio(mm), AST/ALT Ratio. and Right Lobe Liver Diameter (cm)/Albumin Ratio.
2.1 Statistical methods
Data entry and analysis were done using SPSS software v24. Continuous values were described by mean and standard deviation. Categorical values were described by counts and proportions. Univariate analysis for determining the association of various clinical and laboratory variables with the stage of liver fibrosis and the presence or absence of EV was performed using Student's t-test for continuous variables and χ2 test for categorical variables. Differences were considered statistically significant if p-value was less than 0.05. To determine the clinical utility and the diagnostic performance of markers, 2 receiver operating characteristic (ROC) curves were constructed for each of the non-invasive scoring systems that appeared significant in the univariate analysis. Performance of the non-invasive markers was expressed as sensitivity, specificity, positive and negative predictive values (PPV and NPV) and test accuracy.
3 Results
Regarding the demographic data of the studied groups, there were no any significant differences between the studied groups in age and sex. Males predominated females (55% in group I, 60% in group II, 85% in group III and 60% in control group|), while the mean age was 52.0 years, 54.45 years, 52.55 years and 50.59 years in group I, II, III and control group respectively. There was no any significant difference between the studied groups as regard body mass index (BMI) ().
Table 1 Comparison between the different studied groups according to demographic data.
Regarding symptoms of patients at admission, the most common symptoms in group (I) was abdominal distension (55%) followed by dyspepsia (40%) while jaundice and weight loss were present in (30%) of patients. However, 80% of group II patients complained of a dyspepsia, 75% suffered from abdominal distension and 65% of this group had bleeding tendency. In group IIIa, 90% had dyspepsia, 85% had anemia manifestations and 60% had abdominal distention and bleeding tendency while melena was present in 65% of this group.
Regarding the etiology of liver cirrhosis, Group I was mainly HCV positive patients, only one patient with HBV and he was enrolled in group IIIa, all patients in group II were HCV positive, group IIIa was mainly formed of patients with positive shistosomal antibodies titer (). Regarding local abdominal examination of liver, there was no a statistically significant difference between groups I, II and IIIa in liver enlargement. But, there were significant differences between them in spleen enlargement and clinically detected ascites. () The Child-Pugh score of all patients is showed in ()
Table 2 Comparison between the three studied groups according to etiology of liver cirrhosis and fibrosis.
Table 3 Comparison between the three studied groups according to Local abdominal examination.
Table 4 Comparison between the different studied groups according to Child-Pugh classification.
Regarding lab investigations, CBC results showed significant differences between liver cirrhosis groups (I, II and IIIa) and control group IV in all parameters. Within the liver cirrhosis groups, hemoglobin level showed a statistically significant difference between group I and IIIa (p2 < 0.001) and between group II and III (p3 < 0.001) but no significant difference was found between group I and II (p1 = 0.960). Platelets count showed statistically significant differences between group I and II (p1 = 0.037) and between group I and III (p2=0.003) but no significant difference was found between group II and III (p3 = 0.797). There was a significant difference between group I, II and IIIa in comparison to control as regard fasting insulin level (p < 0.001) and HOMA IR (p < 0.001) with no statistically significant difference in fasting blood glucose level (p = 0.924). While HOMA IR showed a statistically significant difference between group I and III (p2 = 0.003) only. () All groups showed a normal renal functions tests with no significant differences between them.
Table 5 Comparison between the different studied groups according to lab investigations.
Regarding liver profile, there were statistically significant differences between each group of patients and group IV of controls in all parameters (p < 0.001). The mean of Serum aspartate aminotransferase (AST) of group I was higher than group II with a statistically significant difference (p1 = 0.010). The mean of serum albumin level showed significant differences between all groups. () The results of coagulation profiles of all groups are showed in ().
Table 6 Comparison between the different studied groups according to liver profiles.
Table 7 Comparison between the different studied groups according to prothrombin activity, prothrombin time and INR.
Regarding ultrasonography, all subjects were cirrhotic except in control group and 60% of subjects in group I and 40% of subjects in group II and IIIa group showed mixed cirrhosis with Schistosomal periportal hepatic fibrosis. Within group I, II and IIIa ascites was not present in 80% of subjects of group I while it was found as mild ascites in 50% of patients of group II and moderate ascites in 35% of patients of group IIIa. Direction of portal blood flow was hepatopetal in 100% of subjects in control and group I while it was non hepatopetal (hepatofugal) in 50% and 65% of subjects of group II and III respectively. Significant difference was found as regard portal blood volume between control group and liver cirrhosis group with median 1241.0 in group of controls. Moreover, within liver cirrhosis groups significant difference was found between group I and II and between group I and III but no significant difference was found between group II and III. In terms of portal vein diameter, statistically significant differences were found between group I and III (p2 < 0.001) and between group II and III (p3 < 0.001) but not between group I and II (p1 = 0.549). () (Appendix A)
Table 8 Comparison between the studied groups according to ultra-sonographic data.
Regarding the classic predictive scores, APRI was higher in group II than group I with a statistically significant difference (P < 0.001). FIB4 was higher in group II than group I with a statistically significant difference (P < 0.001). There was no significant difference between group II and III in their lok score (p3 = 0.909). AST/ALT Ratio and Right lobe liver diameter (cm)/albumin ratio was evaluated also as predictive scores ().
Table 9 Comparison between the different studied groups according to predictive scores.
All patients assigned to the groups using upper GI endoscopy and during it, portal hypertensive gastropathy grading was done and revealed that 55% of patients of group I had no PHG and 55% of patients of group II had mild PHG while 35% of subjects of group III had severe PHG. oesophageal variceal band ligation sessions required for eradication of the large oesophageal varices in group IIIa with mean of 3.0 (±1.45) sessions and duration mean 6.50 (±3.0) weeks. (Appendix B)
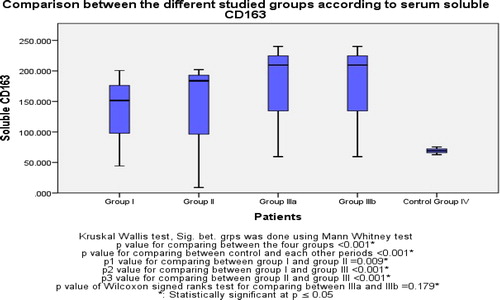
Regarding our main study marker, “soluble CD163” was statistically significant different between control and liver cirrhosis groups with a mean 69.03 (ng/ml) in control and 151.52 (ng/ml), 183.75 (ng/ml) and 209.52 (ng/ml) in group I, II and IIIa respectively. Moreover, within group I, II and IIIa it showed a statistically significant difference between group I and II (p = 0.009) and between group I and IIIa (p < 0.001) and between group II and IIIa (p < 0.001). There was no difference between group IIIa and IIIb (p = 0.179). ()
Regarding the presence of EV, serum soluble CD163 level, HOMA and platelets count are good predictors for presence of oesophageal varices with cut off value > 191.71 for soluble CD163 with 77.50% sensitivity and 77.0% specificity. Soluble CD163, HOMA IR and Platelets to predict presence of EV between (Group II + Group IIIa) vs Group I showed the best sensitivity and specificity 90% and 80% respectively and 90% PPV and 80% NPV. () APRI, FIB4, Lok score, platelets count to spleen diameter ratio and AST/ALT ratio are good predictors. All new variables sensitivity and specificity are showed in (). Moreover, ability of the studied marker soluble CD163 to differentiate between small varices and large varices with cut off value 199.19 with 85.0 % sensitivity and 90.0% specificity. (), ()
Fig. 2 ROC curve for Soluble CD163, HOMA IR and Platelets to predict presence of oesophageal varices between (Group II + Group IIIa) vs Group I.
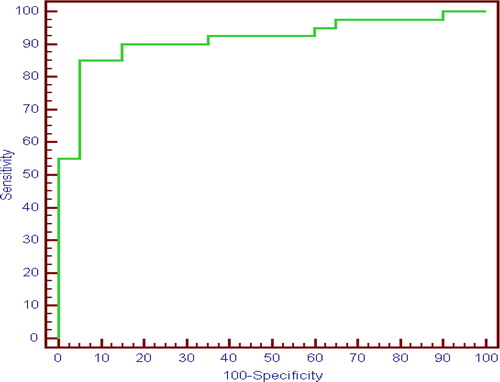
Fig. 3 ROC curve for Soluble CD163 to differentiate small oesophageal varices from large oesophageal varices (Group IIIa vs Group II).
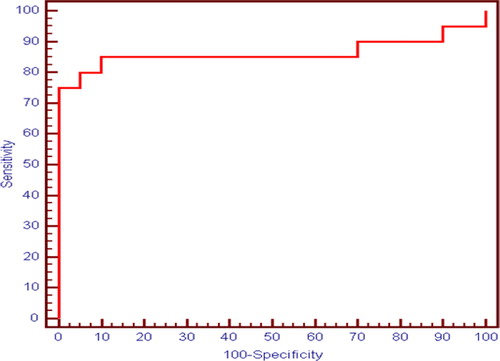
Table 10 Agreement (sensitivity, specificity and accuracy) for different parameters to predict presence of oesophageal varices between (Group II + Group III) vs Group I.
Table 11 Agreement (sensitivity, specificity and accuracy) for soluble CD163 to differentiate small oesophageal varices from large oesophageal varices (Group IIIa vs Group II).
4 Discussion
Several non-invasive methods have emerged in recent years, assessing the potential of various laboratory, clinical, and ultrasonographic parameters, linked directly or indirectly to portal hypertension including: Thrombocytopenia, splenomegalyCitation18. AST/ALT ratio,Citation19 AST to platelets ratio index (APRI).Citation20 platelets count to spleen diameter ratio,Citation21 The right liver lobe diameter/albumin index,Citation22 Transient elastography,Citation23 Forns Index,Citation24 Lok scoreCitation25 and Insulin resistance.Citation26
Insulin resistance which is firstly introduced by Cammà et al. study, which stated that Insulin resistance measured by HOMA-IR, regardless of the presence of diabetes, significantly predicts the presence of EVCitation26.Studies in chronic liver diseases have shown a strong and independent pathogenic link between Insulin resistance (IR) and HCV infection and between IR and the severity of hepatic fibrosis.Citation27 Cammà et al.Citation26 studied 104 patients of Child A HCV induced cirrhosis conclude that HOMA-IR score of greater than 3.5 is the cut-off value with the best sensitivity 61% and specificity 76% for predicting EV presence and HOMA score less than 3.5 (if non-diabetic) could be useful to identify patients at low risk of EV.Citation26 Eslam et al.,Citation28 also concluded that in patients with cirrhosis, the presence of esophageal varices was independently associated with lower platelet count and raised HOMA score with HOMA score correlates with HVPG and independently predict clinical outcomes in these patients. In this study, analysis of the area under the ROC curve (AUROC) revealed that the cut-off value for HOMA-IR score of greater than 2.83 was the optimal value for accurate prediction of EVs with a resulting 67.50% sensitivity, 90% specificity. The differences between the best cut-off values, sensitivity and specificity in this study and Cammà et al.Citation26 study may be attributed to the different ethnic group of the patients, all patients in this study were non-diabetic and non-obese. Where in Cammà et al. study 27 patients were diabetic and 11 patients were obese, and may be due to different genotype of HCV in studied groups where genotype 1 predominate Cammà et al. study and genotype 4 mostly predominate this study.
Fib-4 had been examined for the prediction of EV in patients with cirrhosis, having an AUROC of 0.64 for the prediction of EV at a cutoff value of 3.5, while for the diagnosis of LEV the AUROC was 0.63 and the cutoff value 4.3.Citation29 In another study, for predicting EV, they used a cutoff value of 3.98 and the AUROC was 0.624; for the diagnosis of LEV they used a cutoff value of 6.75.Citation30 Moreover, Eman et al.Citation31 proposed a very low cutoff (2.8) for predicting EV which showed sensitivity 76%, specificity 80%, PPV 92.7% and NPV 50%. However, in this study Fib-4 couldn't differentiate between small EV in group II and large EV in group III and there was no statistically significant difference. While Sebastiani et al.Citation29 and Stefanescu et al.Citation30 proposed a cutoff value 4.3 and 6.75 respectively.
Lok Score had been considered a very satisfactory predictor of EV. At a cutoff value of 0.9, the Lok Score had an AUROC of 0.77 for the diagnosis of EV, while for a cutoff value of 1.5, the AUROC was 0.69 for the prediction of LEV.Citation29 In another prospective study, the best cutoff value for the diagnosis of LEV was 0.8, with an AUROC of 0.731 and a NPV of 86.4%.Citation30 While Eman et al.Citation31 proposed a cutoff value of 0.63 for diagnosis of EV. At this cutoff, the sensitivity was 60%, specificity was 80%, PPV was 78%, NPV was 42.9% and the overall accuracy was 79%. Also, Eman et al.Citation31 proposed a cutoff of 0.72 for the diagnosis of LEV at which sensitivity was 87.5%, specificity was 55.5% and the overall accuracy was 76%. AUROC was 0.72. In the current study, we proposed a cutoff 0.85 which showed sensitivity 87.5%, specificity 55%, PPV 79.5% and NPV 68.8%. However, in this present study Lok Score couldn't differentiate between small EV in group II and large EV in group III with no statistical significant difference. While Sebastiani et al.Citation29 and Eman et al.Citation31 proposed a cutoff value 1.5 and 0.72 respectively.
Mona et al.Citation32 proposed APRI at a cutoff greater than 1.26 could predict the presence of EV (AUROC 0.695) with PPV of 81.42% and APRI at a cutoff greater than 1.47 could predict LEV (AUROC 0.734). These findings are in agreement with the studies by Castéra et al.Citation33, Tafarel et al.Citation34, and Adami et al.Citation35 who proposed APRI at a cutoff 1.3, 1.64, and greater than 1.4, respectively, for prediction of EV. In addition, Sebastiani et al.Citation29 reported APRI at a cutoff of 1.4 for prediction of EV and at a cutoff of 1.5 for detection of LEV. However, Stefanescu et al.Citation30 suggested APRI at a cutoff more than 2.201 with AUROC 0.538 for detection of LEV and Galal et al.Citation36 suggested a cutoff greater than 0.16 for detection of EV and LEV. These different cutoff results indicate the need for further studies in large-scaled studies.
The AST/ALT ratio has been used to predict cirrhosis, and by natural extension studies have been performed to assess its usefulness in predicting oesophageal varices. In a retrospective study by Neblom et al.Citation37, significantly higher AST/ALT ratio were seen in patients with varices compared to those without (ratio: 1.8 versus 1.0, P < 0.0001). In our study ratio was 1.11 in group I without EV and 2.47 in group II and 1.93 in group III. Treeptrasertsuk G et al.Citation38 found an AST/ALT ratio >1.12 to be significantly associated with the presence of varices at initial endoscopy (OR 3.9, P = 0.02 95% CI 1.3–11.8). This cutoff gave a sensitivity of 47.8%, specificity of 87%, PPV 42.3%, and NPV 89.2%, and an AUROC of 0.69. Castéra et al.Citation33 proposed a cutoff of ≥1.0 which demonstrated a sensitivity of 68%, specificity of 89%, PPV 77%, and NPV 83%, with an AUROC 0.83 (0.72–0.94) for predicting the presence of oesophageal varices. In our study, we proposed a cutoff 0.9 which showed sensitivity 77.5%, specificity 75%, PPV 86.1% and NPV 62.5%. Also, in this present study AST/ALT ratio couldn't differentiate between small EV in group II and large EV in group III and there was no a statistically significant difference.
Giannini et al.Citation39 introduced the use of the platelet count/spleen diameter ratio as a tool to predict oesophageal varices. This ratio links thrombocytopenia to splenomegaly to introduce a variable that takes into consideration that thrombocytopenia is mainly due to hypersplenism secondary to portal hypertension. In that study, when a cut-off value of 909 used, the sensitivity was 100%, and the specificity was 93% this agree with Agha et al.Citation40 Cammà et al.Citation26 studied 104 newly diagnosed patients with Child A HCV cirrhosis, identified a value of 792 as the best cutoff for the presence of EVs and ratio greater than 792 Could be useful to identify patients at low risk of EV. And stated these, different results are perhaps related to differences in etiology and class of disease between the two populations as regard Giannini et al. studyCitation39. In one study on Egyptian patients Esmat et al.Citation41 concluded that a cut-off value of 1326.58 for the platelet count/spleen diameter ratio was used with a resulting 96.34% sensitivity, 83.33% specificity and 94% accuracy. In another study also in Egyptian patients Abu El Makarem et al.Citation42 concluded that a cut-off value of 939.7 for the platelet count/spleen diameter ratio was used with a resulting 100% sensitivity, 86.3% specificity and 96.5% accuracy. Monkez et al.Citation43 proposed a cut-off of the platelet count/spleen diameter ratio 750 for accurate prediction of EVs with a resulting 81% sensitivity, 81% specificity and 81% accuracy. In the current study, we proposed a cutoff 643 which showed sensitivity 75%, specificity 85%, PPV 90.9% and NPV 63%. Also, in this present study platelet count/spleen diameter ratio couldn't differentiate between small EV in group II and large EV in group III and there was no statistically significant difference. The difference in the cutoff values between these studies and that of the present work can be explained by their patient sample that included only cirrhotic patients, and none of their patients had evidence of bilharziasis, whereas most of the patients included in the present study had mixed disease etiology: bilharzial and post viral hepatitis C cirrhosis. Both had their insult on the platelets, besides bilharziasis that specifically produces larger even huge spleen. None of these studies included these types of patients that are characteristically prevalent in the Delta region of Egypt. In addition, the absence of interobserver agreement between the sonographers and endoscopists of the different studies which can affect the results. Hence, it is sure to have our different and specific cutoff that needs further larger scale studies in Egypt.
Regarding the right liver lobe diameter /serum albumin ratio Alempijevic et al.Citation44 had counted an original ratio. For the first time they reported the value of the right liver lobe diameter/serum albumin concentration in assessment of portal hypertension. They used serum albumin concentration as a parameter of liver function in combination with right liver lobe size and used this ratio as a non-invasive predictor of oesophageal varices with at a cut-off value of 4.425, the sensitivity was 83.1%, and the specificity was 73.9%. In another study on Egyptian patients Esmat et al.Citation41 concluded that a cut-off value of 4.422 for the right liver lobe diameter/albumin concentration ratio gave sensitivity 91.46%, and the specificity 77.78%. Monkez et al.Citation43 proposed a cut-off value for the right liver lobe diameter/albumin concentration ratio 3.5 for accurate prediction of EVs with a resulting 78.5% sensitivity, 57.1% specificity, and 74% accuracy. On the other hand, El Ray et al.Citation45 found that right liver lobe diameter/serum albumin had no role in prediction of EV presence that agrees with our study. The differences between the best cut-off values, sensitivity, specificity, and accuracy in these studies may be attributed to the different group of patient where all patients in this study were child A, B and C. In the other studies, the patients were child A, also patients were had a different ethnic origin. In addition, the differences between the sonographers of different studies, which can affect the results. This suggests the need for further multicenter studies including a large number of patients with different ethnic background for determining the best cut-off, value for that ratio.
Recent researches has highlighted the important role of liver macrophages (Kupffer cells) in the fibrotic process.Citation46 Macrophage-specific markers may, therefore, prove to be useful for the monitoring of fibrosis development like serum soluble CD163.Citation47,Citation48 These endocytic macrophage surface receptors are shed from activated macrophages during inflammation.Citation49,Citation50 Rødgaard-Hansen et al.Citation48 was the first to indicate that macrophage-related sCD163 may serve as biomarkers for fibrosis. This marker are readily measurable in serum and reflect monocyte/macrophage activation. In the current study, soluble CD163 showed a statistically significant difference between control and liver cirrhosis groups and this agrees with Ying-Ying et al.Citation51 and Gronaek et al.Citation52When applying ROC curves of serum soluble CD163 level, it was a significantly good predictors for presence of oesophageal varices between group I (no EV) and other both groups II and III (those of EV) with cut off value >191.71 ng/ml with 77.50% sensitivity and 77.0% specificity. This agrees with Ying-Ying et al.Citation51 and Waidmann et al.Citation53
An interesting new finding is the ability of the studied marker soluble CD163 to differentiate between small varices and large varices with cut off value 199.19 with 85.0 % sensitivity and 90.0% specificity. This is the first study to correlate sCD163 with grading of EV as noninvasive predictor. No previous studies had correlated this marker after eradication of EV. However, Peter Holland-fischer et al.Citation14 proposed That normalization of the portal hypertension by the TIPS procedure did not normalize sCD163, which remained increased.
After searching the literature, no studies were found to show a relation between bilharsiasis and sCD163.In this study, No significant difference between sCD163 level in group I patients with or without hepatic schistosomiasis with median sCD163 level 100.60 ng/ml in patients without schistosomiasis and 175.61 ng/ml in patients with schistosomiasis. Moreover, No significant difference between sCD163 level in group II patients with or without hepatic schistosomiasis with median SCD163 level 194.15 ng/ml in patients without schistosomiasis and 194.76 ng/ml in patients with schistosomiasis. Also, in group III. We found a model combining serum Soluble CD163, HOMA IR and Platelets to predict presence of oesophageal varices had an area under the curve (AUC) of 0.915 with sensitivity and specificity 90% and 80% respectively. While, ROC curve for Soluble CD163, platelet count /spleen diameter (mm) and FIB4, had sensitivity and specificity 90% and 85% respectively with AUC 0.910.
5 Conclusion
From our results, Serum soluble CD163 is a good noninvasive predictor for the presence of esophageal varices EVs and it may be used for grading of EVs. Serum soluble CD163 level does not change after esophageal varices eradication and it does not be effected by presence or absence of bilharsiasis. Insulin resistance measured by HOMA-IR, Fib-4, Lok Score, APRI, AST/ALT ratio and platelet count/spleen diameter ratio, are good predictors for the presence of EV but not for grading of esophageal varices. Right liver lobe diameter/ serum albumin had no role in prediction of EV presence. Combining of different noninvasive parameters can increase area under the curve AUC for prediction of EVs. A model combining serum Soluble CD163, HOMA IR and Platelets to predict presence of oesophageal varices had the best AUC. Our recommendations are studying serum soluble CD163 on larger sample size, CD163 and risk for variceal bleeding and its correlations with different etiologies of liver cirrhosis, acute liver failure and staging of liver fibrosis.
Competing interests
The authors declare that they have no competing interests.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 23 February 2018
References
- G.Garcia-TsaoA.J.SanyalN.D.GraceW.CareyPrevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosisHepatology (Baltimore, Md).462007922938
- G.D'AmicoR.De FranchisUpper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicatorsHepatology (Baltimore, Md).382003599612
- R.de FranchisRevising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertensionJ Hepatol532010762768
- R.de FranchisEvolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertensionJ Hepatol432005167176
- E.SchwarzenbergerT.MeyerV.GollaN.P.SahdalaA.D.MinUtilization of platelet count spleen diameter ratio in predicting the presence of esophageal varices in patients with cirrhosisJ Clin Gastroenterol442010146150
- A.SarangapaniC.ShanmugamM.KalyanasundaramB.RangachariP.ThangaveluJ.K.SubbarayanNoninvasive prediction of large esophageal varices in chronic liver disease patientsSaudi J Gastroenterol: Off J Saudi Gastroenterol Assoc1620103842
- L.K.WeaverK.A.Hintz-GoldsteinP.A.PioliPivotal advance: activation of cell surface Toll-like receptors causes shedding of the hemoglobin scavenger receptor CD163J Leukocyte Biol8020062635
- S.K.MoestrupH.J.MollerCD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory responseAnn Med362004347354
- A.EtzerodtM.B.ManieckiK.MollerH.J.MollerS.K.MoestrupTumor necrosis factor alpha-converting enzyme (TACE/ADAM17) mediates ectodomain shedding of the scavenger receptor CD163J Leukocyte Biol88201012011205
- D.J.SchaerB.SchleiffenbaumM.KurrerSoluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndromeEur J Haematol742005610
- A.HiraokaN.HoriikeS.M.AkbarK.MichitakaT.MatsuyamaM.OnjiExpression of CD163 in the liver of patients with viral hepatitisPathol Res Pract2012005379384
- A.HiraokaN.HoriikeS.M.F.AkbarK.MichitakaT.MatsuyamaM.OnjiSoluble CD163 in patients with liver diseases: very high levels of soluble CD163 in patients with fulminant hepatic failureJ Gastroenterol4020055256
- H.J.MollerH.GronbaekF.V.SchiodtSoluble CD163 from activated macrophages predicts mortality in acute liver failureJ Hepatol472007671676
- P.Holland-FischerH.GronbaekT.D.SandahlKupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPSGut60201113891393
- A.Loaeza-del-CastilloF.Paz-PinedaE.Oviedo-CardenasF.Sanchez-AvilaF.Vargas-VorackovaAST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosisAnn Hepatol72008350357
- A.Vallet-PichardV.MalletB.NalpasFIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotestHepatology (Baltimore, Md).4620073236
- S.BotaR.SirliI.SporeaA new scoring system for prediction of fibrosis in chronic hepatitis CHepatitis Monthly112011548555
- K.C.ThomopoulosC.Labropoulou-KaratzaK.P.MimidisE.C.KatsakoulisG.IconomouV.N.NikolopoulouNon-invasive predictors of the presence of large oesophageal varices in patients with cirrhosisDigest Liver Dis: Off J Ital Soc Gastroenterol Ital Assoc Study Liver352003473478
- E.GianniniD.RissoF.BottaValidity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver diseaseArch Intern Med16322003218224
- C.-T.WaiJ.K.GreensonR.J.FontanaA simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis CHepatology (Baltimore, Md).382003518526
- E.G.GianniniA.ZamanA.KreilPlatelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation studyAm J Gastroenterol101200625112519
- T.AlempijevicV.BulatS.DjuranovicRight liver lobe/albumin ratio: contribution to non-invasive assessment of portal hypertensionWorld J Gastroenterol: WJG13200753315335
- L.CasteraM.PinzaniJ.BoschNon invasive evaluation of portal hypertension using transient elastographyJ Hepatol562012696703
- X.FornsS.AmpurdanesJ.M.LlovetIdentification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive modelHepatology (Baltimore, Md).362002986992
- A.S.F.LokM.G.GhanyZ.D.GoodmanPredicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohortHepatology (Baltimore, Md).422005282292
- C.CammaS.PettaV.Di MarcoInsulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosisHepatology (Baltimore, Md).492009195203
- D.M.TorresS.A.HarrisonInsulin resistance in chronic hepatitis C, genotypes 1 and 4: the unfortunate realityHepatology (Baltimore, Md).47200821372139
- M.EslamJ.AmpueroM.JoverPredicting portal hypertension and variceal bleeding using non-invasive measurements of metabolic variablesAnn Hepatol122013588598
- G.SebastianiD.TempestaG.FattovichPrediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: results of a multicenter, large-scale studyJ Hepatol532010630638
- H.StefanescuM.GrigorescuM.LupsorA new and simple algorithm for the noninvasive assessment of esophageal varices in cirrhotic patients using serum fibrosis markers and transient elastographyJ Gastr Liver Dis: JGLD2020115764
- E.M.HassanD.A.OmranM.L.El BeshlaweyM.AbdoA.El AskaryCan transient elastography, Fib-4, Forns Index, and Lok Score predict esophageal varices in HCV-related cirrhotic patients?Gastroenterol Hepatol3720145865
- M.ShehataL.AboAliK.El-ShafeyM.El-HossaryA comparative study of Duplex Doppler ultrasound and blood indices as noninvasive predictors of oesophageal varices in cirrhotic patientsTanta Med J4220148391
- L.CasteraB.Le BailF.Roudot-ThoravalEarly detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scoresJ Hepatol5020095968
- J.R.TafarelL.H.TolentinoL.M.CorreaPrediction of esophageal varices in hepatic cirrhosis by noninvasive markersEur J Gastroenterol Hepatol232011754758
- M.R.AdamiC.T.FerreiraC.O.KielingV.HirakataS.M.G.VieiraNoninvasive methods for prediction of esophageal varices in pediatric patients with portal hypertensionWorld J Gastroenterol: WJG19201320532059
- Ghada M.Galal AagEman M.S.MuhammadLaila M.YousefClinical utility of simple fibrosis markers in prediction of oesophageal varices in chronic hepatitis C patients with advanced fibrosisMed J Cairo Univ8020128593
- H.NyblomE.BjornssonM.SimrenF.AldenborgS.AlmerR.OlssonThe AST/ALT ratio as an indicator of cirrhosis in patients with PBCLiver Int: Off J Int Assoc Study Liver262006840845
- S.TreeprasertsukK.V.KowdleyV.A.LuketicThe predictors of the presence of varices in patients with primary sclerosing cholangitisHepatology (Baltimore, Md).51201013021310
- E.GianniniF.BottaP.BorroPlatelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosisGut52200312001205
- A.AghaE.AnwarK.BashirV.SavarinoE.G.GianniniExternal validation of the platelet count/spleen diameter ratio for the diagnosis of esophageal varices in hepatitis C virus-related cirrhosisDigest Dis Sci542009654660
- S.EsmatD.OmarnL.RashidCan we consider the right hepatic lobe size/albumin ratio a noninvasive predictor of oesophageal varices in hepatitis C virus-related liver cirrhotic Egyptian patients?Eur J Intern Med232012267272
- M.A.Abu El MakaremM.E.ShatatY.ShakerPlatelet count/bipolar spleen diameter ratio for the prediction of esophageal varices: the special Egyptian situation: noninvasive prediction of esophageal varicesHepatitis Monthly112011278284
- Monkez M.Yousif HAEMohamed O.WahbaAsmaa M.EshInsulin resistance as a non invasive parameter for prediction of esophageal varices in patients with hepatitis C virus cirrhosisZUMJ202014
- T.AlempijevicV.BulatS.DjuranovicRight liver lobe/albumin ratio: contribution to non-invasive assessment of portal hypertensionWorld J Gastroenterol13200753315335
- A.El RayM.M.AzabI.M.A.El-AzizNon-invasive predictors for the presence, grade and risk of bleeding from esophageal varices in patients with post-hepatitic cirrhosisJ Egypt Soc Parasitol452015421428
- T.A.WynnL.BarronMacrophages: master regulators of inflammation and fibrosisSemin Liver Dis302010245257
- H.J.MollerN.A.PeterslundJ.H.GraversenS.K.MoestrupIdentification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasmaBlood992002378380
- S.Rodgaard-HansenA.RafiqueP.A.ChristensenA soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illnessClin Chem Lab Med522014453461
- U.GaziM.RosasS.SinghFungal recognition enhances mannose receptor shedding through dectin-1 engagementJ Biol Chem286201178227829
- K.A.HintzA.J.RassiasK.WardwellEndotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163J Leukocyte Biol722002711717
- Y.Y.YangM.C.HouM.W.LinCombined platelet count with sCD163 and genetic variants optimizes esophageal varices prediction in cirrhotic patientsJ Gastroenterol Hepatol282013112121
- H.GronbaekT.D.SandahlC.MortensenH.VilstrupH.J.MollerS.MollerSoluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosisAliment Pharm Therap362012173180
- O.WaidmannF.BrunnerE.HerrmannS.ZeuzemA.PiiperB.KronenbergerMacrophage activation is a prognostic parameter for variceal bleeding and overall survival in patients with liver cirrhosisJ Hepatol582013956961
Appendix A
Ultrasound imaging
Appendix B
Upper GI endoscopy and band ligation