Abstract
Background
Intestinal parasites are a major public health problem, and their accurate diagnosis is important. The purpose of this study was to evaluate and compare the efficiency of four concentration techniques for the detection of intestinal parasites under laboratory conditions.
Methods
A total of 800 suspension specimens including 200 samples for each technique were simultaneously and parallel processed for comparing the diagnostic efficiency of the formalin-tween (FTC), formalin-ether (FEC), formalin-acetone (FAC), and formalin-gasoline concentration (FGC) techniques.
Results
Sensitivity of FTC, FEC, FAC, and FGC techniques for diagnosis of intestinal parasites was 71.7%, 55.8%, 70.0% and 56.7%; and their negative predictive values (NPV) were 70.2%, 60.2%, 69.0% and 60.6%, respectively. FTC and FAC techniques with equivalent recovery rates were significantly more sensitive than FEC and FGC techniques for diagnosing helminth ova, but for diagnosis of protozoan cysts they were reversed. Overall diagnostic κ agreement for FTC and FAC techniques was substantial, while for FEC and FGC techniques it was moderate. The κ statistic indicated low to moderate agreement for diagnosis of helminths and moderate to substantial agreement for protozoa.
Conclusion
Tween, acetone and gasoline reagents are more stable, safer, less flammable and of lower cost than that of ether; and promise to be a useful alternative to ether- concentration. Our results demonstrated that the combined use of parasitological techniques is important for the diagnosis of all intestinal parasites. FTC and FGC techniques are superior for the diagnosis of helminth ova and protozoan cysts in stool, respectively. Additional studies are suggested, using a larger sample size and different parasites in the field.
1 Introduction
Intestinal parasites are major contributors to the global burden of disease, affecting especially the population living in the developing countries, and are part of the neglected tropical diseases.Citation1 Generally, soil-transmitted helminths affect approximately 1.5 billion people worldwide, cause considerable morbidity and account for an estimated 5.2 million disability adjusted life years (DALYs).Citation2,Citation3 The prevalence of intestinal protozoa infections (especially G. intestinalis and Entamoeba spp.) vary in different regions of the world. For example, Giardia prevalence is 2–7% in developed countries, whereas it is 20–30% in developing countries.Citation4,Citation5
Detection of intestinal parasites by microscopic examination is a well-described laboratory technique that is widely used for examining stools by various procedures in different institutions of the world. Evaluation of the efficiencies of concentration methods is important in the search for accurate diagnostic techniques to provide adequate patient care, identification of the etiological agent responsible for the disease, assess drug efficacy, monitor the effectiveness of control programs and obtain better understanding of the epidemiology of intestinal parasites.Citation6,Citation7
Since a ‘gold standard’ test (with 100% accuracy) does not exist for detection of intestinal parasites, clinical trials on drug efficacy and diagnostic studies in endemic countries; so a variety of parasitological methods have been utilized in different area of the world.Citation7,Citation8
Although several diagnostic methods such as formalin-acetone concentration (FAC), formalin-gasoline concentration (FGC), and formalin-tween concentration (FTC) techniques are available, formalin-ether concentration (FEC) technique is used as a reliable diagnostic method for helminth eggs, larvae, and protozoan cysts in stool specimens for many laboratories in different parts of the world.Citation9–Citation12 However, the recovery efficiency of this technique is not as high as had been thought for some intestinal parasites. For example, the FEC method may be suboptimal for the detection of Hymenolepis nana and Iodamoeba,Citation9,Citation13 T. trichiura eggsCitation14 and S. stercoralis.Citation10 If the recovery efficiency of the faecal diagnostic techniques is low, this will give rise to increase misdiagnosis (number of false-negative results and underestimation of parasitic diseases) of intestinal parasites. In addition, the use of diethyl ether, an essential reagent of this technique, may be hazardous to laboratory personnel; because it is explosive, contains anaesthetic vapours, has potential toxicity such as respiratory irritation, and causes cardiovascular depression and narcosis.Citation15 Due to these problems, attempts for finding a suitable replacement for diethyl ether should be considered. On the other hand, direct wet mount is the commonly used test for the diagnosis of intestinal parasitic infections generally in the world and particularly in the Middle East.Citation7,Citation10 However, low sensitivity of the direct wet mount technique has been reported in the detection of low intensity infection,Citation10,Citation16 and will significantly increase misdiagnosis of intestinal parasites.
Moreover, other similar studies failed to report the efficiency and/or operational characteristics and Kappa (κ) index in the framework of diagnostic approaches of intestinal parasites, and knowing these performances is very important before a decision is made to use a new suitable technique instead of a previous one (such as formalin-ether). This research assessed and compared four concentration techniques that were not mentioned in the previous reference, in the framework of diagnostic studies of intestinal parasites.
The purpose of this study was to evaluate and compare the efficiency (sensitivity, specificity, positive & negative predictive values), Kappa index of four parasitological concentration techniques for the detection of helminth ova and protozoan cysts.
2 Materials and methods
2.1 Faecal suspension and parasites
Fresh faecal material free of parasites, with individual variations in cellular content, mucus, and consistency, was pooled with 10% formalin in a 1:4 ratio to prepare a standardized specimen. After homogenization, this suspension was divided into five equal portions, one was without infection, and each of the other four portions of this specimen were individually seeded with Entamoeba coli, and Giardia lamblia cysts; ova of Ascaris lumbricoides, and Hymenolepis nana. These organisms were selected because of their frequency of clinical occurrence and variation in size.
In order to have a suitable suspension of low intensity infection, parasite concentrations in unconcentrated faecal suspensions of each parasite combination were determined by direct examination of fifteen 0.02 ml samples. Of these samples, only 1–2 direct wet mounts for respective portions of each parasite suspension were considered to be positive per cover slip, which provided a basis for evaluating concentration efficiency.
2.2 Faecal concentration procedures and examination of sediment
The concentration procedures that we evaluated were the FAC, FTC, FGC and FEC. The sedimentation techniques were simultaneously performed for all suspension specimens. Parallel concentrates for all techniques were prepared for each faecal suspension with and without intestinal parasites.
Overall 800 slides including 200 suspension specimens with and without intestinal parasites for each parasite-concentrator combination were examined. In each procedure, 80 negative stool samples and 120 seeded suspension samples with Entamoeba coli and Giardia lamblia cysts; ova of Ascaris lumbricoides, and Hymenolepis nana, i.e. 30 samples for each mentioned parasite in every procedure were studied. In addition to testing faecal suspension specimens, four similar sets of conical 15-ml centrifuge tubes were separately considered for four concentration procedures. Approximately 7 ml of each suspension specimens with and without intestinal parasites were strained through two layers of gauze into each of the four sets. A 3 ml amount of each parasite-concentrator combination (including acetone, 7.5% tween 20™, gasoline, and diethyl ether) was added to each of the four set conical centrifuge tubes for FAC, FTC, FGC and FEC procedures, respectively. The sets of tubes were closed with a stopper and vigorously shaken for 30 s in order to bring the parasite-concentrator combination in contact with all parts of the faecal suspension materials. The tubes were then centrifuged together at 500g for 2 min (2000 rpm with a table model centrifuge). The plug of debris was loosened with an applicator stick and, together with the liquid, was carefully decanted. A cotton swab was used to wipe the inside of the tube to remove the residual debris plug and excess fluid. The remaining sediment was mixed with one drop of Lugol’s iodine solution, and twenty microliters of the material was applied to a clean glass slide. A cover slip (22 by 22 mm) was placed over the slide, and examined in its entirety at 100× and 400× magnifications, respectively. To eliminate observer bias, the slides were examined by a microscopist who did not know the procedure used for each preparation, and also were not aware of positivity or negativity for parasites.
Therefore, practical conditions and processes/steps for each parasite suspension examined with all techniques were thoroughly identical excepting for reagents (acetone, 7.5% tween 20, gasoline, and diethyl ether).
2.3 Assessment of the parasite morphology and characteristics of solvents
The morphology and appearance of recovered parasite species were determined and also physical and chemical characteristics of solvents (ether, tween, acetone or gasoline) especially in the point of safety and performance for each technique considered.
2.4 Agreement in test results and Statistical analysis
The results were analyzed by 2 × 2 contingency tables and Cohen’s kappa (κ) statistic was calculated to assess the agreement among all diagnostic techniques. Interpretation of κ value was as follows: κ ≤ 0, no agreement; κ > 0–0.20, poor agreement; κ = 0.21–0.40, fair agreement; κ = 0.41–0.60, moderate agreement; κ = 0.61–0.80, substantial agreement; and κ = 0.81–1.00, nearly perfect agreement.Citation17
Frequency of positivity for each intestinal parasite and for each technique was calculated. for post hoc comparison. To evaluate the diagnostic tests, the sensitivity, specificity, Statistical differences were analyzed using ANOVA in conjunction with the Dunnett test positive predictive value (PPV), negative predictive value (NPV) of each technique were determined. The McNemar test was used to examine differences in the proportions of positive results between diagnostic methods. Statistical significance was given for κ values <0.05. Data were analyzed using SPSS version 16 software.
3 Results
Eight hundred suspension specimens were examined for intestinal parasites with four diagnostic methods. These included 320 negative stool samples (80 for each technique), and 480 positive stool samples (120 for each technique).
3.1 Comparison of diagnostic methods
Overall, the sensitivity of FTC, FEC, FAC and FGC techniques for diagnosis of all intestinal parasites was 71.7%, 55.8%, 70.0% and 56.7%, respectively; on the other hand, NPV in those techniques was 70.2%, 60.2%, 69.0% and 60.6% respectively (). The recovery efficiency of formalin-gasoline technique was similar to the formalin-ether method, and both were significantly lower than the two other techniques for the diagnosis of all intestinal parasites (; P < .05).
Table 1 Comparison of the performance of four diagnostic concentration techniques in stool specimens previously identified as positive or negative for the presence of intestinal parasites.
The results of each parasite species seen for each procedure have been given in , and . The analysis of the techniques for diagnosing all helminth species showed that the FTC and FAC techniques revealed higher recovery efficacy than both FEC and FGC techniques (73.3% or more vs. 43.3% or less; P < .01; , ). In contrast, the FEC technique showed a higher sensitivity for diagnosing the protozoan parasites; however, there was no statistical significance (; P > .05).
Fig. 1 Bar graphs showing the recovery of helminth ova and protozoan cysts by the FAC, FTC, FGC and FEC procedures in stool specimens.
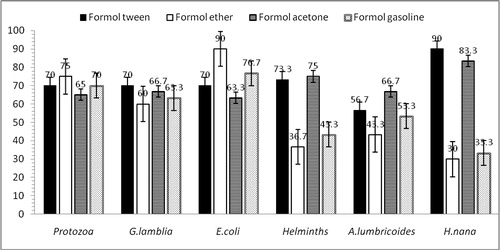
Table 2 Sensitivity and negative predictive value (NPV) of each concentration method for the diagnosis of intestinal helminth species ova and protozoan species cysts.
The recovery of H. nana eggs by FTC method (90%) was similar to FAC method (83.3%), and both were significantly higher than FEC (30%) and FGC (33.3%) procedures (P < .01). The detection rate of A. lumbricoides eggs was better with the FAC procedure (66.7%) compared to other techniques (, ; P > .05).
The most sensitive method for Entamoeba coli diagnosis was FEC method (90.0%), followed by FGC (76.7%; ), and only the FEC technique was significantly higher than that of the FAC technique (P = .021). Performances of different concentration procedures for the diagnosis of Giardia lamblia cysts were almost identical. Overall, the NPV were above 62% (range 62.5–86.9%) with all the four methods for both intestinal protozoan species (E. coli and G. lamblia) and they did not differ significantly ().
There were no false-positive results among the 320 (80 for each technique) specimens previously identified as negative for the presence of intestinal parasites. So, specificity for each technique was 100%.
3.2 Parasite morphology and properties of solvents
The morphology and appearance of the cyst or egg of the individual parasite species, found in all methods, were similar. Also, no distortion or alteration of morphology of the parasites was observed with all solvents (ether, tween, acetone or gasoline). However, clarity of sediment in FEC and FGC methods was identical, and the two methods were slightly better than both FTC and FAC methods. Safety precautions, physical and chemical properties of the reagents are showed in .
Table 3 Comparison of physical and chemical characteristics of diethyl ether, tween, acetone and gasoline.
3.3 Agreement in test results
The overall Kappa index in the FTC and FAC techniques showed substantial agreement (above 65%) for diagnosing all the stool specimens previously identified, while moderate agreement for FEC and FGC techniques (50.3% and 51.1% respectively) was reported ().
Since FEC technique is a widely used for detection of intestinal parasites, Kappa index of each technique for each parasite species was also analyzed with the FEC technique by 2 × 2 contingency table. The agreement between techniques for each parasite species is shown in .
Table 4 Two-way contingency table showing the number of identified positives and the agreement between the formalin-ether concentration (FEC) and each other concentration techniques for the diagnosis of G. lamblia, E. coli, A. lumbricoides, and H. nana in stool specimens.
For the detection of Entamoeba coli, the FEC and all other three techniques revealed substantial agreement (𝜅 value above 60%, P = .000), but the corresponding agreement value for Giardia lamblia was found only between the FEC and FGC techniques (; κ = 0.614, P ≤ .001).
shows that there was moderate agreement between FEC and FGC techniques for detection of Hymenolepis nana (κ = 0.416; P = .003), whereas less agreement value was observed for all of the other methods applied. For diagnosis of Ascaris lumbricoides, there was moderate agreement between FEC and FAC methods (κ = 0.513, P = .000), while between FEC and other techniques results showed fair agreement (κ = 0.275–0.338, P ≤ .001; ).
4 Discussion
4.1 Comparative results of diagnostic methods
The standardized suspension specimen carefully considered free of parasites, but it contained fresh faecal material with some of which had mucus, vegetables and meat fibers, and other debris typical of stool specimens encountered in microbiological laboratories for parasite examination. We analyzed the efficiencies of four sedimentation techniques applied for the diagnosis of intestinal parasites in the specimens previously identified as positive or negative for the first time. Comparison was made with the FEC technique, which is a widely used technique for detection of intestinal parasites, both in epidemiologic surveys and reference laboratories.Citation10,Citation18,Citation19
In the present study, the FGC technique exhibited similar sensitivity and NPV rates to the FEC method for detecting intestinal parasites in all stool samples (). As compared to FGC and FEC methods, both the formalin-tween and formalin-acetone techniques revealed significantly higher sensitivity for the detection of positivity of intestinal parasites (p < .05). Our results reinforce those of studies that demonstrate the accuracy achieved by associating the FTC, FGC and FAC techniques in detection of positivity of intestinal parasites.Citation9–Citation12
There were differences in recovery of the numbers of positive samples by each technique, however, they varied considerably from one species to another. In this study, all techniques revealed the same intestinal parasite species (E. coli, G. lamblia, A. lumbricoides, H. nana), whereas the FTC and FAC techniques were more sensitive than the FEC and FGC techniques for diagnosing helminth species ova especially more likely to correctly identify H. nana, and the differences were statistically significant. As in the present study, previous reports showed that the FTC and FAC techniques are able to detect common human helminth ova including H. nana and A. lumbricoides with an equal or higher sensitivity than currently more widely used methods, such as FEC technique.Citation9,Citation11,Citation12 This might support the idea that FAC and/or FTC method is generally recommended for the diagnosis of helminth infection including ova of A. lumbricoides and H. nana.
In the current study, analysis of the four concentration techniques for the diagnosis of intestinal protozoa showed that the FEC technique had the highest sensitivity and NPV, thus, identified more suspension specimens as positive for the protozoan species cysts. This technique also provided the highest sensitivity (90%) and NPV (86.9%) for the diagnosis of E. coli. On the other hand, the performance of the four concentration techniques for the diagnosis of Giardia lamblia resembled each other. These findings agree favorably with several similar studies,Citation9,Citation10,Citation12,Citation20 and differ from the results reported by Becker et al.,Citation21 where detection of E. coli and G. intestinalis by FLOTAC was found to be more sensitive than FEC technique, whilst FECM was more sensitive for E. histolytica/dispar.
4.2 Agreement in test results
In the present study, the κ index of the four concentration methods for diagnosis of all the stool specimens showed moderate to substantial agreement, substantial agreement (above 65%) for FTC and FAC, and moderate agreement for FEC and FGC methods. The agreement between the diagnostic techniques, as determined by Cohen’s kappa statistic, was generally low to moderate for diagnosis of helminth species. The highest kappa value was found for A. lumbricoides between FEC and FAC (κ = 0.513) techniques, as well as for H.nana between FEC and FGC (κ = 0.416) techniques, whereas in other concentration methods for both helminth species, the kappa values were below 0.40. Similar observations have been made in previous studies in which the agreement between the ether-concentration method and quadruplicate Kato-Katz thick smears was moderate for all helminth species (T. trichiura, κ = 0.54; A. lumbricoides, κ = 0.48; hookworm, κ = 0.47).Citation8 Another study by Utzinger et al. (2010) showed excellent agreement (κ > 0.8) among European reference laboratories for the diagnosis of A. lumbricoides, hookworm, T. trichiura and S. mansoni; moderate agreement (κ = 0.54) for Hymenolepis nana, and lesser agreement was observed for other helminth species discovered.Citation19
In our study, the diagnostic κ agreement between the FEC and all other three methods was substantial for the diagnosis of Entamoeba coli (κ > 0.60), but for Giardia lamblia cysts the corresponding κ values was only observed between FEC and FGC, whilst it was moderate for the other methods. This result is in line with a recent study, which reported that substantial agreement among reference laboratories was found for E. coli (κ = 0.69), but only fair or moderate agreement was found for other Entamoeba species, Giardia intestinalis and Chilomastix mesnili.Citation19 In another study, the diagnostic agreement between FEC and Flotac-400 methods reported was moderate for G. intestinalis (κ = 0.46), and poor for E. histolytica/E. dispar (κ = 0.20).Citation21
4.3 Assessment of parasite morphology and characteristics of reagents
Among variety of reagents employed in stool concentration techniques, ether is one of the most common solvents of fat in the concentration of stool for parasites.Citation22 However, ether offers several disadvantages by virtue of its different physico- chemical characteristics (see ).Citation10,Citation15 Because of its hazardous impacts and low safety, we evaluated tween, acetone and gasoline chemicals as substitute for diethyl ether under laboratory conditions.
Compared with ether, acetone and tween 20™ (the latter widely used in serological works) are stable, safer, less flammable (flash point −9.4 °C and >110 for acetone and tween 20 respectively), of lower cost (a relative cost: half of diethyl ether for acetone and one-fifth of diethyl ether for tween), and they do not produce anaesthetic vapours (see ).Citation9,Citation23 All these properties make them superior to ether for use in concentration of stool by sedimentation method. Although the parasite-concentrator combination (including acetone and 7.5% tween 20) proved to be as good as diethyl ether in maintaining characteristic morphology of helminth eggs and protozoan cysts, however, the amount of fine precipitate in the sediment was more than in ether concentration method and concealed parasites from detection. Similar results have been recorded by other researchers.Citation9,Citation11,Citation12
Gasoline is widely used as fuel for motor vehicles; some use it as diluent and solvent. displays several physico-chemical advantages of this liquid in comparison with ether such as less flammable, less volatile, does not produce anaesthetic vapours, low-toxicity category, easier availability and cost less than one-tenth of ether.Citation10,Citation23,Citation24 According to the result of this study, clarity of sediment, and parasite recovery rates in FEC and FGC methods were identical, and morphology of the individual parasites was well retained in both methods. These data are in accordance with a recent study, which reported that clarity of sediment and morphology of recovered parasite species in both methods resembled each other.Citation10
Since, all practical conditions such as processing steps, the time needed to prepare and demonstrate the samples, period of observation under light microscope, etc. for all techniques resembled those of FEC method, therefore the use of these techniques does not require additional training of the staff.
In conclusion, the recovery efficiency, sensitivity, NPV of FTC and FAC techniques were significantly higher than those of the FEC and FGC techniques for the diagnosis of all intestinal parasites, especially for diagnosing helminth species ova, whilst the FEC and FGC techniques revealed more efficiency rates for the protozoan species cysts. Overall, diagnostic κ agreement for FTC and FAC techniques was substantial, but for FEC and FGC techniques revealed moderate agreement. The agreement between the diagnostic techniques was generally low to moderate for helminth species, and was moderate to substantial for protozoan species. Both helminthic and protozoan parasitic elements were demonstrated in stool, and the morphology was well retained in all methods.
With regard to advantages and disadvantages of each reagent, and the performance evaluation of each concentration methods, this study demonstrated that the combined use of parasitological techniques is important for the diagnosis of all intestinal parasites particularly in the specimens with low intensity infection. We recommended FTC and FGC techniques for the diagnosis of helminth species ova and protozoan species cysts in stools, respectively. Additional studies are suggested, using a larger sample size and different parasite species in the field.
Competing interests
None declared.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The authors thank all their colleagues who cooperated in this investigation.
Financial support
Financial support: This study was financially supported by a grant (No. 1971) from Vice Chancellor for Research, Shahid Beheshti University of Medical Sciences, Iran; hereby is highly appreciated.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 November 2017
References
- World Health Organization. Working to Overcame the Global Impact of Neglected Tropical Diseases. First WHO Report on Neglected Tropical Diseases. Geneva, Switzerland: World Health Organization; 2010 [WHO/HTM/NTD 2010/1].
- C.J.L.MurrayT.VosR.LozanoDisability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010Lancet380201221972223
- R.L.PullanJ.L.SmithR.JasrasariaS.J.BrookerGlobal numbers of infection and disease burden of soil transmitted helminth infections in 2010Parasit Vectors7201437
- S.BaldurssonP.KaranisWaterborne transmission of protozoan parasites: review of worldwide outbreaks – an update 2004–2010Water Res45201166036614
- L.SavioliH.SmithA.ThompsonGiardia and Cryptosporidium join the ‘neglected diseases initiative’Trends Parasitol222006203208
- M.O.HarhayJ.HortonP.L.OlliaroEpidemiology and control of human gastrointestinal parasites in childrenExpert Rev Anti-Infect Ther82010219234
- M.R.TarafderH.CarabinL.JosephE.J.BalolongR.OlvedaS.T.McGarveyEstimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard’Int J Parasitol402010399404
- B.SpeichJ.UtzingerH.MartiComparison of the Kato-Katz method and ether-concentration technique for the diagnosis of soil-transmitted helminth infections in the framework of a randomised controlled trialEur J Clin Microbiol Infect Dis332014815822
- N.A.AhmadiK.PakdadTween as a substitute for diethyl ether in the formalin-ether sedimentation techniqueIran J Publ Health3620079195
- N.A.AhmadiF.-A.DamrajA field evaluation of formalin-gasoline technique in the concentration of stool for detection of intestinal parasitesParasitol Res1042009553557
- S.C.ParijaS.BhattacharyaP.PadhanM.R.ShivaprakashEvaluation of formalin-acetone sedimentation in the concentration of stool for intestinal parasitesTrop Doct332003163164
- R.MethanitikornK.SukontasonK.L.SukontasonS.PiangjaiEvaluation of the formalin-tween concentration technique for parasitic detectionRev Inst Med Trop Sao Paulo452003289291
- A.L.TruantS.H.ElliottM.T.KellyJ.H.SmithComparison of formalin-ethyl ether sedimentation, formalin-ethyl acetate sedimentation, and zinc sulfate flotation techniques for detection of intestinal parasitesJ Clin Microbiol131981882884
- D.GoodmanH.J.HajiQ.D.BickleA comparison of methods for detecting the eggs of Ascaris, Trichuris, and hookworm in infant stool, and the epidemiology of infection in Zanzibari infantsAm J Trop Med Hyg762007725731
- M.V.BoswellV.J.CollinsDiethyl ether and chloroformV.J.CollinsPhysiologic and Pharmacologic Bases of Anesthesia1996Williams & WilkinsPennsylvania650662
- B.LeveckeN.De WildeE.VandenhouteJ.VercruysseField validity and feasibility of four techniques for the detection of Trichuris in simians: a model for monitoring drug efficacy in public health?PLoS Negl Trop Dis32009e366
- J.R.LandisG.G.KochThe measurement of observer agreement for categorical dataBiometrics331977159174
- Ouattara M, N'Guessan NA, Yapi A, N'Goran EK. Prevalence and spatial distribution of Entamoeba histolytica/dispar and Giardia lamblia among schoolchildren in Agboville area (Côte d'Ivoire). PLoS Negl Trop Dis. 2010;4:e574. http://doi:10.1371/journal.pntd.0000574.
- J.UtzingerS.Boter-KleivenF.CastelliMicroscopic diagnosis of sodium acetate – acetic acid-formalin – fixed stool samples for helminthes and intestinal protozoa: a comparison among European reference laboratoriesClin Microbiol Infect162010267273
- B.D.BardaL.RinaldiD.IannielloMini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the fieldPLoS Negl Trop Dis72013e2344https://doi.org/10.1371/journal.pntd.0002344
- S.L.BeckerL.K.LohourignonB.SpeichComparison of the Flotac-400 dual technique and the formalin-ether concentration technique for diagnosis of human intestinal protozoon infectionJ Clin Microbiol49201121832190
- M.CheesbroughDistrict Laboratory Practice in Tropical Countries, Part 11998Cambridge University PressUK
- R.J.LewisSrHawley's Condensed Chemical Dictionary13th ed.1997Van Nostrand ReinholdNew York
- Spencer AB, Colonna GR. Fire Protection Guide to Hazardous Materials. 13th ed. Vol 325. National Fire Protection Association, Quincy, NFPA; 2002.
