Abstract
Objectives
Unilateral middle cerebral artery occlusion (MCAO) is an established rat model for stoke studies. It induces focal cerebral ischemia, prior to necrotic and apoptotic loss of tissue in a circumscribed cortical area, paralleled by temporary motor impairment.
Methods
Here we examined tissue samples from the peri-infarct zone of rats that had survived unilateral MCAO for up to 90 min. With immunohistochemistry we stained sections for proliferation markers Ki 67 and PCNA and for intermediate filament protein nestin. Electron microscopy was employed to assess ultrastructural changes.
Results
All MCAO animals developed pronounced lesions in the motor cortex. Numerous cells in the immediate peri-infarct area and scattered cells which seem to have migrated into the infarcted lesion stained positively for Ki 67 and PCNA. Electron microscopy revealed that cells in the lesion site proliferate along the blood vessels. Most of these cells had the ultrastructural features of fibrillary astrocytes while some of the cells were clearly neurons. Endothelia were in part fenestrated. Some of the surrounding cells showed immunostaining for PCNA, indicating proliferation. Oligodendroglia and myelination could not be seen in the lesion site. Single neuronal contacts exhibited the ultrastructural features of synapses. Reformation of cortical layers could not be observed.
Conclusions
We concluded that in spite of extensive proliferation; neuronal and glial regeneration occurs after MCAO only to a small extent. Revascularization seems to be an important initial step. The observed functional recovery of experimental animals may be due to neuronal plasticity in young rats rather than structural regeneration.
1 Introduction
Stroke is one of the most common causes of mortality and morbidity in modern society. Although much progress has been made toward understanding the mechanistic basis of stroke; therapeutic options are still limited.Citation1 In general, the adult central nervous system (CNS) possesses little capacity for regeneration after injury, including ischemia.
Various animal models have been established to study the pathophysiology of cerebral ischemia and the effects of neuroprotective agents.Citation2 Desirable animal models are those that replicate features of human cerebrovascular syndromes. MCAO in rat using a thin filament which is inserted through the internal carotid artery allows defined times of ischemia. For this approach, intraluminal suturing without craniotomy is possible.Citation3,Citation4 Unilateral MCAO is known to induce focal cerebral ischemia, prior to necrotic and apoptotic loss of neuronal tissue in a circumscribed cortical area. This is paralleled by temporary motor impairment. In a previous studyCitation5, we observed extensive necrosis and apoptosis in the lesion site in rats subjected to 60 min MCAO followed by 7 days of reperfusion. Apoptotic markers were observed not only in the tissue surrounding the lesion site but also on the contralateral side of the cortex, indicating degeneration along commissural projections. Nevertheless these animals showed remarkable functional recovery prior to sacrifice.Citation5
Following ischemic injury, neural tissue recovery is accompanied by formation of reactive astrogliosis. This process is vital for isolating necrotic tissue from uninjured surroundings, but concurrently, it markedly impedes regenerative processes. Shortly after ischemia, a series of ionic, neurotransmitter and oxidative radical imbalances occur that lead to the activation of microglia and subsequently to an increased number of reactive astrocytes. These cell types release cytokines and other soluble products, that play an important role in subsequent processes, including apoptosis of oligodendrocytes and neurons.Citation6–Citation8
One of the histochemical markers of cellular regeneration is proliferating cell nuclear antigen [PCNA].Citation9 PCNA was originally identified in cell nuclei during DNA synthesisCitation10,Citation11 and during repair of damaged DNA strands.Citation12 Another immunohistochemical indicator of cell proliferation is Ki-67. Ki-67 is a ubiquitous human nuclear protein expressed in G1-, S-, and G2-phases of the cell cycle but not in the G0-phase.Citation13
The majority of cerebral stem cells are located in the subventricular zone of all cerebral ventricles and in the hippocampal subgranular layer. These cells are the source of both neuronal and glial cells.Citation14 They migrate into the lesion site upon activation through chemical signals released from damaged tissue. Neural stem cells express certain proteins that are typically present during brain development. One of these markers is nestin, an intermediate filament protein, which is expressed in the astrocytes and in radial glial cells in the developing brain.Citation15 Upon focal ischemia in rat, nestin positive cells from the ipsilateral subventricular zone have been shown to differentiate into glial cells. Therefore, in the adult brain, nestin expression seems to occur in both proliferating cells and reactive astrocytes.Citation16,Citation17
Therefore, detailed study of the cellular and histochemical changes associated with cortical ischemia in rat was done. Tissue samples from the lesion site of animals that survived 90 min of MCAO and 7 days of reperfusion were subjected to immunohistochemistry for PCNA, Ki67 and nestin. Electron microscopy was used to characterize the ultrastructural features of cells occurring in cortical lesions.
2 Materials and methods
2.1 Animals
Histological samples were taken from animals subjected to 90 min of MCAO. Briefly, intact young adult male Wistar rats (n = 20), weighing 200–220 g were obtained from the local breeding colony, (Jena University Hospital, Germany). Animals were housed under conditions of natural illumination with food and water available ad libitum. Animals were divided into 2 groups randomlyCitation1 the sham operated group (n = 10)Citation2; the ischemic group (n = 10). These experiments were performed in accordance with protocol # 02-040/10, Landesverwaltungsamt Thüringen, Germany.
2.2 MCAO animals
10 rats were subjected to MCAO for 90 min by unilateral ligature of the left middle cerebral artery via insertion of an intraluminal thread.Citation3,Citation18 After a survival time of 7 days, rats were killed by prolonged ether anesthesia. Animals were subjected to cardiac perfusion with 4% paraformaldehyde and 0.25% glutaraldehyde in 0.1M sodium phosphate buffer pH 7.2 containing 0.9% NaCl phosphate buffered saline (PBS). After perfusion, brains were rapidly removed and postfixed overnight in the same fixative. Brains were sectioned into 3 mm thick frontal sections with a razor blade, the lesion site was identified and dissected. Tissue samples intended for electron microscopy were postfixed in 2% osmium tetroxide (OsO4) for 1 h. All tissue blocks were dehydrated through ascending ethanol series and embedded in EPON (Fluca, Munich, Germany). Propylene dioxide (Sigma, Munich, Germany) served as intermedium. Epoxy resin was allowed to polymerize for 2 days at 55 °C.
Tissue blocks were sectioned on an Ultracut microtome (Reichert; Vienna, Austria) into serial 1 μm thick sections for immunohistochemistry. OsO4 fixed samples were used to prepare ultrathin sections (70 nm) on the same microtome. These sections were collected onto collodium coated copper grids.
2.3 Immunohistochemistry
Semithin sections were immersed in 10% sodium methoxide (Sigma, Munich, Germany) for 2 min to remove epoxy resin, followed by brief washes in methanol and benzene (mixed 1:1). After washing in acetone (2x 2 min) sections were immersed in PBS.
Anti Ki67 (rabbit polyclonal antibody, sc-15402), Santa Cruz, USA) was diluted 1:200 in PBS. Sections were incubated over night at 4 °C, followed by washing in PBS and incubation with anti- rabbit IgG, diluted in PBS 1:100 for 1 h at room temperature. After washing in PBS, sections were incubated with rabbit peroxidase-anti-peroxidase complex (PAP) for 1 h at room temperature. Sections were again washed in PBS and stained with 3,3′-diaminobenzidine (DAB) and H2O2 (all reagents obtained from Sigma, Munich, Germany).
For PCNA staining we used a rabbit polyclonal antibody (Chemicon, Temecula, USA- PC 474) diluted 1:1000 in PBS. Visualization of immune signals was performed as described above.
Anti-Nestin (mouse monoclonal antibody, Chemicon, Temecula, USA- clone 10C2-catalogue no. MAB5326) was used at a dilution of 1:1000 overnight, followed by anti-mouse IgG, mouse PAP-complex and DAB staining.
Stained sections were washed in tap water, dehydrated through ascending ethanol series, cleared in xylene and mounted with Entellan® (Merck, Darmstadt, Germany). Stained slides were examined with an Olympus BX 50 photo microscope with interference contrast illumination. A digital Olympus DP10 camera and Olympus DP-software were used for assessment of images.
2.4 Electron microscopy
Ultrathin sections were dried on copper grids, then were contrasted with 2% uranyl acetate in H2O for 10 min at room temperature. After brief rinsing in double distilled water, sections were immersed in 1% aqueous lead citrate solution for 2 min at room temperature. After washing again in H2O, grids were air-dried and examined with an EM 902 elecron microscope (Zeiss, Oberkochen, Germany). Samples were examined under 80 kV and cooled with liquid nitrogen.
3 Results
Animals survived 90 min of MCAO well. After showing severe motor impairment for the first two days, all rats recovered almost completely within 7 days. However extensive testing of motor skills was not performed in this study.
Brains of MCAO rats showed after a survival time of 7 days macroscopically visible tissue damage in the respective portion of the motor cortex. Lesions had an approximate diameter of 2.5 mm.
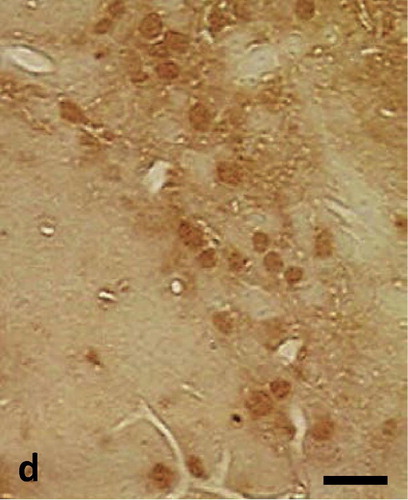
Semithin sections of the peri-infarct zone showed clear indications of tissue damage. The layers of the motor cortex had disappeared. Pyramidal and granular cells could not be identified. Numerous small blood vessels developed within the lesion cavity, surrounded by cells with extended processes. Morphology of cells was quite heterogeneous (). These cells showed neuron or glia like appearance. Morphological signs of mitosis could be observed in some of these cells. Cell bodies and neuronal processes immunostained for nestin could be observed in the ischemic lesion. Staining was confined to the perinuclear cytoplasm while nuclei remained unstained. Numerous nestin positive cell processes were observed (). Many of the cells within the lesion contained nuclear immunostaining for PCNA and Ki67. Such staining was absent in the peri infarct tissue (). Immunostaining for Ki67 was also found in cortical lesions. Distribution of nuclear Ki67 staining was similar to PCNA staining ().
Fig. 1a Methylene blue stained semithin section of the peri-infarct zone (a) and the ischemic lesion (b) of a rat motor cortex, 7 days after MCAO. Cortical layers have disappeared in both parts. There are numerous blood vessels in the lesion site (arrows). Cells within the infarcted area seem to be more numerous that in the peri -infarct tissue. Scale bar = 50 μm.
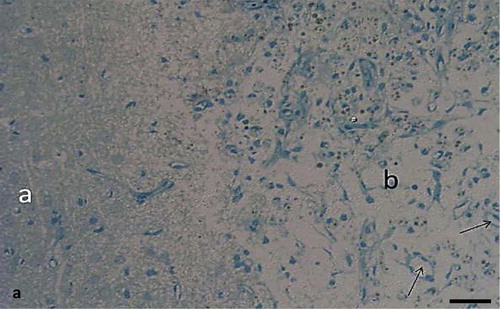
Fig. 1b Semithin sections of the infarcted zone show perikarya with nestin immuostained cytoplasm while nuclei remain unstained (arrows). Numerous nestin positive varicosities can be seen (open arrows). Scale bar = 20 μm.
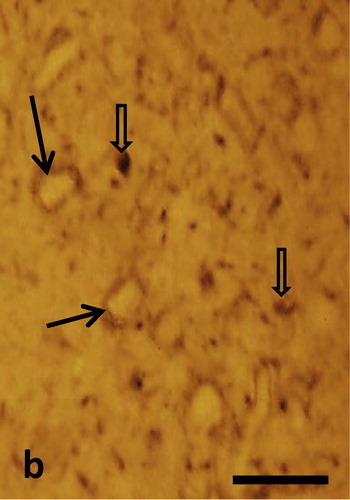
Fig. 1c Immunostaining for PCNA in a cortical sample 7 days after MCAO: Numerous PCNA positive nuclei occur in the infarcted lesion (b). Such staining is absent in the periinfarctal tissue (b). Scale bar = 25 μm.
Fig. 1d Semithin section of infarcted cortical tissue immunostained for Ki67 reveals numerous immunoreactive nuclei. Scalebar = 10 μm.
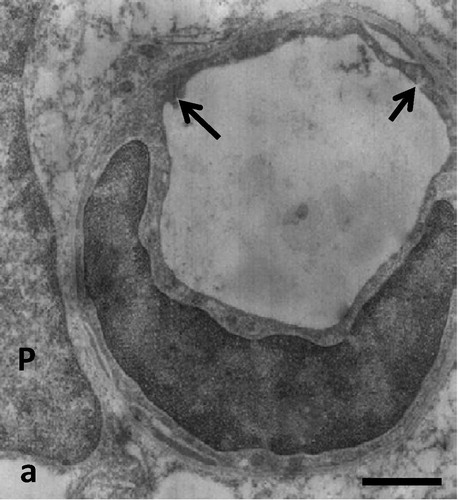
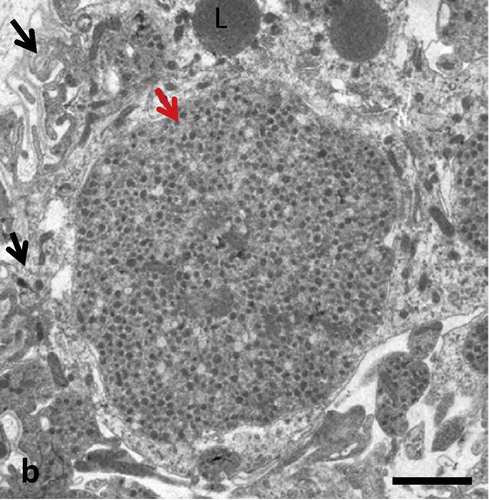
Electron microscopy revealed that blood vessels were lined with fenestrated endothelia and pericytes within abundant intercellular spaces (). Some cells within the lesion site showed the ultrastructural features of fibroblasts surrounded by their collagen fibers. Blood vessels were engulfed by cells containing glial filaments and numerous mitochondria typical of fibrillary astrocytes (). Some of these glial cells showed signs of mitosis as they appeared immature with relatively small cytoplasm and large nuclei. Well-developed oligodendrocytes could not be observed and signs of myelination were not visible. There were abundant cross sections of glial and neuronal processes in the lesion site as determined by their content of either glial filaments and lipid droplets or neurotubules and secretory vesicles (). Mature neuronal cell bodies could only occasionally be observed. These cells and their processes contained neurofilaments, neurotubules, rough endoplasmic reticulum and dense core vesicles with various diameters. Such neuronal processes seemed to develop large varicosities ().
4 Discussion
Our findings indicate that some neuronal and glial regeneration occurs in the young rat cerebral cortex within 7 days after a transient ischemic insult. Fibroblasts, blood vessels, astrocytes and single neurons seem to develop in the lesion site. Whether regeneration would further proceed with a longer survival time has to be studied in future experiments. In a previous study,Citation5 we compared 30, 60 and 90 min of ischemia prior to reperfusion. Necrosis of cortical tissue and extensive expression of apoptotic markers in both the lesion site and on the contralateral side of the cortex were seen. This was in contrast to the observed functional recovery of MCAO rats a few days after the experiment. In the present morphological and histochemical study we did not perform extensive testing of motor skills and behavior however we observed functional improvement similar to our previous findings.Citation5
In most parts of normal adult mammalian brains, neurons are not replaced after cell death.Citation19 Neuronal and glial stem cells are known to occur in the adult rat brain in specific regions, including the subventricular zone (SVZ) of the lateral wall of the lateral ventricles, and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus.Citation20–Citation23 The regenerative capacity of the adult brain may require a series of coordinated cellular processes; neural precursor cell proliferation and migration into injured sites, neuronal and glial differentiation, cell survival, and the integration of new neurons into existing neural circuits. Therefore the efficiency of spontaneous regeneration in the injured mammalian brain may be rather low.Citation24
Our immunohistochemical findings however confirm morphological observations, proliferation of brain tissue seems to occur in MCAO rats within the experimental time frame of 7 days in the peri-infarct zone, mostly associated with sprouting blood vessels. The neuroectodermal stem cell marker nestin is a type VI intermediate filament protein expressed mostly in nerve cells. It has been implicated in radial growth of axons. Our observation of nestin immunoreactivity in 7 days old MCAO lesions suggests that neuronal and glial processes are spontaneously extended. Immunostaining for proliferation markers PNCA and Ki-67 was found in numerous cells in the lesion site suggesting de novo proliferation of various cell types.
Similar observations were made in brain after perinatal hypoxia.Citation25 Such cellular proliferation in the lesion site may result from proliferation of surviving cells or of stem cells that cross the endothelial membrane and spread out into the lesion. The process of neurogenesis after stroke was studied by several groups who observed proliferation peaks one week after an ischemic insult but increases have been observed as early as 2 days after hypoxia/ischemia.Citation26–Citation28 Some cells proliferating after one week appeared to migrate from the SVZ into the damaged striatum to differentiate into phenotypically mature neurons.Citation24 Robust compensatory neurogenesis was observed in the rat neocortex upon perinatal hypoxia/ischemia.Citation29 Post-stroke neurogenesis was found in close association with the vasculature.Citation30,Citation31 There is no evidence that newly formed neurons in the adult neocortex develop functionally relevant connections although scattered synapses were observed. However there are several studies demonstrating that certain brain regions indeed generate new cells, and that the continuous “rewiring“ of the brain is an important physiological function.Citation32 However we assume that functional recovery of animals after MCAO observed in this study is not due to neuronal regeneration but due to the fact that the rat brain is capable of life long plasticity. This may be especially true for young rats, used in this study. Further studies are needed to clarify whether the treatment with specific neurotrophic factors can enhance morphological and functional recovery.
Our electron microscopic observations indicated the presence of astrocyte- like cells and of cells with neuron- like morphology. There is the possibility that these cells just survived infraction to some extent. Their close apposition to blood vessels which may have recently sprouted due to their fenestrated endothelia, together with the expression of proliferation markers suggest that central nervous tissue had spontaneously been formed after induction of ischemia. Following cerebral ischemia or other types of brain insult, neuronal plasticity is reactivated in surviving cells in order to compensate for cell death.Citation33 The possible mechanisms for this include; dendritic reorganization, axonal sprouting, and activation of endogenous pluripotent cells that can differentiate into neurons as revealed by nestin immunoreactivity. Such regeneration is known to occur in cerebellar granule cells throughout life. Newly formed neurons could be observed in various brain regions under experimental conditions, e.g. the administration of growth factors.Citation34,Citation35 It is well known that an ischemic lesion is confined to the respective brain areas and does not increase in size due to anterograde and retrograde degeneration. It is likely that the newly developed neuronal cells liberate neurotropic factors like Glia- derived neurotrophic factor (GDNF) that keep the cortical lesion confined. Here we observed that neurons within the lesion have developed axonal varicosities with secretory vesicles in a neuroendocrine- like fashion. Whether these neurons are capable of liberating their content in a paracrine way to additionally affect tissue regeneration has to be addressed in further studies. Fenestrated endothelia of blood vessels suggesting a disruption of the blood brain barrier in a neurohemal organ-like fashion.
On a cellular level, there are at least two main processes of neural repair after stroke: post-stroke axonal sprouting and neurogenesis. Axonal sprouting after stroke has been shown to occur within the cortical lesion and in the respective contralateral cortex, in the ipsilateral striatum, red nucleus and cervical spinal cord.Citation36 Axonal sprouting after stroke requires a molecular growth program in which a neuron must respond to injury, then elaborate a growth cone, extend an axon and establish new synapses.Citation37,Citation38 Blood- borne neuronal stem cells may migrate into the lesion.
Reestablishment of cortical layers was not be observed in the present study and it is therefore unlikely that functionally relevant neuronal circuits have developed. None of the experimentally regenerated central neurons have been shown to get integrated in functional circuits. This finding, however, was contradicted by Lu et al., 2004.Citation39 Scheff et al., 2005 reported that following cerebral injury, surviving neurons have been shown to form new synapses to compensate for the lost contact surfaces.Citation40
Clearly the functional recovery observed in the present study is unlikely to be caused by the rather minor neuronal and glial proliferation. Perhaps owing to the young age of the studied rats, other brain regions may have been recruited into takeover of lost cerebral function. It has been published that one possible explanation for the discrepancies between experimental stroke models and clinical studies may be the role that age plays in brain recovery.Citation41 It will be interesting to see whether very old rats behave similarly or whether their recovery is less.
Various experimental settings have been used to assess the recovery of sensorimotor functions, spontaneous activity and memory after ischemia in aged rats. Overall, the results indicate that aged rats have the capacity to recover behaviorally after cortical infarcts albeit to a lower extent than the young counterparts.Citation42,Citation43
The fact, that neuronal and glial regeneration occurs spontaneously as a possible consequence of revascularization gives rise to hope. Experiments used neuronal and glial growth factors immediately after the MCAO, through intralesion injections may provide novel clinical benefits. Such benefits are not only to induce cellular regeneration in stroke patients but also to limit tissue damage or to stop and delay the slow process of anterograde and retrograde degeneration.Citation44,Citation45 The use of stem cells to replace neurons lost after stroke potentially offers an encouraging approach to treatments aimed at improving recovery of tissue and function.Citation46 Such treatment might utilize the endogenous reserves of stem cells located in the subventricular zone or the subgranular zone of the hippocampus. One major concern however with any therapy designed to boost neurogenesis following stroke is the age dependent reduction of neuronal proliferation.Citation44,Citation47,Citation48 Although functionally relevant neuronal circuits may not develop; proliferating neuronal and glial cells may be the source of neurotropic factors that maintain survival of the remaining brain regions.
Conflict of interests
The auhors state that there is no conflict of interests to be reported in context with this study and with this manuscript.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 6 March 2018
References
- H.F.ZhuD.WanY.LuoJ.Li ZhouL.ChenX.Y.XuCatalpol increases brain angiogenesis and up-regulates VEGF and EPO in the rat after permanent middle cerebral artery occlusionInt J Biol Sci62010443453
- M.HeM.ChenJ.WangRelationship between glutamate in the limbic system and hypothalamus-pituitary-adrenal-axis after middle cerebral artery occlusion in ratsChin Med J116200314921496
- C.RedeckerW.WangF.J.MarcO.W.WitteWidespread and long-lasting alterations in GABAA- receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processesJ Cereb Flow Metab22200214631475
- Z.Zea LongaP.R.WeinsteinS.CarlsonReversible middle cerebral artery occlusion without craniectomy in ratsStroke2019898491
- A.E.DiefG.F.JirikowskiK.RagabH.IbrahimIpsilateral and contralateral cortical apoptosis in rats after unilateral middle cerebral artery occlusionAnatomy220083948
- D.S.TianQ.DongD.J.PanY.HeZ.Y.YuM.J.XieAttenuation of astrogliosis by suppressing of microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury modelBrain Res11542007206214
- Y.YangF.Y.JalalJ.F.ThompsonE.J.WalkerE.Candelario-JalilL.LiTissue inhibitor of metalloproteinases-3 mediates the death of immature oligodendrocytes via TNF-a/TACE in focal cerebral ischemia in miceJ Neuroinflammation82011108
- L.C.PettigrewM.S.KindyS.ScheffFocal cerebral ischemia in the TNF alpha-transgenic ratJ Neuroinflammation5200847
- L.MuskhelishviliJ.R.LatendresseR.L.KodellE.B.HendersonEvaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNAJ Histochem Cytochem5112200316811688
- E.LeonardiS.GirlandoG.SerioF.A.MauriG.PerroneS.ScampiniPCNA and Ki67 expression in breast carcinoma: correlations with clinical and biological variablesJ Clin Pathol451992416419
- G.D.BowmanM.O'DonnellJ.KuriyanStructural analysis of a eukaryotic sliding DNA clamp-clamp loader complexNature4292004724730
- J.EssersA.F.TheilC.BaldeyronNuclear dynamics of PCNA in DNA replication and repairMol Cell Biol25200593509359
- T.ScholzenJ.GerdesThe Ki-67 protein: from the known and the unknownJ Cell Physiol1822000311322
- C.ZhaoW.DengF.H.GageMechanisms and functional implications of adult neurogenesisCell1322008645660
- M.KálmánB.M.AjtaiA comparison of intermediate filament markers for presumptive astroglia in the developing rat neocortex: immunostaining against nestin reveals more detail, than GFAP or vimentinInt J Dev Neurosci192001101108
- T.NakagomiA.TaguchiY.FujimoriIsolation and characterization of neural stem/progenitor cells from poststroke cerebral cortex in miceEur J Neurosci29200918421852
- C.C.ShenY.C.YangM.T.ChiaoW.Y.ChengY.S.TsueiJ.L.KoCharacterization of endogenous neural progenitor cells after experimental ischemic strokeCurr Neurovasc Res72010614
- T.N.HaefelinO.W.WittePeri-infarct and remote excitability changes after transient middle cerebral artery occlusionJ Cereb Blood Flow Metab2020024552
- W.JiangW.GuT.BrännströmR.RosqvistP.WesterCortical neurogenesis in adult rats after transient middle cerebral artery occlusionStroke32200112011207
- P.TaupinF.H.GageAdult neurogenesis and neural stem cells of the central nervous system in mammalsJ Neurosci Res692002745749
- A.D.GarciaN.B.DoanT.ImuraT.G.BushM.V.SofroniewGFAP expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrainNat Neurosci7200412331241
- D.SunR.J.ColelloW.P.DaughertyT.H.KwonM.J.McGinnH.B.HarveyCell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injuryJ Neurotrauma22200595105
- N.KanekoK.SawamotoAdult neurogenesis and its alteration under pathological conditionsNeurosci Res632009155164
- A.ArvidssonT.CollinD.KirikZ.KokaiaLindvallNeuronal replacement from endogenous precursors in the adult brain after strokeNat Med82002963970
- R.J.FellingM.J.SnyderM.J.RomankoNeural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemiaJ Neurosci2616200643594369
- R.L.ZhangZ.G.ZhangL.ZhangM.ChoppProliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemiaNeuroscience10520013341
- Y.LiJ.ChenM.ChoppCell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in ratsJ Neurol Sci1932002137146
- M.IwaiK.SatoH.KamadaTemporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbilsJ Cereb Blood Flow Metab232003331341
- Z.YangM.V.CoveyC.L.BitelL.NiG.M.JonakaitS.W.LevisonSustained neocortical neurogenesis after neonatal hypoxic/ischemic injuryAnn Neurol612007199208
- J.J.OhabS.FlemingA.BleschS.T.CarmichaelA neurovascular niche for neurogenesis after strokeJ Neurosci2620091300713016
- L.WangZ.G.ZhangR.L.ZhangS.R.GreggA.Hozeska-SolgotY.LeTourneauMatrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migrationJ Neurosci26200659966003
- D.BavelierD.M.LeviR.W.LiY.DanT.K.HenschRemoving brakes on adult brain plasticity: from molecular to behavioral interventionsJ Neurosci3020101496414971
- C.A.BlizzardJ.A.ChuckowreeA.E.KingFocal damage to the adult rat neocortex induces wound healing accompanied by axonal sprouting and dendritic structural plasticityCereb Cortex212011281291
- H.J.BuschI.R.BuschmannG.MiesC.BodeK.A.HossmannArteriogenesis in hypoperfused rat brainJ Cereb Blood Flow Metab232003621628
- R.IssaA.Al QteishatN.MitsiosExpression of basic fibroblast growth factor mRNA and protein in the human brain following ischaemic strokeAngiogenesis820055362
- N.DancauseS.BarbayS.B.FrostExtensive cortical rewiring after brain injuryJ Neurosci4420051016710179
- I.E.BonillaK.TanabeS.M.StrittmatterSmall proline rich repeat protein IA is expressed by axotomized neurons and promotes axonal out growthJ Neurosci22200213031315
- D.FischerZ.HeL.I.BenowitzCounteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth stateJ Neurosci24200416461651
- D.LuA.GoussevJ.ChenAtorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injuryJ Neurotrauma2120042132
- S.W.ScheffD.A.PriceR.R.HicksS.A.BaldwinS.RobinsonC.BrackneySynaptogenesis in the hippocampal CA1 field following traumatic brain injuryJ Neurotrauma222005719732
- H.AyW.J.KoroshetzM.VangelConversion of ischemic brain tissue into infarction increases with ageStroke36200526322636
- T.M.MarkusS.Y.TsaiM.R.BollnowRecovery and brain reorganization after stroke in adult and aged ratsAnn Neurol582005950953
- C.L.RosenV.A.DinapoliT.NagamineT.CroccoInfluence of age on stroke outcome following transient focal ischemiaJ Neurosurg1032005687694
- S.LanfranconiF.LocatelliS.CortiGrowth factors in ischemic strokeJ Cell Mol Med15201116451687
- A.LarpthaveesarpD.M.FerrieroF.F.GonzalezGrowth factors for the treatment of ischemic brain injury (growth factor treatment)Brain Sci52015165177
- S.I.SavitzM.ChoppR.DeansS.T.CarmichaelD.PhinneyL.WechslerStem cell therapy as an emerging paradigm for stroke (STEPS) IIStroke42201115
- J.M.MirichN.C.WilliamsD.J.BerlauP.C.BrunjesComparative study of aging in the mouse olfactory bulbJ Comp Neurol4542002361372
- M.J.JosephJ.CaliaperumalL.C.SchlichterAfter Intracerebral hemorrhage, oligodendrocyte precursors proliferate and differentiate inside white-matter tracts in the rat striatumTransl Strok Res72016192208