Abstract
Objective
The use of alloxan as a diabetogenic agent at 150 mg/kg BW has been characterized by low percentage induction and instability of the hyperglycemia induced. The present study examined its time course effects with a view to suggesting the probable effective dose of the compound for animal studies.
Methods
Forty adult Wistar rats were equally randomized into two groups (I and II) and were injected with single intraperitoneal dose of alloxan, 170 and 200 mg/kg BW respectively. Blood glucose concentration (BGC) was monitored in consecutive phases of hourly for 3 h, 3 h interval for 15 h, 6 h interval for 12 h and 9 h after. Changes with time in biomarkers of oxidative stress (SOD, CAT, GST and MDA) and pancreas histopathology were studied.
Results
Alloxan at the evaluated doses produced a multiphasic blood glucose response. One hour post alloxan injection, 90% of group I and 85% of group II animals exhibited diabetic hyperglycemia (glucose level ≥ 200 mg/dL). Groups I and II respectively produced peak levels of hyperglycemia (586.8 and 575.9 mg/dL) at 9 and 12 h post alloxan administration. Hypoglycemia which is characteristic of experimental diabetes was noted between the 15th and 21st hour in both groups (I and II) and resulted in 5 and 10% mortality respectively. At 36th hour, hyperglycemia was restored and apparently sustained. Changes in biomarkers of oxidative stress showed patterns similar to that of blood glucose, and the histopathological examination of the pancreas mainly indicated focal area of a necrotic islet and multifocal area of mild infiltration in both groups.
Conclusion
The data obtained shows that alloxan at the investigated doses produced sustained hyperglycemia at 21st and 24th hour post administration, and 170 mg dosage of the compound is apparently a better diabetogenic dose, particularly in terms of reduced animal mortality.
1 Introduction
Alloxan (5,5-dihydroxyl pyrimidine-2,4,6-trione) which is an organic compound, a urea derivative is a carcinogen and cytotoxic glucose analogue.Citation1 It has the molecular formula, C4H2N2O4 and a relative molecular mass (Mr) of 142.06 g/Mol. Alloxan is one of the common diabetogenic agents used to assess the anti-diabetic or hypoglycemic potential of both pure compounds and plant extracts in studies involving diabetes.Citation2 Compared to other diabetogenic compounds such as streptozotocin, dithizone, monosodium glutamate, gold thioglucose and anti-insulin serum, it appears to be the most used compound for the induction of diabetes in animal experimental studies, probably because of its affordability and availability.Citation3
Like other diabetogenic agents, alloxan causes diabetes by a mechanism which basically involves partial degradation or damage of the beta (β) cells of pancreatic islets, and subsequent compromise in the quality and quantity of insulin produced by these cells.Citation4
The use of alloxan as a diabetogenic agent in experimental animals was first reported by Dunn Sheehan and McLetchie in their study in which they successfully induced diabetes in experimental rabbits.Citation5
Several studies have thereafter reported the effective use of alloxan for induction of diabetes in experimental animals, particularly at a dosage of 150 mg per kg of body weight (single intraperitoneal administration).Citation6–Citation9
However, in previous studies on diabetes in our laboratory, we observed that single administration of alloxan at this dosage (150 mg/kg BW) has a low success rate as most of the animals in a study group do not attain a diabetic blood glucose level (Value ≥ 200 mg/dL).Citation10 This often leads to time, energy and animal loss, since a new batch of normoglycemic animals have to be injected with alloxan in order to achieve the required number of diabetic animals statistically relevant for the study. Increase of alloxan dosage to 200 mg/kg BW appeared to be very effective as most of the animals exhibited a blood glucose level greater than 200 mg/dL. This however came with the problem of higher mortality rate. Mortality from diabetes has been adduced to either initial hypoglycemic shock or emergence of diabetic complications or direct kidney tubular cell toxicity.Citation11 The placement of alloxan-treated animals on 5–10% glucose solution in a bid to prevent hypoglycemic shock is often practiced but this intervention appears not to be significantly helpful, and thus the problem of mortality persists. High mortality rate is a major setback in diabetic studies. First it increases the financial burden of the study as several animals, more than required have to be used in order to carry the study to a meaningful and conclusive end. Secondly, it does not allow for proper evaluation of the antidiabetic potential of the investigated compound or test drug.
These challenges collectively necessitated the need to examine the time course effects of alloxan using doses above 150 mg/kg BW (170 and 200 mg/kg BW). It is expected that the information obtained from this study will be helpful in suggesting the most effective dose of alloxan for induction of diabetes in experimental animals, vis-à-vis onset of experimental diabetes, period of stable hyperglycemia and mortality rate. It will also assist researchers with the precise time or period when stable diabetes is achieved by the drug, and hence, the appropriate time to commence treatments in diabetic studies involving alloxan. The latter will go a long way in minimizing mortality in the experimental animals, particularly if the investigated extract or test compound is effective. This is because mortality occurrence and rate increase in the diabetics when treatment is delayed; even when such treatments have the potential to curtail or reverse diabetes and its associated complications.
2 Materials and methods
2.1 Chemicals
Alloxan monohydrate was obtained from Sigma Co St. Louis, Missouri, USA.
2.2 Animal management and experimental design
Forty adult male Wistar rats (mean body weight of 124.39 g) were purchased from the animal breeding unit of the Department of Anatomy, University of Ibadan. The rats were randomized into two groups (I and II, n = 20). All procedures for maintenance and sacrifice (care and use) of animals were carried out according to the criteria outlined by the National Academy of Science published by the National Institute of HealthCitation12 and approved by the Ethical Committee of the Faculty of Sciences, Lead City University. The animals were handled humanely, kept in plastic suspended cages, placed in a well ventilated and hygienic rat house under suitable conditions of temperature and humidity. They were provided rat pellets, served water ad libitum and subjected to natural photoperiod of 12 h light and 12 h dark cycle. The animals were allowed two weeks of acclimatization prior to the commencement of the study.
2.3 Alloxan preparation and administration
Fresh alloxan solution was prepared by dissolving an appropriate amount of alloxan monohydrate (Sigma Chemical Co. USA) in an appropriate volume of normal saline (0.9%). Groups I and II animals were administered 170 and 200 mg/kg BW single intraperitoneal dose of alloxan respectively.
2.4 Monitoring of blood glucose concentration (BGC)
The accucheck active glucometer with disposable test strips was used to determine blood glucose level in rats. Using aseptic precautions, blood samples were collected through the tail of the animals. The tail in each case was first wiped with surgical spirit and then nibbed with a pair of sharp scissors. A Test Strip was fully inserted into the glucometer before applying a drop of blood to fully cover the test area inside the grey target. The principle of the test is based on a glucose oxidase/peroxidase reaction, which is specific for β-d-glucose. The test area of the strip is designed in such a way that when a drop of blood is placed on the top surface, color change occurs which is determined by the glucometer and this is proportional to the concentration of glucose in the blood sample. After collection of blood, the nibbled side of the tail was rubbed with cotton wool soaked in surgical spirit to protect the animal from infection and to arrest further bleeding. Blood glucose levels were monitored at different time intervals consecutively. First phase monitoring was done hourly for 3 h, second phase was monitoring was at 3 h interval for 15 h, third phase at 6 h interval for 12 h and last phase was monitored once after 9 h.
Two animals were taken from each group and sacrificed (by cervical dislocation) at 6 h interval. The pancreas was harvested and processed in each case for biochemical assays and histopathological examination as described below.
2.5 Isolation and preparation of pancreas for biochemical analysis
The pancreas was rinsed with 1.15% KCl solution. Part of the organ was prepared for histopathological examination by storing in freshly prepared 10% (v/v) formalin solution and the remaining was stored in ice-cold homogenizing buffer (Tris-HCl).
2.6 Histopathological examination of pancreas
The pancreas preserved in 10% formalin solution in each case was first gross-examined for any observable lesion or tissue derangement before it was processed using the automatic tissue processor. The technique involved dehydrating the fixed tissue placed in tissue baskets within their respective labels and passing through graded alcohol (70, 90, 95, and 100%). The tissues were removed after dehydration and immersed in xylene solution to clear the alcohol and facilitate molten wax impregnation. The tissues were finally sectioned through rotary microtome (at 5µ thickness), stained with Haematoxylin and Eosin (H&E) and then examined microscopically using standard techniques by Hulland.Citation13
2.7 Homogenization of pancreas
Weighed part of the organ was mixed with 4 times volume Tris-HCl (0.1 M, pH 7.4), the mixture was homogenized using Potter Elvehjem type homogenizer. The homogenate was centrifuged at 5500 revolution per minute (rpm) for 20 min using cold Centrifuge and the supernatant obtained was used to assay for lipid peroxidation, protein concentration, superoxide dismutase (SOD), catalase (CAT), and glutathione-s-transferase (GST) activities.
2.8 Biochemical assays
Protein concentration was estimated by the procedure of Lowry et al.Citation14 SOD activity was determined by the method of Misra and Fridovich.Citation15 The procedure described by Sinha was employed in the estimation of catalase activityCitation16 and GST activity was estimated spectrophotometrically at 25 °C according to the procedure of Habig et al.Citation17
2.9 Statistical analysis of data
Data analysis was performed using statistical software, Graphpad Prism, version 6.4. The statistical significance of difference between groups was analyzed using the one-way analysis of variance (ANOVA) followed by independent-sample t test. The level of significance was set at p < 0.05. The results are presented as the mean ± SD.
3 Results
3.1 Blood glucose concentrations in rats administered 170 and 200 mg/kg/BW alloxan
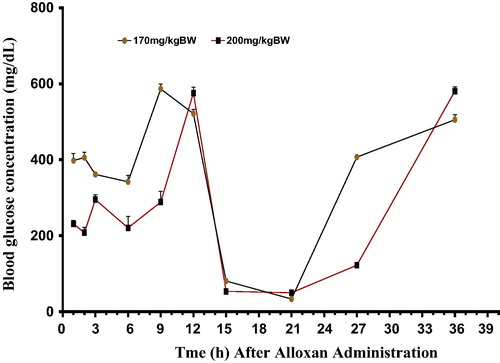
shows the effects of alloxan monohydrate at doses of 170 and 200 mg/kg BW on blood glucose level of rats. At the studied doses, alloxan produced a multiphasic blood glucose response. Following its administration, significant alterations characterized by inconsistent increase and decrease in blood glucose level (BGL) was observed at different times of the study. However, right from the first hour post alloxan injection, both groups of animals exhibited diabetic glucose level (≥200 mg/dL), with lower dose of alloxan (170 mg/kg BW) surprisingly producing higher blood glucose level of 398.2 mg/dL compared to 231.2 mg/dL recorded in animals treated with 200 mg/kg BW.
There was a drop in diabetic blood glucose level within the first 6 and 2 h in the animals injected with 170 and 200 mg/kg dosage of the drug respectively. On the contrary, both doses at the 9th and 12th h after administration respectively produced peak levels of diabetic blood glucose (586.8 and 575.9 mg/dL). This phase was succeeded by another sharp decline between the 15th and 21st h of the study. This was followed by a very steep rise in blood glucose at 24 h in animals treated with 170 mg/kg dosage whereas this increase was gradual in the animals administered 200 mg/kg dosage until the 27th h when it became very steep. By the 36th h. Animals treated with higher dose of alloxan (200 mg/kg/BW) exhibited a higher level of diabetic glucose (581.3 mg/dL) compared to those administered 170 mg/kg/BW (505.2 mg/dL).
3.2 Catalase activity in rats administered 170 mg/kg/BW and 200 mg/kg/BW alloxan
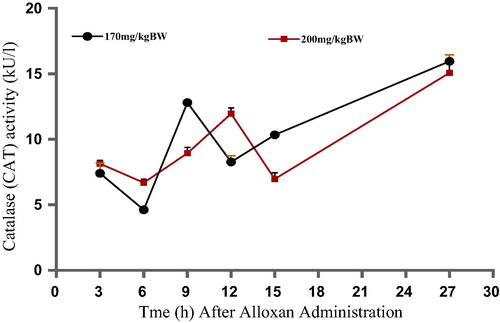
depicts the effects of alloxan monohydrate on pancreatic catalase activity (CAT) of experimental rats. Both doses (170 and 200 mg/kg BW) of alloxan caused an initial decrease in CAT activity up to the 6th h after administration. This was followed by a sudden increase at the 9th h and till the 12th h in 170 and 200 mg/kg BW groups respectively. The observed increase was steeper in the former. By the 12th h, Animals treated with the lower dosage (170 mg/kg BW) exhibited a sharp drop in CAT activity whereas those treated with higher dosage (200 mg/kg BW) showed the same level of decline by the 15th h of the study. The highest level of CAT activity was observed in both groups of animals at the 27th h of the study.
3.3 Superoxide dismutase activity in rats administered 170 mg/kg/BW and 200 mg/kg/BW alloxan
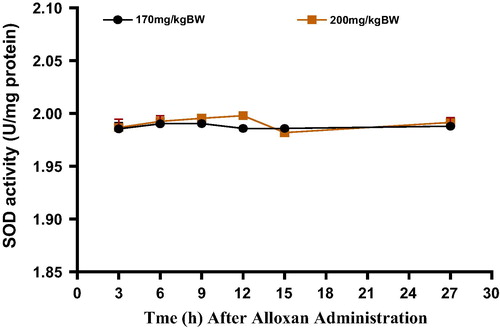
The effects of alloxan monohydrate on superoxide dismutase (SOD) activity in the pancreas of rats at the administered doses (170 and 200 mg/kg BW) are summarized in . In the group of animals treated with 170 mg/kg BW, a slight increase in SOD activity was observed between 3 and 6 h after alloxan administration.
This activity was maintained till the 9th h, followed by a decrease in the activity at 12th h and this level of activity was maintained till the end of the experiment (27th h). In the 200 mg/kg BW treated group of animals, SOD activity in the pancreas was slightly and gradually increased up to the 12th h after alloxan administration. Thereafter, a decrease in SOD activity was noted at the 15th h of the study, and this was followed by another phase of slight increase in the enzyme activity.
3.4 Malondialdehyde (MDA) concentration in rats administered 170 and 200 mg/kg/BW alloxan
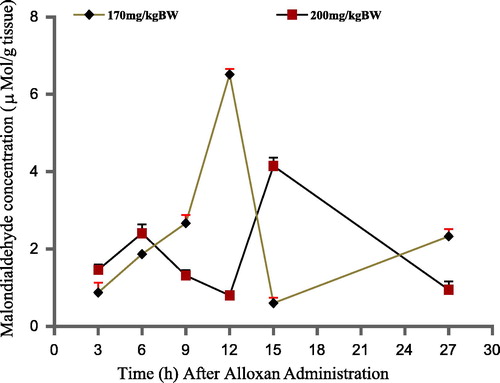
The effects of alloxan monohydrate on the physiological level of Malondialdehyde (MDA) of rats at the administered doses (170 and 200 mg/kg BW) are represented by . The effects did not follow any uniform or specific pattern of increase or decrease. Rather, at both doses, the diabetogenic compound caused an inconsistent increase and decrease in pancreatic MDA concentration with time. At a lower dose (170 mg/kg BW), alloxan administration caused gradual increase in the level of MDA in experimental rat up to 9 h and a sudden drastic upsurge, reaching a peak MDA level at 12 h. This was immediately followed by a steep decrease by 15 h and finally another gradual increase in the concentration of MDA was observed up to the 27 h. At higher dose of 200 mg/kg BW, the drug had similar effect on MDA up to 6 h showing a gradual increase in Malondialdehyde concentration but producing higher amount of MDA. This was followed by a decline till 12 h with highest level of Malondialdehyde reached at 15 h, then a sharp decline up to 27 h.
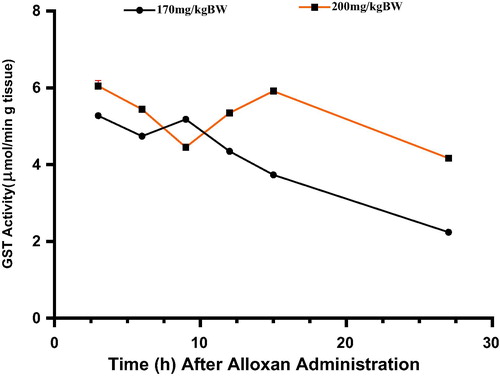
3.5 Glutathione-S-transferase (GST) activity in rats administered 170 and 200 mg/kg/BW alloxan
shows the effects of alloxan monohydrate on Glutathione-S-transferase (GST) activity in the pancreas of experimental rats. Alloxan showed similar effects on pancreatic GST activity at the investigated doses of 170 and 200 mg/kg BW. Post alloxan injection, GST activity initially decreased up to 6 and 9 h respectively in groups of animals administered 170 and 200 mg/kg dosage of the drug. This was followed by increased GST activity phase in both groups. The period of increased activity was brief in the 200 mg/kg BW treated group, notable at the 9th h and for a longer period in the 170 mg/kg BW treated counterparts, notable from the 12th to the 15th h of the study. This phase of increased GST activity was subsequently ended by decline in GST activity in both groups.
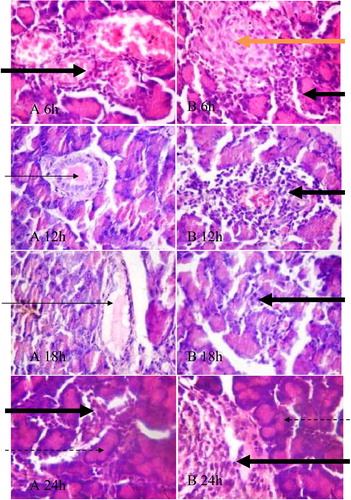
3.6 Photomicrographs of thin sections (5 µm) of pancreas of rats treated with 170 and 200 mg/kg BW alloxan; harvested at different time interval and stained with H &E (400×)
The effects of alloxan on the histology of the pancreas of rats treated with 170 and 200 mg/kg BW of the diabetogenic drug for different period of time are represented by . The results obtained generally showed mild disseminated congestion, focal area of a necrotic islet and multifocal area of mild infiltration by inflammatory cells along with normal exocrine acini, intralobular and interlobular ducts which in some cases contain pancreatic secretion. Nonetheless, the histopathological changes in the pancreas appeared not to have varied with time in both groups.
Fig. 6 Plates show focal area of a necrotic islet (orange arrow) and multifocal area of mild infiltration by inflammatory cells (black arrows), intralobular and interlobular ducts are essentially normal and in some cases contain pancreatic secretion (slender arrow) and normal exocrine acini (dotted arrows). A = 170 mg/kg BW alloxan treated rat, B = 200 mg/kg BW alloxan treated rat. 6 h, 12 h, 18 h and 24 h represent post alloxan treatment time.
4 Discussion
Lack of curative drugs for diabetes supports the need for continuous research on the disease and its related aspects, and the use of experimental animals for such purpose greatly extricate the risks humans are exposed to in clinical studies. In a bid to understanding the pathophysiology of diabetes and ultimately find a ‘perfect drug antidote‘ for the condition, alloxan is widely used for induction of diabetic hyperglycemia in different animal models.Citation18 However, its use at a common dosage of 150 mg/kg BW has a couple of limitations, particularly in terms of animal mortality, low percentage induction and instability of the hyperglycemia induced. The present study examined the time course effects of alloxan with a view to suggesting the probable effective dose of the compound for animal studies.
The doses of alloxan (170 and 200 mg/kg BW) examined in this study produced a multiphasic blood glucose response characterized by marked fluctuations in concentration. This observation is consistent with the previous reports on the use of alloxan for induction of experimental diabetes.Citation1,Citation10,Citation11,Citation19 The inconsistent increase and decrease in blood glucose level (BGL) observed in rats at different times in this study may reflects the anomaly of alloxan as a diabetogenic drug as proposed by Jain and Arya.Citation20 It could also be due to interplay of time dependent physiological changes in factors such as number of functional beta cells, insulin concentration and status of pancreatic antioxidant system, which are either directly or indirectly capable of influencing the concentration of blood glucose sequel to alloxan injection. This view is corroborated by the claim of SzkudelskiCitation11 that the understanding of changes in beta cells of the pancreas as well as in the whole organism after alloxan or streptozotocin treatment is essential for using these compounds as diabetogenic agents.
In the present study alloxan, both at 170 and 200 mg dosages induced diabetic hyperglycemia (blood glucose ≥ 200 mg/dL or 11.1 mmol/L) in rats one hour post its administration. Previous authors have also noted the immediate diabetogenicity of alloxan following its administration to experimental animals. LenzenCitation1 in his study titled ‘Alloxan and streptozotocin diabetes‘ informed that notable hyperglycemia commenced in experimental rats one hour after alloxan treatment (150 mg kg BW). Similar report ‘that alloxan administration to rats causes immediate hyperglycemia which reaches its peak within two or three hours was earlier communicated by Goldner and Gomori.Citation21 According to Szkudelski,Citation11 Alloxan is a hydrophilic and unstable substance with a half-life of 1.5 min at neutral pH and 37 °C. This implies that the time for alloxan degradation (metabolism) is sufficiently short enough to allow it to reach the pancreas very fast and in deleterious amount. This possibly explains the one hour post alloxan diabetic effect noted in this study.
Besides, alloxan toxicity in the pancreas is preceded by its rapid uptake by the insulin-secreting beta cells and this has been proposed to be one of the important features determining alloxan diabetogenicity.Citation1,Citation11 This occurs through cyclic reduction of alloxan, leading to the production of reactive species, particularly hydroxy radicals (OH∗) which ultimately destroy the beta cells of the pancreas and consequently affects insulin production and release.Citation1,Citation11,Citation20 Inhibition of insulin secretion, a condition that may invariably lead to hypoinsulinemia and hyperglycemia has been linked with early post alloxan effect.Citation1
Post alloxan effect was more evident in this study between the 9th and 12th h, as 170 and 200 mg/kg BW treated animals respectively exhibited peak levels of hyperglycemia (586.8 and 575.9 mg/dL) during this period (9 and 12 h post alloxan administration). This was however followed by hypoglycemia which is characteristic of experimental diabetes, between the 15th and 21st hour, and at 36th hour, hyperglycemia was restored and apparently sustained in both groups. This observation agrees with the earlier report of Goldner and Gomori,Citation21 who hinted ‘that alloxan-induced hyperglycemia in rats is followed by a severe and fatal hypoglycemia, which after a duration of several hours yields to a final hyperglycemia.
Arguably, the period of peak hyperglycemia noted in this study (9th h for 170 mg/kg BW alloxan and 12th h for 200 mg/kg BW alloxan) indicates maximal inhibition of insulin secretion and action following optimal uptake of the compound by the secreting granules of pancreatic beta cells. As postulated by Banerjee,Citation22 subsequent to uptake, alloxan induces the rupture of secretary granule and cell membrane, leading to flooding of the circulation with insulin, a phenomenon which accounts for severe hypoglycemia as noted in this study. Nonetheless, the period of hyperglycemia (15–21 h) in this study is at variance with that reported by LenzenCitation1 in which severe hypoglycemia was evident within 4–8 h in animals administered 150 mg/kg BW alloxan. The time difference of hypoglycemia in the two studies may be due to the difference in dose of alloxan or species of animals used.
Hypoglycemia in diabetes is a fatal phenomenon which usually results in mortality.Citation21 The mortality of animals recorded in this study occurred during the period of severe hypoglycemia, with 170 and 200 mg/kg BW alloxan causing 5 and 10% mortality respectively within 36 h. These values are quite low when compared to previous report which associates 25, 33.3 and 40% mortality with 150, 160 and 170 mg/kg BW alloxan injection respectively within 15 days.Citation10 The different durations of the two studies is obviously responsible for the significant variation in degree of mortality observed.
As previously stated, reactive species play very important role in the diabetogenicity of alloxan.Citation1 In the presence of an intracellular thiol especially glutathione, alloxan undergoes a cyclic reaction to generates reactive oxygen species such as superoxide radical (O2∗), and hydroxy radical (OH∗) through the auto-oxidation of its reduction product, dailuric acid. These radicals, particularly OH∗ caused oxidative damage to the beta cells of the pancreas as evident by inflammation and necrosis of pancreatic islet cells in rats injected with both 170 and 200 mg/kg BW alloxan in this study. It was expected that the severity of histological damage in the pancreas should worsen with time. However, the histopathological changes observed in the experimental animals appeared not to have varied with time in both groups. This is probably as a result of the short duration of alloxan exposure (27 h) in the animals.
Usually, superoxide radical (O2∗) generated in tissues through metabolism is catalytically converted to hydrogen peroxide (H2O2) and molecular oxygen (O2) by superoxide dismutase (SOD). H2O2 when accumulated is toxic to body tissues or cells. Also, it is converted to deleterious OH∗ in the presence of Fe2+ through Fenton reaction. In other to prevent this phenomenon, catalase (another antioxidant enzyme) breaks down H2O2 into water and oxygen, consequently curtailing free radical-induced damage. In fact, catalase deficiency has been suggested to increase the possibility of developing diabetes and related complications.Citation23 Unfortunately, pancreas unlike other tissues such as liver and kidney has very low contents of these antioxidant enzymes, making it highly sensitive or susceptible to alloxan metabolism.Citation24
In the present study, treatment of rats with alloxan at both 170 and 200 mg/kg BW caused marked changes (irregular increase and decrease) in SOD and CAT activities as well as Malondialdehyde (MDA) level in the pancreas. This may be a reflection of the normal cellular antioxidant response/attempt to combat beta cell oxidative damage or suppression of the antioxidant system, triggered by alloxan administration. The changes in the activities of these enzymes (SOD and CAT) and level of MDA noted in this study corroborates the reports of previous studies on chemical - induced diabetes.Citation25,Citation26 The decrease in catalase activity observed from 9 to 15 h in both groups suggest that hyperglycemia may suppress catalase activity by increasing metabolic reactions, this postulation supports the previous report on the expression of CAT in the pancreas of hyperglycemic rat by Suarsana et al.Citation27 Malondialdehyde is a metabolic index of lipid peroxidation. High level of Malondialdehyde observed around 9–15 h in both groups of animals depicts increased level of free radicals produced in the pancreas of the animals. Beta cell damage by hyperglycemia-induced lipid peroxidation has been suggested by Suarsana et al.Citation27
5 Conclusion
In summary, comparing the diabetogenic effects of alloxan at 170 mg/kg BW and 200 mg/kg BW through intraperitoneal route of administration did not show significant (P < 0.05) difference between the two doses, particularly in respect to the stability of the hyperglycemia induced. However, in terms of the severity of induced hyperglycemia (diabetes) and low animal mortality rate, the use of alloxan as a diabetogenic compound in animal studies at a dosage of 170 mg/kg BW is apparently more effective. Nonetheless, a longer duration of the study beyond 36 h is necessary to ascertain the stability of the final phase of hyperglycemia noticed in this study.
Conflict of interest statement
The author unanimously declare that there is no conflict of interest concerning this article
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 5 June 2018
References
- S.LenzenU.PantenAlloxan: history and mechanism of actionDiabetologia311988337342
- E.U.EtukAnimals models for studying diabetes mellitusAgric Biol J North Am12010130134
- S.WangF.ZhuAntidiabetic dietary materials and animal modelsFood Res Int312016315331
- N.MiraziM.RezaeiM.MirhoseiniHypoglycemic effect of Satureja montanum L. hydroethanolic extract on diabetic ratsJ HerbMed Pharmacol520161722
- J.S.DunnN.G.McLetchieExperimental alloxan diabetes in the ratThe Lancet2421943384387
- A.T.El-AlfyA.A.AhmedA.J.FataniProtective effect of red grape seeds proanthocyanidins against induction of diabetes by alloxan in ratsPharmacol Res522005264270
- M.F.AhmedS.M.KazimS.S.GhoriAntidiabetic activity of Vinca rosea extracts in alloxan-induced diabetic ratsInt J Endocrinol2220102010
- Abayomi AI, Adewoye EO, Olaleye SB, Salami AT. Effect of Magnesium pre-treatment on Alloxan induced hyperglycemia in rats. Afr Health Sci. 2011;11.
- H.Y.BakoJ.S.MohammadP.M.WazirT.BulusM.Y.GwarzoM.M.ZubairuLipid profile of alloxan-induced diabetic wistar rats treated with methanolic extract of Adansonia digitata fruit pulpSci World J920141924
- M.MisraU.AimanAlloxan: an unpredictable drug for diabetes induction?Indian J Pharmacol442012538
- T.SzkudelskiThe mechanism of alloxan and streptozotocin action in B cells of the rat pancreasPhysiol Res502001537546
- NIH Publication Number 04-4158 February 2010 Available at <http://www.win.niddk.nih.gov>.
- T.J.HullandHandbook of histopathological techniquesCan Veter J8196716
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- H.P.MisraI.FridovichThe role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutaseJ Biol Chem247197231703175
- A.K.SinhaColorimetric assay of catalaseAnal Biochem471972389394
- W.H.HabigM.J.PabstW.B.JakobyGlutathione S-transferases the first enzymatic step in mercapturic acid formationJ Biol Chem249197471307139
- Rafieian-Kopaei M, Nasri H. The ameliorative effect of Zingiber officinale in diabetic nephropathy. Iran Red Crescent Med J 2014;16.
- I.F.FederiukH.M.CaseyM.J.QuinnM.D.WoodK.W.WardInduction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatmentComp Med542004252257
- D.JainR.AryaAnomalies in alloxan-induced diabetic model: it is better to standardize it firstIndian J Pharmacol43201191
- Goldner MG, gomori G. Studies on the mechanism of alloxan diabetes. Endocrinology 1944 35:241–8.
- S.BanerjeeRelation of scurvy to the adrenalin content of the adrenal glands of guinea pigsJ Biol Chem1591945327331
- Góth L. Catalase deficiency and type 2 diabetes. Diabetes Care 2008;31:e93-.
- V.BurkartT.KoikeH.H.BrennerY.ImaiH.KolbDihydrolipoic acid protects pancreatic islet cells from inflammatory attackInflamm Res119936065
- D.V.GodinS.A.WohaiebM.E.GarnettA.D.GoumenioukAntioxidant enzyme alterations in experimental and clinical diabetesMol Cell Biochem841988223231
- V.M.BhorN.RaghuramS.SivakamiOxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic ratsInt J Biochem Cell Biol3620048997
- NyomanSuarsanaIwan HarjonoUtamaI. MadeKardenaImmunohistochemical expression of insulin and glucagon, superoxide dismutase and catalase activity in pancreas in hyperglycaemia conditionAsian J Biochem112016177185