Abstract
Objective
To study the role of urinary interferon gamma-induced protein 10 (IP-10) and urinary soluble CD 25 (sCD 25) as diagnostic and prognostic markers of lupus nephritis (LN) and their relation to the LN class in renal biopsy.
Subjects and methods
This study included 45 lupus patients fulfilling the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria: 25 patients with active LN during activity and during follow up (3 months later) as [group A] and 20 patients without any signs of activity [group B]. (20) age and sex matched healthy subjects were enrolled as control group [group C]. Urine samples were collected at baseline and at follow up. Urinary IP-10 and sCD25 were measured by ELISA.
Results
Urinary IP-10 and sCD25 levels were higher in group A compared to groups B and C (P < 0.001 for both). In patients with active nephritis, urinary IP-10 and sCD25 correlated positively with serum creatinine (P < 0.001 for both), proteinuria (p = 0.010; p = 0.007), anti ds-DNA (p = 0.002; p < 0.001), SLEDAI score (global) (P < 0.001 for both), and renal SLEDAI score (p = 0.002; p < 0.001) respectively. The urinary IP-10 and sCD25 levels were highest in patients with class (IV) LN and lowest in class (II) patients with a statistically significant difference. In patients achieving remission with treatment, both markers decreased significantly.
Conclusion
Urinary IP-10 and sCD25 are potential biomarkers for early recognition and follow up of LN.
Keywords:
1 Introduction
Lupus nephritis (LN) is a serious complication of systemic lupus erythematosus (SLE) affecting 23–66% of lupus patients.Citation1 But with the use of immunosuppressive regimens, renal affection and patient survival are improving.Citation2 LN pathogenesis is a complicated process, including glomerular deposition of autoantibodies, complement activation, cellular proliferation, release of chemokines and proinflammatory cytokines leading to inflammation and fibrosis.Citation3 Although, kidney biopsy provides evidence for LN diagnosis, prognosis, activity, chronicity and planning of therapy, it is an invasive procedure with its own complications. Thus, the discovery of new biomarkers can facilitate early diagnosis, assessing disease severity and predicting disease outcome.Citation4
C-X-C motif chemokine 10 (CXCL10) also known as interferon γ-induced protein 10 (IP-10) is one of the CXC chemokine family.Citation5 CXCL10 chemoattracts macrophages, dendritic cells, NK cells and activated T lymphocytes toward sites of inflammation.Citation6 CXCL10 is a pleiotropic molecule capable of exerting potent biological functions, including promoting the chemotactic activity of CXCR3+ cells, inducing apoptosis, regulating cell growth and proliferation as well as angiogenesis in infectious and inflammatory diseases and cancer.Citation7,Citation8 Serum CXCL 10 levels are elevated in lupus patients and correlate with disease activity.Citation9
CD25 represents the α-chain of the interleukin-2 receptor (IL-2Rα), a low affinity binding receptor.Citation10 IL-2 is secreted after T-cell activation playing an important role in growth, proliferation and differentiation of T cells converting them to effector T cells. sCD25 can be measured in serum as well as in body fluid as a marker of T-cell activation.Citation11 sCD25 levels increase during SLE flares and decline with treatment and clinical improvement suggesting that sCD25 can be considered a promising candidate biomarker for disease activity in patients with SLE, especially among those with kidney involvement.Citation12
2 Subjects and methods
2.1 Subjects
This study included 45 patients with SLE fulfilling the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for diagnosis of SLECitation13 classified into 25 patients with active lupus nephritis during activity and during follow up (3 months later) as [group A] and 20 patients without any signs of activity as [group B]. Twenty age and sex matched healthy subjects were enrolled as control group [group C]. Patients were recruited from the outpatient clinic and the inpatient ward of the internal medicine department at Alexandria Main University Hospital after the approval of the ethical committee. Exclusion criteria were diabetes mellitus, sepsis, hepatitis C virus infection, renal transplantation and tuberculosis. The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and informed consent was obtained from each patient.
2.2 Methods
All groups were subjected to full history taking, full clinical examination and assessment of disease activity by applying the score of SLE disease activity index (SLEDAI).Citation14 Assessment of renal disease activity was done by applying the renal SLEDAI score.Citation15 Laboratory investigations included CBC, ESR, serum creatinine, blood urea, 24 h urinary protein, complete urine analysis, serum complement levels C3 and C4, serum ANA and serum anti ds-DNA titres by specific enzyme-linked immunosorbent assay (ELISA) method (ORGENTEC Diagnostika CO., Mainz, Germany). Renal biopsy was performed to all SLE patients with clinical and laboratory evidence of renal involvement. Determination of the activity and chronicity indices was performed according to the scheme of the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification of lupus nephritis.Citation16
In group A patients, induction therapy was started using intravenous methyl prednisolone (0.51 gm for 3 days) and intravenous cyclophosphamide (0.5 gm) every 2 weeks for 3 months (Euro-Lupus regimen).Citation17 Patients were assessed 3 months later (follow up) to determine their response to treatment. Complete remission was defined when serum creatinine returned to previous baseline and urine protein:creatinine ratio declined to <500 mg/g. Partial remission was defined as having stabilization (±25%) or improvement of serum creatinine, but not to normal, and ˃50% reduction in urine protein:creatinine ratio.Citation18
2.3 Urine sample collection and biochemical analysis
Urine was collected in a sterile container and centrifuged for 20 min at 2000–3000 r.p.m. Supernatant was removed. If precipitation appeared, centrifugation of the sample was done again. The levels of urinary IP-10 and sCD25 were measured by ELISA (Assay Kit CO., Sunnyvale, CA, USA) according to the manufacturer’s instructions.
2.4 Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. Qualitative data were described using number and percent and were compared using Chi square test. Student’s t test and F-test (ANOVA) were used to compare between normally distributed quantitative data, which were expressed in mean ± standard deviation (SD). Mann–Whitney test and Kruskal Wallis test were used to compare abnormally distributed quantitative variables, which were expressed in median, minimum and maximum. Correlations between two quantitative variables were assessed using Spearman coefficient. Receiver operating characteristic (ROC) analysis and difference between area under the curve (AUC) were performed by MedCalc, version 15.8. Significance of the obtained results was judged at the 5% level. P value < 0.05 was considered as significant.
3 Results
3.1 Baseline characteristics of patients
The study included 45 SLE patients (active nephritis, inactive SLE) (5 males, 40 females) with a mean age of (27.96 ± 7.98) years in group A, (30.25 ± 10.05) years in group B and (24.70 ± 6.42) years in group C (healthy controls). There was no statistically significant difference between different groups regarding age and sex. Clinical and laboratory data of patients and control group are shown in .
Table 1 Demographic, clinical and laboratory characteristics of patients in different groups.
3.2 Urinary IP-10 and sCD25 levels
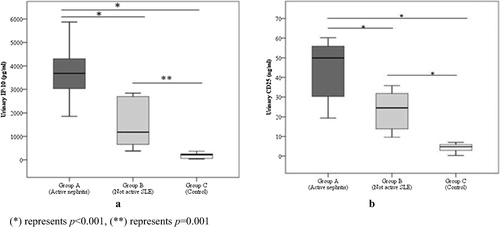
Urinary IP-10 and sCD25 levels were significantly higher in group A (active nephritis) than in groups B (inactive) and C (control) (P < 0.001) and both were higher in group B than group C with P values of (0.001) and (<0.001) respectively ().
3.3 Correlation between urinary IP-10 and sCD25 with different parameters
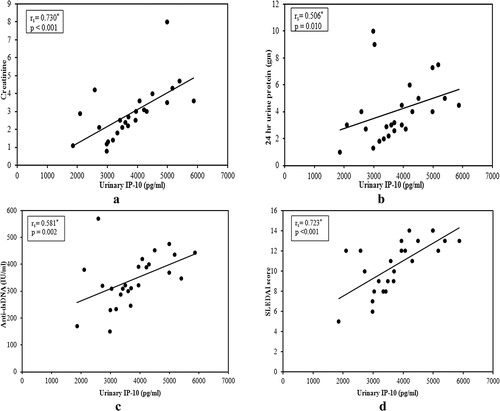
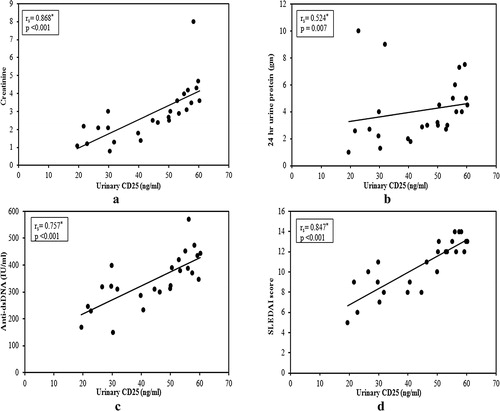
In active nephritis group (A), there was significant positive correlation between urinary IP-10 and sCD25 with serum creatinine (a and a), proteinuria (b and b), anti ds-DNA (c and c) and SLEDAI score (global) (d and d). Also, renal SLEDAI score showed a positive correlation with both markers (r = 0.586, p = 0.002; r = 0.844, p < 0.001). In addition, there was negative correlation between urinary IP-10 with serum C3 and C4 but it was insignificant (r = −0.376, p = 0.064; r = −0.162, p = 0.439). On the other hand, the correlation between urinary sCD25 with serum C3 and C4 was significant (r = −0.479, p = 0.015; r = −0.492, p = 0.012).
3.4 Renal biopsy and its relation to the urinary markers
Renal biopsy was done for all patients in group A (active nephritis) with 2 (8%) patients had class II LN, 7 (28%) had class III (±V) LN, 14 (56%) had class IV (±V) LN and 2 (8%) had class V LN. Activity index of renal biopsy ranged from 5 to 12 with a median value of 8, while the chronicity index ranged from 1 to 5 with a median value of 3.
Urinary IP-10 and sCD25 were higher in patients with diffuse proliferative GN (class IV) than in focal proliferative GN (class III) patients while those with the non-proliferative forms of lupus nephritis (class II and V) had the lowest levels of IP-10 (p = 0.011) and sCD25 (p = 0.001), with a statistically significant difference (). In addition, there was a positive correlation between urinary IP-10 and sCD25 with activity (r = 0.677, p < 0.001; r = 0.736, p < 0.001) and chronicity indices (r = 0.647, p < 0.001; r = 0.677, p < 0.001) of renal biopsy.
Table 2 Urinary levels of IP-10 (pg/ml) and CD25 (ng/ml) in different histological classes of renal biopsy.
3.5 Follow up (3 months later)
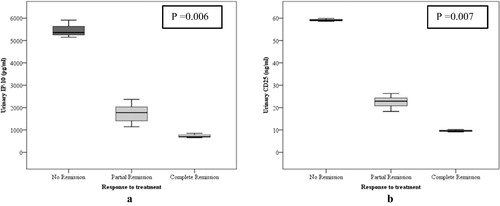
With treatment, 22 patients (from 25 in active nephritis group) achieved complete (n = 3) or partial (n = 19) remission with the urinary IP-10 and sCD25 levels decreasing significantly from the baseline levels. A similar decrease was not found in the other three patients who did not achieve remission (p = 0.006, p = 0.007 respectively) (). Patients who achieved complete remission were having classes II and III LN, while all those without remission were having class IV+V LN.
Follow up urinary IP-10 and sCD25 had a positive significant correlation with serum creatinine (p = 0.001, p < 0.001), 24 h urinary protein (p = 0.001, p = 0.012) and SLEDAI score (p = 0.001, p < 0.001).
3.6 ROC curves
Roc curve analysis for urinary IP-10 and sCD25 showed that both can significantly discriminate between active lupus nephritis and lupus patients without activity at a cut off level > 2716 pg/ml for urinary IP-10 with a sensitivity of 88% and specificity of 80% and at a cut off level > 27.9 ng/ml for sCD25 providing sensitivity of 84% and specificity of 65% (), ().
Fig. 5 ROC curve for urinary IP-10 and urinary CD25 to diagnose active LN vs SLE without activity (Group A vs B).
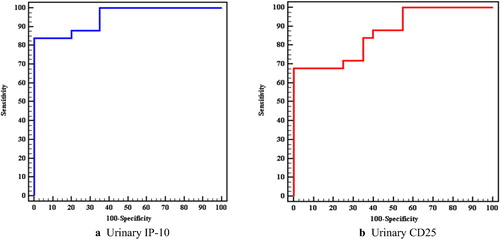
Table 3 Agreement (sensitivity, specificity) for urinary IP-10 and urinary CD25 to diagnose active LN vs SLE without activity (Group A vs B).
4 Discussion
Renal involvement in SLE contributes significantly to patient morbidity and mortality.Citation19 Hence, it is essential to find a noninvasive biomarker that could be used for early diagnosis of LN flares and for follow up.Citation20 This study illustrated the potential utility of urinary IP-10 and sCD25 as biomarkers of LN.
We found higher levels of urinary IP-10 and sCD25 in patients with active nephritis compared to inactive SLE patients and healthy controls.
CXCL10 promotes migration of T cells to sites of inflammation and down-regulates angiogenesis, together with its receptor, CXC receptor 3 (CXCR3).Citation5 In renal transplantation, many studies have shown the potential role of CXCL10 (IP-10) as an early marker of acute rejection. Rabant et al.Citation21 concluded that there is an association between urinary CXCL10:Cr ratio and tubulointerstitial and microvascular inflammation of the renal allograft. A lot of studies assessed serum IP-10 as a marker of active SLE and active lupus nephritis.Citation22–Citation24 A study by Lit et al.Citation23 showed that plasma concentrations of IP-10 were higher in SLE patients than in healthy individuals.
Similar findings of urinary IP-10 in lupus nephritis were reported by Marie et al.Citation25 in their study which included thirty patients with lupus nephritis and another 30 without evidence of lupus nephritis. Avihingsanon et al.Citation26 studied 26 patients with LN. They examined the urinary mRNA levels of IP-10, CXCR3, transforming growth factor-β (TGF-β), and vascular endothelial growth factor and concluded that class IV lupus nephritis patients had higher levels than other classes. Also, Abujam et al.Citation27 studied 136 patients with SLE from whom 78 were active (46 active renal and 32 active non-renal) finding no difference in serum levels of monocyte chemoattractant protein 1(MCP-1) and IP-10 between active renal and active non-renal SLE; only urinary levels were higher. It seems that the increased urinary excretion of IP-10 in active LN is mainly due to active local inflammation in the kidneys.
The present study illustrated the correlation between urinary IP-10 and different activity markers in active lupus nephritis finding a significant positive correlation with serum creatinine, proteinuria, anti ds-DNA, SLEDAI score (global) and renal SLEDAI score. With follow up, patients who achieved remission had significant lower levels of urinary IP-10 than baseline levels with persistent or higher levels in patients without remission. This finding corroborates with that reported by Abujam et al.Citation27 and Avihingsanon et al.Citation26 in their studies. Also, follow up urinary IP-10 showed a positive significant correlation with serum creatinine, 24 h urinary protein and SLEDAI score. However, Abujam et al.Citation27 found no correlation between change in SLEDAI and change in the IP-10 in the follow up period.
Urinary IP-10 levels were highest in class IV LN and lowest in class II LN and correlated well with activity and chronicity indices of renal biopsies.
In LN pathogenesis, T lymphocytes have a significant role. IL-2 is secreted after T-cell activation promoting T cell proliferation by binding to a high affinity receptor composed of three transmembrane proteins, α, β and γ chains with CD25 representing the α-chain of the interleukin-2 receptor (IL-2Rα).Citation28 Many studies reported that the CD25 region is associated with type1 diabetes and autoimmune thyroid diseases mainly Graves disease (GD).Citation29,Citation30 CD25 is highly expressed in CD4+CD25+T regulatory cells(Tregs) and is important for the production and function of Tregs, which actively suppress auto reactive T cells in the periphery.Citation31 Serum sCD25 had been studied as a marker of active SLE and active lupus nephritis by many authors.Citation32,Citation33
In this study, patients with active LN had higher levels of urinary sCD25 than inactive patients and healthy controls. Gupta et al.Citation34 in their study which included 119 patients [57 active nephritis (AN), 43 inactive disease (ID), 19 active non renal (ANR)] collected urine and serum samples for sCD 25 at baseline from all patients and at 3-monthly follow-up from patients with AN. Urinary sCD25 values in (AN) at baseline were significantly higher than inactive and control, but this relation was insignificant between AN and ANR group. At baseline, mean serum sCD25 levels for patients in the three groups of patients showed no significant difference. The levels of urinary sCD25 did not correlate with levels of serum sCD25. Similar findings had been reported by Tsai et al.Citation35 who studied urine samples from 23 patients with lupus nephropathy (active, inactive) and 15 patients with proteinuria who did not have (SLE) for the presence of cytokines, soluble interleukin 2 receptors (sIL-2R), and free light chain immunoglobulins reporting higher levels of sCD25 in patients with active nephritis.
The increased urinary sCD25 in active LN is mainly due to local production by infiltrating mononuclear or mesangial cells in the inflamed kidney not just filtration from the circulation through the damaged glomeruli. This is supported by the findings from studies showing that the urinary not serum levels of sCD25 can differentiate active LN from inactive disease.
Like IP-10, we evaluated the correlation between urinary sCD25 and different activity markers in active lupus nephritis finding a significant positive correlation with serum creatinine, proteinuria, anti ds-DNA, SLEDAI score (global) and renal SLEDAI score and a negative significant correlation with C3 and C4. However, Gupta et al.Citation34 found no correlation between urinary sCD25 and urine protein:creatinine ratio. Tsai et al.Citation35 stated that, the sIL-2R concentration in protein from urine was inversely correlated with the 24 h protein excretion in patients with SLE. Like urinary IP-10, we found that follow up levels of urinary sCD25 decreased significantly in patients achieving remission without a similar decrease in those without remission.
The highest urinary sCD25 levels were in patients with diffuse proliferative GN (class IV) and the lowest levels were in class II patients with a good correlation with activity and chronicity indices of renal biopsies. This might be explained by having more intense inflammation and more T lymphocyte activation in proliferative classes (III and IV) releasing more sCD25.
The strengths of our study are that we are among the earliest to study the relation between urinary IP-10 and sCD25 with the histopathological renal class in renal biopsy and the correlation with the different activity markers of LN. Another strength point is that we followed up our patients for 3 months and studied the relation with the response to treatment. Limitations of our study may be the small sample size and not including active non renal group.
5 Conclusion
Urinary IP-10/CXCL10 and sCD25/ IL-2Rα are potential biomarkers for early recognition and follow up of lupus nephritis and are highest in proliferative classes of LN.
Conflict of interest
The authors declare that they have no conflicts of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 11 December 2018
References
- S.C.SimmonsM.L.SmithA.Chang-MillerM.T.KeddisAntinuclear antibody-negative lupus nephritis with full house nephropathy: a case report and review of the literatureAm J Nephrol422016451459
- F.A.HoussiauC.VasconcelosD.D’CruzEarly response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term follow up of patients in the Euro-Lupus Nephritis TrialArthritis Rheum50200439343940
- S.L.GurevitzJ.A.SnyderE.K.WesselJ.FreyB.A.WilliamsonSystemic lupus erythematosus: a review of the disease and treatment optionsConsult Pharm282013110121
- C.C.LiuS.ManziJ.M.AhearnBiomarkers for systemic lupus erythematosus: a review and perspectiveCurr Opin Rheumatol172005543549
- L.MingliG.ShanchunM.H.JacquelineCXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implicationsCytokine Growth Factor Rev222011121130
- M.EnderlinE.V.KleinmannS.StruyfTNF-alpha and the IFN-gamma-inducible protein 10 (IP-10/CXCL-10) delivered by parvoviral vectors act in synergy to induce antitumor effects in mouse glioblastomaCancer Gene Ther162009149160
- R.A.ColvinG.S.CampanellaJ.SunA.D.LusterIntracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 functionJ Biol Chem27920043021930227
- N.KandaT.ShimizuY.TadaS.WatanabeIL-18 enhances IFN-gamma-induced production of CXCL9, CXCL10, and CXCL11 in human keratinocytesEur J Immunol372007338350
- S.NarumiT.TakeuchiY.KobayashiK.KonishiSerum levels of IFN- inducible protein-10 relating to the activity of systemic lupus erythematosusCytokine12200015611565
- C.J.HessN.FellerF.DenkersCorrelation of minimal residual disease cell frequency with molecular genotype in patients with acute myeloid leukemiaHaematologica9420094653
- M.ReddyE.EirikisC.DavisH.M.DavisU.PrabhakarComparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune functionJ Immunol Methods2932004127142
- G.I.GaborT.EdwardL.LarissaE.L.PeterBiomarkers in systemic lupus erythematosus: II markers of disease activityArthritis Rheum50200420482065
- P.MichelleO.Ana-MariaS.GracielaAlarcónG.CarolineDerivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosusArthrit. Rheum.64201226772686
- D.A.IsenbergA.RahmanE.AllenDisease activity index for patients with SLERheumatology442005902906
- M.PitashnyN.SchwartzX.QingUrinary lipocalin-2 is associated with renal disease activity in human lupus nephritisArthritis Rheum56200718941903
- J.J.WeeningV.D.D'AgatiM.M.SchwartzThe classification of glomerulonephritis in systemic lupus erythematosus revisitedJ Am Soc Nephrol22004241250
- F.A.HoussiauC.VasconcelosD.D’CruzThe 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamideAnn Rheum Dis6920106164
- Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int. 2012:139–274.
- Y.H.DesmondN.L.KarPathogenesis of renal disease in systemic lupus erythematosus—the role of autoantibodies and lymphocytes subset abnormalitiesInt J Mol Sci16201579177931
- N.SchwartzL.SuL.C.BurklyUrinary TWEAK and the activity of lupus nephritisJ Autoimmun272006242250
- M.RabantL.AmroucheX.LebretonUrinary C-X-C motif chemokine 10 independently improves the noninvasive diagnosis of antibody-mediated kidney allograft rejectionJ Am Soc Nephrol26201528402851
- Q.FuX.ChenH.CuiAssociation of elevated transcript levels of interferon-inducible chemokines with disease activity and organ damage in systemic lupus erythematosus patientsArthritis Res Ther102008R112
- L.C.LitC.K.WongL.S.TamE.K.LiC.W.LamRaised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosusAnn Rheum Dis652006209215
- K.O.KongA.W.TanB.Y.ThongEnhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosusClin Exp Immunol1562009134140
- M.A.MarieR.E.Abu KhalilH.M.HabibUrinary CXCL10: a marker of nephritis in lupus patientsReumatismo652013292297
- Y.AvihingsanonP.PhumesinT.BenjachatMeasurement of urinary chemokine and growth factor messenger RNAs: a noninvasive monitoring in lupus nephritisKidney Int692006747753
- A.AbujamBS.S.CheekatlaS.AggarwalUrinary CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritisLupus222013614623
- R.M.ThomasC.IrisInterleukin-2 receptor signaling: at the interface between tolerance and immunityImmunity332010153165
- A.VellaJ.D.CooperC.E.LoweLocalization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphismsAm J Hum Genet762005773779
- O.J.BrandC.E.LoweJ.M.HewardAssociation of the interleukin-2 receptor alpha (IL-2Rα)/CD25 gene region with Graves’ disease using a multilocus test and tag SNPsClin Endocrinol662007508512
- A.L.BayerA.YuD.AdeegbeT.R.MalekEssential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal periodJ Exp Med2012005769777
- E.M.DavasA.TsirogianniI.KappouD.KaramitsosI.EconomidouP.C.DantisSerum IL-6, TNFα, p55 srTNFα, p75srTNFα, srIL-2α levels and disease activity in systemic lupus erythematosusClin Rheumatol1819991722
- D.DejicaSerum soluble IL-2 receptor as a marker of lymphocyte activation in some autoimmune diseases: effect of immunosuppressive therapyRoum Arch Microbiol Immunol602001183201
- R.GuptaA.YadavR.MisraA.AggarwalUrinary sCD25 as a biomarker of lupus nephritis disease activityLupus242015273279
- C.-Y.TsaiT.-H.WuK.-H.SunW.M.LinC.L.YuIncreased excretion of soluble interleukin 2 receptors and free light chain immunoglobulins in the urine of patients with active lupus nephritisAnn Rheum Dis511992168172