Abstract
Background
Different anesthetic techniques may have different effects on the immune response of patients undergoing the same type of surgery. The aim of the work is to evaluate the effects of two anesthetic techniques: total intravenous anesthesia (TIVA) with propofol versus volatile induction and maintenance anesthesia (VIMA) with sevoflurane, on cellular immune functions in patients undergoing meniscectomy.
Patients and methods
50 Adult patients scheduled for meniscectomy were randomly assigned into two groups: TIVA group (Group T: n = 25), and VIMA group (Group V: n = 25). A blood sample was collected 24 h before surgery (Sbasal). The first sample on the day of operation was collected just before the induction of anesthesia (S1), then 3 h later after induction (S2), and lastly 24 h after surgery (S3). CRP levels were assayed and T-lymphocyte subpopulations were investigated by flow-cytometry.
Results
CRP was increased at (S1) and reached its maximum levels at (S3) in both groups. CRP levels at (S3) were significantly higher in TIVA compared to VIMA groups. A significant decrease in the percentage of CD3, CD4 and decreased CD4/CD8 ratio occurred at (S2) and (S3) in both groups but more significantly with TIVA. CD8 showed no significant changes in both groups. NK cells decreased significantly at (S2) and (S3) in both groups, with no significant differences between the two groups. The percentage of HLA-DR showed no significant changes all through the study time in both groups.
Conclusion
VIMA with sevoflurane is less immunosuppressive than TIVA with propofol in patients undergoing minor surgery.
1 Introduction
General anesthesia and surgical stress have a major role in affecting patients’ cellular immunity [Citation1]. Different anesthetic techniques may have different effects on the immune response of patients undergoing the same type of surgery [Citation2]. Since both anesthesia and surgery affect the immune system in many ways, a large number of studies have been paying concern to the perioperative immune responses [Citation1]. Moreover, the effect of anesthetics per se on the immune system has taken a lot of attention [Citation3].
A large number of in vitro cellular immunity studies were performed upon T-lymphocytes to explore the effect of inhalational anesthetics upon them, and they revealed inhibition of T-lymphocytes functions [Citation4,Citation5]. In vivo investigations of T-lymphocyte functions revealed that the effect of both anesthesia and surgery lead to combined suppression of the proliferative response as well as the number of circulating T-lymphocytes [Citation6]. However, other studies have shown no alteration of T lymphocytic functions in individuals working in the field of anesthesia [Citation4,Citation7].
Propofol is a commonly used intravenous anesthetic which is characterized by its short duration of action and has been effectively used for induction and maintenance of anesthesia [Citation8].
Sevoflurane is a general inhalational anesthetic that can be used for inhalational induction and maintenance of general anesthesia due to its non-irritant and pleasant odor [Citation9]. Sevoflurane depressed the release of inflammatory mediators and reduced neutrophil accumulation in an in vitro study incorporating a model of endotoxin-induced injury in alveolar epithelial cells [Citation10]. In an animal study, the impact of sevoflurane anesthesia on the immune system was evaluated without surgery. In that study, sevoflurane decreased the number of peripheral blood lymphocytes and stimulated T-helper (CD4) lymphocytes [Citation11].
The aim of the present study is to evaluate the effects of two anesthetic techniques: total intravenous anesthesia (TIVA) with propofol versus volatile induction and maintenance anesthesia (VIMA) with sevoflurane, on cellular immune functions in patients undergoing meniscectomy.
2 Patients and methods
After the approval of our local ethics committee, 50 adult patients (American Society of Anaesthesiologists class I and class II) scheduled for meniscectomy with a written informed consent were enrolled in the present study. Exclusion criteria were preexisting chest diseases (determined by clinical examination, chest X-ray), insulin-dependent diabetes mellitus, and renal, hepatic, or cerebrovascular diseases. Patients with preoperative signs of infection (such as white blood cells (WBCs) count >12,000/μL, body temperature >38 °C, and C-reactive protein [CRP] >5 mg/dL), immune system related disorders, endocrine pathologies, allergic reactions, pregnancy or contraceptive bills, and steroids or other immunologic therapies were also excluded.
Patients were randomly assigned into two groups: TIVA group (Group T), VIMA group (Group V). Patients were given consecutive numbers and those with odd numbers received TIVA while those with even numbers received VIMA.
All patients were premedicated with 0.1 mg/kg midazolam and 0.5 mg atropine intramuscularly 1 h before surgery. All patients were also given a loading dose of fentanyl 1 μg/kg i.v. and 100% oxygen via a face mask for 2–3 min before induction. Supplementary doses of fentanyl were administered intraoperatively as required.
In Group T, induction was performed using propofol 1–2.5 mg/kg and anesthesia was maintained with propofol 4–8 mg/kg/h. In Group V, induction was done using a face mask with sevoflurane starting at 8% with an initial fresh gas flow (FGF) of 6 L/min for 1–3 min and reaching down to a FGF of 3 L/min during maintenance together with 1.5–3.5% sevoflurane. For all patients, rocuronium (Esmeron) 0.7 mg/kg i.v. was administered to facilitate tracheal intubation. Muscle relaxation was maintained using incremental doses of rocuronium (0.07 mg/kg) as required. Patients were ventilated to maintain an end-tidal PCo2 of 35–45 mm Hg throughout the whole procedure. Depth of anesthesia was monitored by clinical signs and hemodynamic responses to surgical stimuli. When adequate depth was achieved, maintenance dose of either propofol or sevoflurane was kept constant. ECG, pulse oximetry, capnography and non-invasive blood pressure monitoring were also applied to all patients.
After skin closure, abrupt discontinuation of either sevoflurane or propofol and reversal of residual muscle relaxation were done and the lungs were ventilated with 100% oxygen at a flow rate of at least 6 L/min.
2.1 Blood samples collection
For all patients four venous blood samples were collected in two tubes with ethylendiamine tetra-acetic acid (EDTA) one for fresh flow-cytometry analysis and the other for CRP analysis. A sample was collected 24 h before surgery (Sbasal) to achieve the basal findings of the patient. The first sample on the day of operation was collected just before the induction of anesthesia (S1), the second sample was collected 3 h after induction (S2) and the third sample was collected 24 h after surgery (S3).
2.2 CRP detection
After being centrifuged, plasma was stored at −20 °C until assayed. CRP levels were assayed by immunoturbidimetric method with Roche kits, using modular analytics P module (Roche Diagnostics, Manheim, Germany). The sensitivity, dynamic range, and accuracy of this test were the following: 0.425%, 1–280%, and 4.61% for CRP. The total protein concentrations of blood samples were measured and the level CRP was corrected using the changes in the total protein concentrations, in order to eliminate the effect of hemodilution on the test results postoperatively.
2.3 Flow cytometric analysis
The immune system represented by T-lymphocytes and its subpopulation and monocytes were investigated by FACSCalibur flow-cytometer (Becton Dickinson Biosciences, San Jose, USA). A selected panel (CD3, CD4, CD8, CD56, CD14 and HLA-DR) had been analyzed in all cases. The antibodies’ combination for flow cytometry was labeled with FITC and PE. All monoclonal antibodies used were purchased from Becton Dickinson. Cells were stained by whole blood protocol [Citation12]. Peripheral blood 50 μL aliquots were immuno-stained with Moab (20 min at 4 °C), then incubated for 10 min in 2 mL of diluted (1:10) lysing solution, washed three times in phosphate-buffered saline (PBS) containing 1% BSA, incubated in 10% human AB serum, and stained cells re-suspended in 0.5 mL PBS. Isotype controls were run with each sample to distinguish the positive cells from the negative cells.
A total of 10,000 events were acquired for analysis using a FACSCalibur flow-cytometer equipped with CellQuest (BD Biosciences). A First gate (G1) on the lymphoid population according to typical light scatter characteristics was done to determine the positivity of CD3 (T-lymphocytes), CD4 (T-helper), CD8 (T-suppressor) and CD56 (NK cells). A second gate (G2) was done on monocyte population with higher light scatter characteristics than the lymphoid population to determine the expression of monocytoid HLA-DR. A marker was considered “+” if expressed by at least 20% of the analyzed cells in the lymphocytes gate.
2.4 Statistical analysis
According to a pilot study (10 patients in each group), we calculated that 25 patients per group were sufficient to give P < 0.5 significance with a confidence interval 95% with a power of 80%.
Statistical analysis was performed using the SPSS software (SPSS, Chicago, IL, USA). All values were given as mean ± standard deviation (SD) or as median [range]. Paired and non-paired t-tests, Mann Whitney U, and Wilcoxon tests were applied. Differences were considered significant at (P < 0.05).
3 Results
There were no significant differences regarding the general data of the patients included in the present study (). There were no significant differences regarding patients’ ages, body weights and anesthesia time between the two groups.
Table 1 Patients’ general data.
CRP levels were increased just before the induction of anesthesia (S1) in both groups and remained significantly higher than base line levels (Sbasal) all through the study time. The levels obtained 24 h after surgery (S3) were significantly higher than those obtained 3 h after induction of anesthesia (S2) in both groups. CRP levels in propofol group were increased 24 h after the induction of anesthesia more significantly than in the sevoflurane group at the same time ().
Table 2 Comparison between the levels of CRP (mg/dl) in the two groups in the different samples.
Flow-cytometric analysis detecting T-lymphocytic subpopulations in the present study showed significant decrease in the percentage (mean ± SD) of CD3 and CD4 T lymphocytes as well as decreased CD4/CD8 ratio 3 h after induction of anesthesia and these changes remained 24 h after the induction of anesthesia. The decreases in CD3, CD4, and CD4/CD8 ratio in propofol group were more significant than those in the sevoflurane group. The percentage of CD8 T lymphocytes showed no significant changes in both groups. NK cells (CD56) percentage showed a significant decrease 3 h after the induction of anesthesia and remained significantly decreased 24 h later in both groups, with no significant differences between the two groups ().
Table 3 Comparisons between the percentages of lymphocytes subpopulations in the two groups in different samples.
The percentage of Human leukocyte antigen (HLA-DR) on monocytes showed no significant changes all through the study time, and there were no significant differences between the two groups ().
Table 4 Comparisons between the percentages of monocytoid HLA-DR in the two groups in different samples.
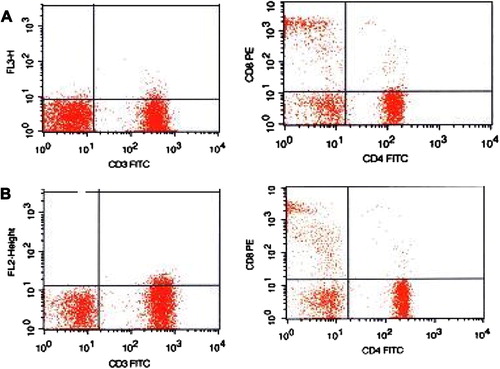
shows flowcytometric analysis plotting the expression of CD3, CD4 and CD8 for 2 patients 24 h after surgery. Patient (A) was on TIVA and patient (B) was on VIMA.
4 Discussion
The present study compared the immuno-modulatory effects of two different anesthetic techniques: total intravenous anesthesia (TIVA) with propofol versus volatile induction and maintenance anesthesia (VIMA) with sevoflurane in patients undergoing meniscectomy and revealed that VIMA with sevoflurane produced less immuno-suppressive effects than TIVA with propofol. In the current study, Both techniques lead to immune-suppression, as shown by increased C-reactive protein (CRP) and disturbances in T-lymphocytes subpopulation of the peripheral blood, in the form of decreased CD3, T-helper lymphocytes (CD4), and natural killer cells (NK) percentages as well as decreased T-helper/T-suppressor lymphocytes (CD4/CD8) ratio during the postoperative period. However, this immune-suppression was more prominent with propofol compared to sevoflurane.
There may be too many factors that might lead to the impairment of postoperative immunity when anesthesia and surgery are combined; including systemic illness, pathology involved, length of surgery, anesthetics and anesthetic techniques [Citation2]. Some might suggest that performing similar immuno-modulatory research in patients performing major surgeries would reflect different results. However, one of the targets of the current study was to limit the effect of surgery, thus, meniscectomy was chosen (being a simple procedure with small incisions with minimal stress and pain).
Alterations in acute phase reactants (APR) might occur in response to systemic inflammation which occurs in association with many conditions such as infection or trauma. Normal values of C-reactive protein (CRP) may be 0–1.0 mg/dL [Citation13]. The effect of anesthesia on acute phase reactants was evaluated in many studies with controversial results [Citation14,Citation15]. Elisena and coworkers [Citation10] compared the effect of anesthesia with sevoflurane to propofol in one lung ventilation (OLV), their study showed significant increase in CRP levels with propofol compared to attenuate and non significant increase in CRP with sevoflurane. They even suggested a possible anti-inflammatory effect of sevoflurane. In the current study, CRP was elevated in both groups, yet it was more significantly elevated with propofol compared to sevoflurane.
Macrophages engulf antigens. Every antigen shows itself on the surface of the macrophages. T-lymphocytes respond to that by expressing antibodies on their membranes. These antibodies are named “clusters”. CD stands for “clusters of differentiation”, meaning that the lymphocytic subpopulation can be differentiated from each other through the antibodies expressed on their membranes [Citation13].
CD3 is a protein found on the surface of T-cells. This protein is necessary for the activation of an immune response of T-cells [Citation16]. An in vitro study compared the effects of sevoflurane, isoflurane, and desflurane on human CD3 T-lymphocytes from healthy volunteers. In that study, sevoflurane induced both time and concentration dependent suppression of CD3T-lymphocytes. They thought that sevoflurane possess an anti-inflammatory effect that might be attributed to an intracellular molecular mechanism which was only detected with sevoflurane but not with isoflurane or desflurane [Citation17]. In the present study, CD3 decreased in both groups; however, it was more significantly decreased with propofol compared to sevoflurane.
T-lymphocytes have two major types, one presenting the CD4 antigen on its surface (T-helper) and the other presenting the CD8 antigen (T-suppressor) [Citation18]. The ratio of CD4/CD8 is a valuable measure to assess the disturbances affecting the immune system. Ratios of CD4/CD8 1.5–2.5 are considered normal [Citation19]. Most of the researches concerned with studying the levels of T-lymphocytes in minor surgeries showed negligible alterations in CD4/CD8 ratio [Citation20]. However; suppressor lymphocyte activity, which attenuates host defensive mechanisms was found to be increased with sevoflurane with resultant lowering of CD4/CD8 ratio [Citation21]. In their study, Durlu and coworkers [Citation21] stated that although CD4/CD8 ratio was decreased with sevoflurane and increased with isoflurane during the postoperative period, these changes were statistically insignificant. In the present study, depression of CD4/CD8 ratio occurred during the postoperative period in both groups, with more significant suppression accompanied with propofol compared to sevoflurane.
A study by Mustafa and his colleagues [Citation22] thought that the immune-suppression produced by TIVA with propofol is related to the high lipid content of propofol preparations and may be attributed to its release of cytokines. However, this study differs from the current study in that we compared total intravenous general anesthesia with propofol versus total inhalation general anesthesia with sevoflurane, while that study compared two intravenous agents (propofol vs thiopental).
However, another study [Citation23] was not in agreement with the results obtained in the current study. In that study compared the immunological effects of sevoflurane were compared to that of propofol in patients undergoing laparoscopic cholecytectomy. They found that propofol group was accompanied by a higher ratio of CD4/CD8 and lower cortisol levels indicating that propofol might even exert an immunoprotective effect. This study might be criticized for small sample size (14 patients in each group) which might not be enough to support such conclusions.
One of the T-lymphocytic subpopulations is the natural killer (NK) cells which improve bacterial clearance by priming macrophages to assist clearing the next microbial challenges [Citation24], besides its cytotoxic effect against malignant cells, microbial infections and some primitive normal cells [Citation25]. NK cell activity is thought to be suppressed by anesthetics [Citation26]. However; some in vivo and in vitro studies revealed that the effect of anesthesia alone on the NK cells is limited and reversible [Citation27,Citation28]. In the current study, NK cells decreased during the postoperative period for both TIVA and VIMA techniques. However, more significant decrease in NK cells occurred with total intravenous general anesthesia with propofol versus total inhalational general anesthesia with sevoflurane.
The mission of the human immune system is to defend the body against exterior invasion. In order to perform this function properly, the immune system must be able to distinguish between the subject’s own cells and other invading organisms. This function can be done through the molecules of the major histo-compatibility complex (MHC) [Citation2]. One of these molecules is the human leukocyte antigen (HLA). HLA molecules, and the genes encoding them, can be divided into three categories: class I, class II, and class III. HLA-DR belongs to class II molecules. The function of class II molecules is to serve antigenic peptide fragments to CD4 T-lymphocytes during the beginning of immune responses [Citation29]. Monocytes and macrophages express class II HLA-DR. This HLA-DR expression on monocytes is one of the keys of immune system responses because of the crucial role that HLA-DR plays in presenting antigen to T lymphocytes [Citation30].
The degree of suppression of (HLA-DR) expression signifies the degree of alteration of immune status in patients after stressful insult [Citation31], and may express potential risk of infection [Citation32] severe immuno-suppression [Citation29], or immuno-incompetence [Citation33]. Also, the stress of anesthesia and surgery might lead to suppression of monocytes with resultant decrease in monocytic HLA-DR expression [Citation29].
Asadullah and coworkers [Citation32] compared the degree of suppression of HLA-DR expression during intravenous anesthesia with propofol, to balanced anesthesia with isoflurane or sevoflurane. Their results showed no significant alterations all through the study time except at the third day postoperatively when a decrease in the HLA-DR expression occurred. They thought that the depression of HLA-DR expression was a sign of immune-suppression caused by infectious complications rather than anesthesia. On other hand, in another study done by Tuna and coworkers [Citation2], an increase in monocytic HLA-DR expression occurred in the third postoperative day which might be attributed to the improvement of the immune function. In the present study, control values for monocytic HLA-DR expression were the patients’ own preoperative values. Suppression of HLA-DR expression was not detected in both groups all through the study times indicating that neither propofol nor sevoflurane affects HLA-DR expression on monocytes.
5 Conclusion
VIMA with sevoflurane is less immunosuppressive than TIVA with propofol in patients undergoing minor surgery. Further studies will be needed to ensure that these results could help anesthetists to select safer anesthetic circumstances especially for immuno-compromised patients performing different types of surgeries.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- Y.HoryT.IbukiT.HosokawaY.TanakaThe effect of neurosurgical stress on peripheral lymphocyte subpopulationsJ Clin Anesth15200318
- N.TunaM.TunaD.KocaF.AticiA.AriboganThe effects of three neurosurgical anaesthetic methods on HLA-Dr expressionTurk J Med Sci3220026571
- M.BauerH.RensingT.ZiegenfussAnesthesia and perioperative immune functionAnaesthesist471998538556
- A.KoenigU.D.KoenigB.BinholdH.StoeckelDifferences in lymphocyte mitogenic stimulation pattern depending on anaesthesia and operative trauma: II. Combined neurolept anaesthesiaEur J Anaesth419872533
- G.W.StevensonS.HallP.J.MillerG.AlvordJ.B.LeventhalF.SelenyH.C.StevensonThe effect of anesthetic agents on human immune system function. I. Design of a system to deliver inhalational anesthetic agents to leukocyte cultures in vitroJ Immunol881986277283 Methods
- B.WaltonAnaesthesia, surgery and immunologyAnaesthesia331978322348
- M.SaloM.VapaavuoriPeripheral blood T- and B-lymphocytes in operating theatre personnelBr J Anaesth481976877880
- I.SmithP.F.WhiteM.NathansonR.GouldsonPropofol: An update on its clinical useAnesthesiology81199410051043
- E.D.KharaschM.D.KarolC.LanniR.SawchukClinical sevoflurane metabolism and disposition; sevoflurane and metabolite pharmacokineticsAnesthesiology82199513691378
- D.C.ElisenaP.S.MarcW.MoritzZ.P.MarcoW.WalterS.DidierS.C.RalphK.RichardN.A.ThomasS.R.EdithS.R.DonatB.Z.RothU.MartinB.BeatriceAnesthetic-induced improvement of the inflammatory response to one-lung ventilationAnesthesiology110June 200913161326
- N.R.PuigP.FerreroM.L.BayG.HidalgoJ.ValentiN.AmerioG.ElenaEffects of sevoflurane general anesthesia: immunological studies in miceInt Immunopharmacol21Jan 200295104
- V.C.MainoM.A.SuniJ.J.RuitenbergRapid flow cytometric method for measuring lymphocyte subset activationCytometry201995127133
- C.GabayI.KushnerAcute-phase proteins and other systemic responses to inflammationN Engl J Med3401999448
- P.SheeranG.M.HallCytokines in anaesthesiaBr J Anaesth7821997201219
- V.Brix-ChristensenE.TonnesenI.J.SorensenT.V.BilfingerR.G.SanchezG.B.StefanoEffects of anaesthesia based on high versus low doses of opioids on the cytokine and acute-phase protein responses in patients undergoing cardiac surgeryActa Anaesthesiol Scand42119986370
- M.M.DavisA new trigger for T-cellsCell11032002285287 Review
- Torsten L, Patrick S, David D, Frank M, Matjaz H, Martin R, Alexander H, Rene S, Burkhard M, Klaus G, Heike LP, Benedikt HJP. Sevoflurane inhibits phorbol-myristate-acetate-induced activator protein-1 activation in human T lymphocytes in vitro: potential role of the p38-stress kinase pathway. Anesthesiology, Sept 2004: 101-3-710-721.
- A.AmadoriR.ZamarchiG.De SilvestroG.ForzaG.CavattonG.A.DanieliM.ClememtiL.Chieco-BianchiGenetic control of the CD4/CD8 T-cell ratio in humansNat Med1199512791283
- T.LiZ.QiuY.HanZ.WangH.FanW.LuJ.XieX.MaA.WangRapid loss of both CD4+ and CD8+ T lymphocyte subsets during the acute phase of severe acute respiratory syndromeChin Med J1162003985987
- M.A.ProcopioA.J.RassiasJ.A.DeLeoJ.PahlL.HildebrandtM.P.YeagerThe in vivo effects of general and epidural anesthesia on human immune functionAnesth Analg932001460465
- N.DurluY.BatislamO.OzatamerThe effects of isoflurane and sevoflurane on immune system in minor surgical interventionsJ Ankara Med School2432002105112
- AltindisMustafaAribasEmel TürkKartAliVatansevCelalettinComparison of immunological effect of propofol and thiopentone on the immune systemJ Turgut Özal Med Center41997187192
- J.Fu-haiW.Yu-lanY.Jian-pingEffects of propofol anesthesia and sevoflurane anesthesia on the differentiation of human T-helper cells during surgeryChin Med J12442011525529
- M.J.ScottJ.J.HothS.A.GardnerJ.C.PeytonW.G.CheadleNatural killer cell activation primes macrophages to clear bacterial infectionAm Surg692003679686
- R.B.HerbermanC.W.ReynoldsJ.R.OrtaldoMechanism of cytotoxicity by natural killer (NK) cellsAnnu Rev Immunol41986651680
- B.BeilinY.ShavitJ.HartB.MordashovS.CohnI.NottiH.BesslerEffects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative periodAnesth Analg821996492497
- C.D.M.GriffithM.B.KamathEffect of halothane and nitrous oxide anaesthesia on natural killer lymphocytes from patients with benign and malignant breast diseaseBr J Anaesth581986540543
- G.M.WoodsD.M.GriffithsReversible inhibition of natural killer cell activity by volatile anaesthetic agents in vitroBr J Anaesth581986535539
- C.H.WakefieldP.D.CareyS.FouldsJ.R.MonsonP.J.GuillouChanges in major histocompatability complex class 2 expressions in monocytes and T cells of patients developing infection after surgeryBr J Surg801993205209
- E.BenjaminiS.LeshowitzImmunology, a short course1991Wiley-Niss IncNew York pp. 175–82
- O.KimA.MonselM.BertrandP.CoriatJ.CavaillonM.Adib-ConquyDifferential down-regulation of HLA-DR on monocyte subpopulations during systemic inflammationCrit Care142010R61
- K.AsadullahC.WoiciechowskyW.DöckeImmunodepression following neurosurgical proceduresCrit Care Med23199519761982
- R.Y.LinM.E.AstizJ.C.SaxonE.C.RackowAltered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, CD11b, CD14, and IL-2R expressionChest10431993847853