Abstract
Background
Endotracheal tube tolerance is a major concern in patients who require postoperative retention of endotracheal tube following major head and neck cancer surgeries. This study investigates the role of Dexmedetomidine for endotracheal tube tolerance, opioid sparing effect and sedation.
Methods
Prospective randomised double blind control study. Sixty-four patients scheduled for head and neck surgery lasting longer than 2 h who require post operative endotracheal tube retention were randomly allocated into two groups of 32 each. The Dexmedetomidine group (Group D, n = 32) received Dexmedetomidine 1 μg kg−1 diluted to 10 ml, i.v. over 10 min prior to reversal and thereafter 0.2 μg kg−1 h−1 i.v. infusion till extubation while the control group (Group P, n = 32) received 10 ml normal saline i.v. over 10 min prior to reversal and volume-matched normal saline infusion as placebo till extubation. The two groups of patients were compared for haemodynamic changes during administration of the bolus drug, after reversal and in the ICU. The quality of endotracheal tube tolerance, analgesic requirement and sedation were also compared between the two groups in the ICU.
Results
Group D tolerated the endotracheal tube better than Group P based on cough (p < 0.01) and nurses’ judgement (p < 0.01). Group D showed 72% decrease in morphine requirement (p < 0.05), experienced arousable sedation (p < 0.05) and the vital parameters were better preserved (p < 0.05) than Group P.
Conclusions
Patients receiving Dexmedetomidine tolerated the endotracheal tube better, required lesser morphine, were adequately sedated, arousable, stable haemodynamics and lacked respiratory depression.
Introduction
Postoperative airway obstruction is a relatively common complication after anaesthesia for head and neck surgery [Citation1]. Prolonged surgery, tissue oedema and possible post operative bleeding can result in airway compromise.
In a patient undergoing lengthy head and neck surgical procedure, the trachea may remain intubated and the patient sedated overnight in the intensive care unit [Citation2]. The goal is to prevent pain, provide sedation and limit reflex responses to the endotracheal tube. Tolerance of the endotracheal tube is facilitated by intravenous hypnotics (e.g., propofol) and opioids (e.g., fentanyl, morphine) in low doses.
The danger of respiratory depression with these drugs often necessitates their discontinuation before extubation leading to patient anxiety and discomfort. The possibility of continuing sedation throughout extubation without significant respiratory impairment makes Dexmedetomidine a favourable option [Citation3,Citation4].
.1 Aims and objectives
Major head and neck cancer surgery patients require to retain the endotracheal tube postoperatively for 16–20 h. Endotracheal tube tolerance is a major concern in this context.
Dexmedetomidine has been proved to be effective for its opioid sparing effect, rousable sedation and lack of respiratory depression with haemodynamic stability [Citation3–Citation8]. This study investigates whether Dexmedetomidine will be a better alternative than routinely used drugs for alleviating discomfort and pain due to endotracheal tube maintaining high degree of patient rousability.
Besides the primary objective, the study also investigates the effect of Dexmedetomidine on the haemodynamic variables and the overall ease of patient management.
Materials and methods
The study was approved by the institutional review board and ethics committee (Ref:HEC.No.29/2010).
A prospective randomised double blind control study was initiated. The study population included 64 Patients, ASA I and II in the age group 20–70 yrs scheduled for elective head and neck surgery lasting longer than two hours who need to retain the endotracheal tube in the post operative period. Of these, 32 patients received Dexmedetomidine (Group D) and 32 patients received placebo saline (Group P) (see ). Written informed consent was taken from each patient.
.1 Inclusion criteria
ASA I and ASA II patients in the age group 20–70 yrs scheduled for elective head and neck surgery lasting longer than 2 h who require to retain the endotracheal tube in the post operative period were included in the study.
.2 Exclusion criteria
Patients below 20 yrs and above 70 yrs of age, ASA grade more than II, patient with arrhythmias, patient with congestive heart failure, patient with dementia, patient with heart block and pregnant patients were excluded.
.3 Premedication
All patients were kept nil orally from 12 midnight prior to day of surgery. All patients were premedicated with Tab. Alprazolam 0.25 mg – 0.5 mg, Tab. Pantoprazole 40 mg and Tab. Domperidone 10 mg orally previous night and 6 am on the day of surgery.
.4 Anaesthesia technique
On arrival at the OT, baseline heart rate, NIBP, SPO2 values were recorded. The patients were premedicated with Inj. Midazolam 1 mg i.v., Inj. Glycopyrrolate 0.2 mg i.v. and Inj. Fentanyl 2 μg kg−1 i.v. Two drops of Xylometazoline nasal drops 0.1%, were instilled into each nostril.
General anaesthesia was induced with propofol 2–2.5 mg kg−1 i.v. Inj. Vecuronium 0.1 mg kg−1 was given i.v. to facilitate nasal endotracheal intubation using 7.5 mm cuffed tube for males and 7 mm cuffed tube for females. The endotracheal tube was secured after confirming the position of the tube by auscultation and ETCO2. Following placement of the endotracheal tube, anaesthesia was maintained with 66% Nitrous oxide in oxygen 2:1 ratio at fresh gas flow of 1.5 litres/min and Isoflurane 0.5 – 2% using closed circuit. Inj. Vecuronium was given in 1 mg increments to maintain neuromuscular blockade. No additional opioid was given after induction.
Intraoperatively, Inj. Diclofenac 75 mg was given as slow intravenous infusion. Inj. Ondansetron 4 mg intravenous was given as antiemetic. Nitroglycerin i.v. infusion was used in titrated doses to maintain mean arterial pressure of 60 mmHg. Warming blankets were used to maintain normothermia. ECG, NIBP, SPO2, ETCO2 were monitored throughout the procedure.
Ten minutes prior to administration of reversal, the study drug and placebo were given according to group allocation as per computer generated random numbers in a double blinded manner. Group D received a bolus of Dexmedetomidine 1 μg kg−1 diluted to 10 ml with normal saline i.v. over 10 min. Group P received a bolus of 10 ml normal saline i.v. over 10 min.
Heart rate and B.P were recorded at the start of the bolus drug injection and at 1 min, 3 min, 5 min, and 10 min after administration of the drug. At the end of surgery, neuromuscular block was antagonised with Neostigmine 0.05 mg kg−1 i.v. and glycopyrrolate 0.01 mg kg−1 i.v. Subsequently 1 min, 3 min, 5 min, 10 min and 15 min after reversal, the heart rate and blood pressure were recorded. Coughing after reversal was also noted. Morphine 0.05 – 0.1 mg kg−1 i.v. bolus was given for intolerance to endotracheal tube, pain and agitation. Ephedrine 6–12 mg i.v. bolus was given for hypotension [decrease in systolic pressure >20% from baseline]. Atropine 0.6 mg i.v. bolus was given for bradycardia. [Heart rate <45/min.] The patient was then shifted to the post surgical intensive care unit with the nasal endotracheal tube in situ.
On arrival at ICU, Dexmedetomidine i.v. infusion at 0.2 μg kg−1 h−1 or volume matched normal saline i.v. infusion was started as per group allocation and continued till extubation. In the ICU, the nurses were blinded to the intravenous drug infused. Heart rate, NIBP and SpO2 were recorded every 15 min for the 1st hour, then hourly for 5 h and subsequently every 2 h till extubation. The sedation level, coughing, morphine requirement and endotracheal tube tolerance of the patients, as judged by the nurses, were also recorded. Inj. Morphine 0.05 – 0.1 mg kg−1 i.v. bolus was given for intolerance to endotracheal tube, pain and agitation. Ephedrine 6–12 mg i.v. bolus was given for hypotension [decrease in systolic pressure >20% from baseline]. Atropine 0.6 mg i.v. bolus was given for bradycardia. [Heart rate <45/min.]
.5 Statistical analysis
The minimum group size of 64 was calculated in order to achieve a study power of 80% with α error of 5%. The data was presented as mean (SD). Statistical analysis was done by software SPSS (Statistical package for social sciences) version 11.0. Statistical analysis was performed using appropriate application of statistical tools-Chi square test, independent ‘t’ test and Fisher’s exact test. P < 0.05 was considered significant.
Results
The two groups were comparable in patient characteristics, baseline vital signs and duration of surgery [].
able 1 Patient demographics and baseline cardiorespiratory parameters of Group D (Dexmedetomidine) and Group P (Control).
Prior to administration of the reversal drug, as the bolus drug was administered the heart rate between the two groups was comparable till 3 min and thereafter there was a significant decrease in heart rate in patients of group D compared to group P (p < 0.05). The systolic blood pressure was comparable at all times, but at 10 min, patients of group D had significantly lower systolic blood pressure compared to group P (p < 0.01).
After administration of the reversal drug, the patients in group D had significantly lower heart rate (p < 0.01), systolic blood pressure (p < 0.01) and diastolic blood pressure up to 15 min compared to group P. There was significantly lesser coughing at 1 min and 3 min in group D compared to group P (p < 0.05). The frequency of bolus morphine i.v. administered was significantly lesser in the patients of group D compared to group P (p < 0.01).
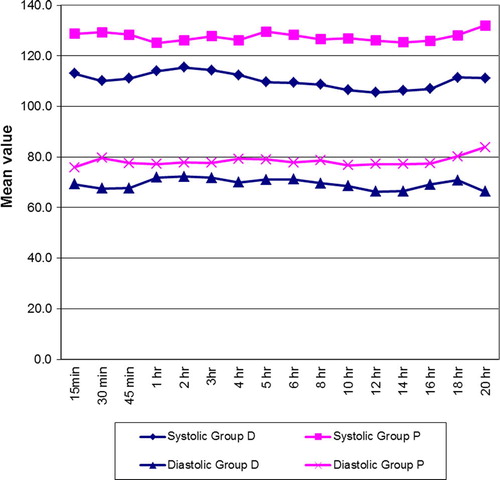
In the ICU, as the patients received drug infusion, the patients in group D had significantly lower heart rate compared to group P (p < 0.01) []. The patients in group D had lower systolic and diastolic BP compared to group P [].
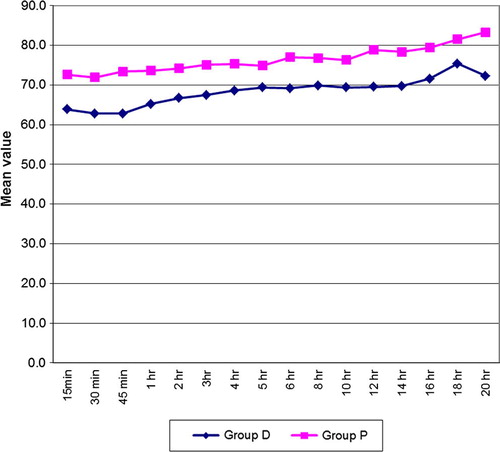
Patients in group D maintained respiratory rate better than group P (p < 0.05). Group D showed better oxygen saturation than group P (p < 0.05).
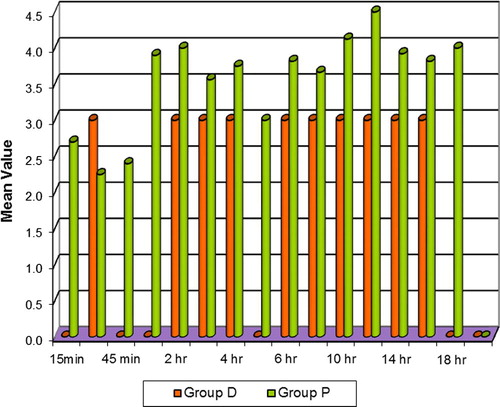
The frequency and dose of morphine requirement in the ICU was significantly lesser in group D compared to group P (p < 0.05) []. Coughing was significantly lesser (p < 0.01) and milder in Group D than group P especially at 45 min, 3 h, 5 h, 12–18 h (p < 0.05) in the ICU [].
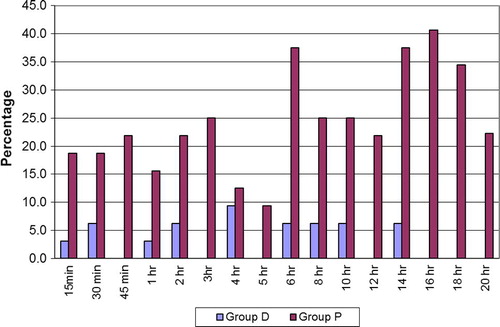
The patients in group D were better sedated and easily arousable in the ICU compared to group P, which was statistically significant (p < 0.05) at 15 min, 30 min and 18 h, (p < 0.01) at 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, 10 h, 14 h and 16 h.
The nurses judged that patients in group D tolerated the endotracheal tube better than group P in the ICU. (p < 0.01) [].
Discussion
We conducted this prospective randomised study to determine the role of Dexmedetomidine as a better alternative than routinely used drugs for endotracheal tube tolerance, analgesia and sedation.
Dexmedetomidine is a highly selective α2 agonist that possesses hypnotic, sedative, anxiolytic, sympatholytic, analgesic and anaesthetic sparing effects without causing significant respiratory depression [Citation9–Citation12]. It causes dose-dependent decrease in arterial blood pressure and heart rate associated with decrease in plasma catecholamines [Citation13].
As the bolus drug was administered, the patients in Group D had a significantly lower heart rate than Group P from 3 min onwards. None of the patients in Group D required intervention for bradycardia. Patients in Group D had a lower systolic BP at all times and the difference was statistically significant at 10 min. Four patients in Group D required intervention for hypotension.
Following administration of the reversal drug, there was a significant decrease in heart rate in the patients of Group D at all times for 15 min compared to the Group P (p < 0.01). The systolic BP after reversal was significantly lower in Group D compared to Group P at all times for 15 min (p < 0.01). There was significantly lesser coughing experienced at 1 min (p < 0.01) and 3 min (p < 0.05) by patients of Group D. Morphine bolus requirement as rescue opioid was less in Group D at all times till 10 min after reversal. This difference was most significant at 1 min (p < 0.01) when only 9.4% of patients in Group D required morphine bolus compared to 40.6% of patients in Group P.
In the ICU, the mean heart rate in the patients of Group D was lower than the control group which was highly significant at 15 min through 1 h (p < 0.01) and significant (p < 0.05) at most times through 20 h till extubation []. The patients of Group D had lower systolic and diastolic BP than Group P. The difference between the groups was significant at 15 min after the start of the infusion through the 20 h till extubation (p < 0.01) []. The patients of Group D maintained normal respiratory rate and had higher oxygen saturation (p < 0.01) than Group P and the difference was significant at most times through 20 h. The mean total morphine requirement in Group D was 72% lesser than Group P []. There was significantly lesser coughing (p < 0.01) and less intense cough in patients of Group D []. The patients of Group D were adequately sedated compared to Group P and this was significant throughout. None of the patients were sedated to the point of non arousal. Group D tolerated the endotracheal tube better than Group P as judged by the nurses [].
Following bolus drug administration, heart rate changes in our study were similar to the study conducted by Jain et al. [Citation14]. The trials by Martin et al. [Citation4], Guler et al. [Citation15] showed bradycardia of which few required intervention. Study by Guler et al. [Citation15] found a significant decrease in systolic BP at 3 min and 5 min after administration of Dexmedetomidine, whereas no significant change was noted in systolic BP in the study done by Jain et al. [Citation14]. In our study, four patients in Group D required intervention for hypotension.
Following reversal, our results are in accordance with studies by Martin et al. [Citation4] but differs from previous studies [Citation14,Citation15] where increase in heart rate was noted in both groups, but to a lesser extent in the group that received Dexmedetomidine. The lower systolic BP noted in Group D was consistent with trials by Martin et al. [Citation4]. Trial by Jain et al. [Citation14] showed no change in systolic BP in the Dexmedetomidine group and a mild increase in the control group whereas trial by Guler et al. [Citation15] stated increase in systolic BP in both groups but to a lesser extent in the Dexmedetomidine group. The increase in heart rate and BP in the Dexmedetomidine group in previous studies [Citation14,Citation15] may be due to the fact that patients underwent extubation whereas in our study the patients retained the endotracheal tube. Extubation like intubation is an acute stimulus associated with haemodynamic changes.
In the ICU, our observations of heart rate and B.P. were in accordance with trials by Martin et al. [Citation4], the only difference being a higher incidence of bradycardia and hypotension in their study. Blood pressure and heart rate reductions in the patients receiving Dexmedetomidine were predictable for an alpha2 adrenoreceptor agonist and stayed within the clinically acceptable range for most of the patients. Villella and Nascimento [Citation16] observed that Dexmedetomidine infusion in the postoperative period improves haemodynamic stability.
Patients who are at high risk of postoperative cardiovascular complications may benefit from Dexmedetomidine’s predictable reductions in blood pressure, heart rate [Citation9,Citation17], and plasma catecholamine concentrations [Citation13,Citation18]. In a study by Talke and Mangano [Citation19], reducing the heart rate and blood pressure appeared to reduce the risk of perioperative ischaemia and hypoxia. Studies by Mangano [Citation20], the MSPI Europe Research Group [Citation21], and Oliver et al. [Citation22] demonstrated that perioperative therapy with alpha2 – adrenoreceptor agonists decreased both the incidence of myocardial infarction and mortality in the perioperative period. Data by Mangano et al. [Citation23] supported by Raby et al. [Citation24] demonstrated that it was the reduction of heart rate and not beta-adrenergic blockade per se, that was beneficial. Lawrence et al. [Citation25] demonstrated that the sympatholytic effect of Dexmedetomidine decreased heart rate and contractility, increased coronary blood supply to the left ventricle by prolonged diastole, and decreased myocardial oxygen consumption.
The trials conducted by Venn et al. [Citation26] show that Dexmedetomidine appears to have no clinically important adverse effects on respiratory rate and gas exchange when used in spontaneously breathing ICU patients after surgery. Unlike our study, Martin et al. [Citation4] found no significant difference in oxygen saturation (p = 0.846) and mean respiratory rate between the two groups.
Our finding of reduced morphine requirement in Group D correlates with findings by Martin et al. [Citation4]. The studies by Alex Bekker et al. [Citation27] and Hassan et al. [Citation28] support our finding of opioid sparing effect of Dexmedetomidine. The analgesic sparing effect of Dexmedetomidine has been demonstrated in earlier studies [Citation29–Citation31]. Our results of endotracheal tube tolerance are consistent with the study by Martin et al. [Citation4].
The major limitations of the study are the absence of a pain scale and the subjective assessment of endotracheal tube tolerance.
Conclusion
Dexmedetomidine is efficacious for postoperative endotracheal tube tolerance. It has opioid sparing effect and can be used as a sole sedative agent without the danger of respiratory depression. Dexmedetomidine prevents postoperative hypertension and tachycardia and promotes easier management of patients with endotracheal tube in the surgical ICU. Although close monitoring is needed to detect hypotension and/or bradycardia Dexmedetomidine has a useful role in the multimodal approach to management of post surgical patients.
Author’s contribution
| . | Dr. Renu Ramakrishnan – Study design, acquisition, handling, analysis and interpretation of data, drafting of article. | ||||
| . | Dr. Rachel Cherian Koshy – Study conception and design, critical review of article, final approval of version to be published. | ||||
| . | Dr. Rajasree O. – Patient recruitment, critical review of article. | ||||
Conflict of interest
The authors declare that there are no conflicts of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- Dane A.HassaniSanjay M.BhanankerPostoperative airway obstruction after airway tumor debulkingJ Anesth2032006237239
- R.GargV.DarlongR.PandeyJ.PunjAnesthesia for oncological ENT surgeries: reviewInternet J Anesthesiol2012009
- D.A.JarvisS.R.DuncanI.S.SegalM.MazeVentilatory effects of clonidine alone and in the presence of alfentanil, in human volunteersAnesthesiology761992899905
- E.MartinG.RamsayJ.MantzS.T.J.Sum-PingThe role of α2 adrenoreceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unitJ Intensive Care Med1820032941
- Z.P.KhanC.N.FergusonR.M.JonesAlpha-2 and imidazoline receptor agonists: their pharmacology and therapeutic roleAnaesthesia541999146165
- J.FlackeB.BloorW.FlackeReduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary artery bypass surgeryAnaesthesiology6719871119
- J.S.GabrielV.GordinAlpha 2 agonists in regional anesthesia and analgesia [review]Curr Opin Anaesthesiol142001751753
- P.L.BaileyR.J.SperryG.K.JohnsonRespiratory effects of clonidine alone and combined with morphine, in humansAnaesthesiology74119914348
- B.C.BloorD.S.WardJ.P.BellevilleM.MazeEffects of intravenous dexmedetomidine in humans. II. Hemodynamic changesAnesthesiology77199211341142
- J.E.HallT.D.UhrichJ.A.BarneyS.R.ArainT.J.EbertSedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusionsAnesth Analg902000699705
- R.AantaaJ.KantoM.ScheininA.KallioH.ScheininDexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgeryAnesthesiology731990230235
- J.P.BellevilleD.S.WardB.C.BloorM.MazeEffects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rateAnesthesiology77199211251133
- T.J.EbertJ.E.HallJ.A.BarneyT.D.UhrichM.D.ColincoThe effects of increasing plasma concentrations of dexmedetomidine in humansAnaesthesiology932000382394
- D.JainR.KhanM.MaroofEffect of Dexmedetomidine on stress response to extubationInternet J Anesthesiol2009 21-1
- G.GulerA.AkinZ.TosunE.EskitascogluA.MizrakA.BoyaciSingle dose dexmedetomidine attenuates airway and circulatory reflexes during extubationActa Anaesthesiol Scand49200510881091
- N.R.VillelaP.NascimentojuniorDexmedetomidine in anaesthesiologyRev Bras Anestesiol53200397113
- J.JalonenL.HalkolaK.KuttilaEffects of dexmedetomidine on coronary hemodynamics and myocardial oxygen balanceJ CardiothoracVasc Anesth91995519524
- P.TalkeC.A.RichardsonM.ScheininD.M.FisherPostoperative pharmacokinetics and sympatholytic effects of dexmedetomidineAnesthAnalg85199711361142
- P.O.TalkeD.T.ManganoAlpha2-adrenergic agonists and peri-operative ischaemiaAnaesth Pharmacol Rev11993310315
- D.T.ManganoPerioperative cardiovascular morbidity: new developmentsBaillere’s Clin Anaesthesiol131999335338
- Perioperative sympatholysis. Beneficial effects of the alpha 2-adrenoceptor agonist mivazerol on hemodynamic stability and myocardial ischemia. McSPI-Europe Research GroupAnesthesiology861997346363
- M.OliverB.GoldmanD.JulianI.HolmeEffect of mivazerol on perioperative cardiac complications during non-cardiac surgery in patients with coronary artery disease: the European Mivazerol Trial (FMIT)Anesthesiology911999951961
- D.ManganoE.LayugA.WallaceI.TateoEffect of atenolol on mortality and cardiovascular morbidity after non cardiac surgery: multicenter study of Perioperative Ischaemia Research GroupN Engl J Med335199617131720
- K.RabyS.BrullF.TimimiThe effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgeryAnesth Analg881999477482
- C.J.LawrenceF.W.PrinzenS.De LangeThe effect of dexmedetomidine on the balance of myocardial energy requirement and oxygen supply and demandAnesth Analg821996544550
- Richard M.VennJohnHellandR.Michael GroundsRespiratory effects of dexmedetomidine in the surgical patient requiring intensive careCrit Care42000302308
- A.BekkerM.SturaitisM.BloomM.MoricJ.GolfinosE.ParkerThe effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomyAnesthAnalg107200813401347
- Hassan S.BakhameesM.YasserM.HalaM.NevienSultanAl TemyattEffects of Dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypassMiddle East J Anaesth1932007
- R.M.VennC.J.BradshawR.SpencerD.BrealeyE.CaudwellC.NaughtonA.VedioM.SingerR.FeneckD.TreacherS.M.WillattsR.M.GroundsPreliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unitAnaesthesia54199911361142
- J.JalonenM.HynynenA.KuitunenH.HeikkilaJ.PerttilaM.SalmenperaDexmedetomidine as an anesthetic adjunct in coronary artery bypass graftingAnesthesiology861997331345
- M.AhoA.M.LehtinenO.ErkolaA.KallioK.KorttilaThe effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomyAnesthesiology7419919971002