Abstract
Background
High-frequency oscillatory ventilation (HFOV) is one of lung protective strategies in acute respiratory distress syndrome (ARDS). It is not recommended to be used as initial mode of ventilation. Previous studies showed conflicting results for late use of HFOV (after prolonged period of conventional mechanical ventilation (CMV)). This study investigated the use of HFOV as an early therapy (after 24 h of CMV) in the management of ARDS due to burn.
Methods
70 burned ARDS patients were ventilated by CMV during the first 24 h (Day 0). Then, patients were randomly allocated into two equal groups (35 each):
Group 1 (G 1 or CMV): they continued on CMV.
Group 2 (G2 or HFOV): HFOV was instituted for 72 h (Days 1, 2, 3). Then, patients were shifted to CMV on Day 4 to continue on CMV. Ventilator settings, gas exchange parameters, hemodynamics, sedatives, vasoactive and paralytic requirements, barotraumas and hospital mortality were recorded and compared between the two groups.
Results
In Day 0: Demographic data, ventilator settings, gas exchange parameters, and hemodynamics showed no significant difference between both groups. Days 1, 2, 3: there was statistically significant decrease of FiO2 and OI accompanied by an increase in PaO2, PaO2/FiO2 and PaCO2 in G2. Day 4: while both groups on CMV, G2 patients showed statistically significant decrease in PEEP and mPaw with same gas exchange findings on Days 1, 2, 3 between two groups. During the study period, Hypotension was observed following HFOV in G2 and was most significant in Day 1. G2 showed statistically significant increase in barotraumas and required more midazolam, atracurium and norepinephrine. There was no statistically significant difference in 30 days mortality between both groups.
Conclusions
Early HFOV therapy is effective in improving oxygenation in burn patients with ARDS, but it failed to reduce hospital mortality.
Introduction
Acute respiratory distress syndrome (ARDS) is characterized by severe deterioration in oxygenation following acute insult, as severe inflammation causes permeability changes in the alveolar capillary membrane leading to fluid shifts into the alveolar and interstitial spaces [Citation1]. It is manifested by hypoxemia and bilateral infiltrates on chest radiographs with normal pulmonary capillary wedge pressures. ARDS can be classified into mild (PaO2/FiO2 ⩽ 300 mmHg with PEEP 5 cm H2O), moderate (PaO2/FiO2 ⩽ 200 mmHg with PEEP 5 cm H2O) and severe (PaO2/FiO2 ⩽ 100 mmHg with PEEP 5 cm H2O) [Citation2].
Burn is associated with an average mortality rate of 0.8% [Citation3]. It may predispose to ARDS in critically ill burn patient due to factors leading to lung injury such as smoke inhalation, sepsis, ventilator-induced lung Injury or a systemic inflammation in response to burn [Citation4,Citation5]. Lung protection plan in ventilating ARDS patients is to maintain low inspiratory driving pressures, with lower tidal volumes (4–6 ml/kg) and the use of limited airway pressure, with the synchronized prevention of alveolar collapse through the use of high PEEP to keep end-expiratory pressures above the lower inflection point on the static pressure–volume curve of the respiratory system [Citation6]. Conventional mechanical ventilation (CMV) may lead to tidal hyperinflation and shear stress effect, even when it is administered according to a ‘lung protective strategy’ that limits tidal volumes and plateau pressure [Citation7].
In spite of advances in critical care and understanding of ARDS pathophysiology, ARDS mortality remains as high as 48% as was reported by Villar et al., [Citation8] and 41% as was reported by Wang et al. [Citation9].
So, a number of ventilation modes have been recommended like high-frequency oscillatory ventilation (HFOV) which requires the use of a specific designed ventilator that has been approved by Food and Drug Administration. HFOV theory is to deliver a continuous distending mean airway pressure (mPaw), around which oscillations of predefined amplitude at a high frequency (usually between 3 and 15 Hz) are obtained by using a diaphragm. These pressure oscillations result in very small tidal volumes (1–4 ml/kg) smaller than the anatomical dead space. Therefore, theoretically, HFOV is the ideal “lung protective” ventilation strategy since it provides very low pressure swings, thus minimizing volutrauma and atelectrauma and maximizing alveolar recruitment [Citation10].
Using HFOV in ARDS patients after prolonged period of CMV has been tried by many researchers [Citation10–Citation19]. They used it as a rescue therapy for patients who remained hypoxemic and required high levels of inspired oxygen, or those who have plateau pressures >35 cm H2O despite 4 ml/kg tidal volumes on CMV, and the results were conflicting. Others [Citation11–Citation18] demonstrated improvement of oxygenation on HFOV in ARDS patients with increased risk of barotraumas and unfavorable hemodynamics due to high airway and intrathoracic pressure and a decreased venous return [Citation19–Citation21].
.1 Aim of the study
The aim of this study was to evaluate the efficiency and complications of HFOV as an early therapy (after 24 h of CMV) for a determined period (72 h) for management of ARDS in adult burn patients without smoke inhalation injury compared to CMV.
.2 Outcome measures
The primary outcome included determination of hospital mortality (30-day mortality) [Citation22]. Secondary outcomes included assessment of gas exchange parameters and adverse events (barotraumas and unfavorable hemodynamic).
.3 Patients and methods
The study was performed on 70 burn patients with ARDS (PaO2/FiO2 ratio of 200 mmHg or less) who were admitted to ICU in north zone in KSA from 2007 to 2011. Approval of the hospital institutional review board and obtaining a written consent from every patient or next of kin were done.
Inclusion criteria: male or female, age 18–60 years old, burn patients with 40% TBSA burned or more.
Patients were excluded when the patient’s age was less than 18 or over 60 years and weight was less than 35 kg, asthmatic, pregnant and the patients with smoke inhalational injury that was diagnosed by fiber-optic bronchoscopy when suspected.
Tracheal intubation was done to all included patients in the first 24 h from the onset of burn as was suggested by Cancio LC [Citation23].
In the first 24 h (Day 0), patients were ventilated by lung protective strategy (LPS) [Citation24] (tidal volumes 4–6 ml/kg, RR 12–22 breaths per minute, plateau airway pressure <32 cm H2O and PEEP 10–12 cm H2O). The patients were divided randomly using computer generated number and concealed using sequentially numbered, sealed opaque envelope technique into two equal groups (35 patients each):
Group 1 (CMV): patients continued on CMV by LPS for days 1, 2, 3 and 4.
Group 2 (HFOV): patients were shifted to HFOV strategy for 72 h for days 1, 2 and 3. Then, patients were shifted to CMV on day 4 either for weaning or to continue on LPS.
G2 Patients were assessed after 3 h on HFOV, if they could not achieve improvement of oxygenation parameters or could not tolerate HFOV during study period (Days 1, 2, 3) due to severe hypoxemia, severe hypercarbia, severe barotrauma and severe hypotension, HFOV was terminated and they were shifted to CMV and excluded from the study.
Institution of HFOV
Initial patient preparation was confirmation of ET tube patency and airway suctioning. Continuous infusion of midazolam (0.02–0.1 mg/kg/h), atracurium (0.4–0.8 mg/kg/h), norepinephrine (if needed: 0.05–1 μg/kg/min) and correction of intravascular volume status were done. Any imaging (CT) or interventions outside ICU (any operating theater procedure), bronchoscopy, echocardiography, insertion of arterial and central venous lines were performed before starting HFOV.
HFOV ventilator ()
HFOV was delivered using an adult high-frequency oscillatory ventilator (3100B, Viasys (Care Fusion), Yorba Linda, CA, USA).
Starting HFOV settings: A protocol for the adjustment of HFOV was used [Citation25]:
| #x2022; | FiO2 was started at 1.0 and decreased to maintain an oxygen saturation of 88% or more. | ||||
| #x2022; | Frequency of 4–6 Hz. | ||||
| #x2022; | Inspiratory time of 33%, may be increased to 50%. | ||||
| #x2022; | Bias flow of 30–40 L/min. | ||||
| #x2022; | A power was adjusted to obtain oscillation (wiggles) from shoulders till the level of mid-thigh. | ||||
| #x2022; | Adjustment of mPaw 4–5 cm H2O higher than plateau pressure for CMV settings in hemodynamic stable patients and only 2–3 cm H2O higher in hemodynamic unstable patients. | ||||
| #x2022; | ET suction by closed suction system. | ||||
Patient care and monitoring during HFOV
| #x2022; | Chest wiggling should be equal on both sides of the chest and from shoulders till mid thighs. Patients should be sedated and relaxed. | ||||
| #x2022; | Daily chest X-ray for detecting overinflation or pneumothorax. | ||||
| #x2022; | Daily arterial blood gases. | ||||
| #x2022; | keeping SPO2 > 88%. | ||||
| #x2022; | Hemodynamic monitoring (HR and invasive BP) and vasopressor requirements were recorded. Any hypotension or hypovolemia was properly managed. | ||||
Weaning from HFOV
Patients were considered ready for weaning when FiO2 requirement was ⩽0.4 with a PaO2 ⩾ 60 mmHg and mPaw had reached 20–22 cm H2O for at least 12–24 h.
| #x2022; | Weaning started by gradually decreasing FiO2 and then decreasing mPaw in increments of 1–2 cm H2O to avoid lung derecruitment. Those patients were converted to CMV (Day 4) (LPS with plateau pressure similar to that on HFOV). Then, patients were weaned gradually from CMV. | ||||
The recorded data included patients’ demographic data, gas exchange parameters (FiO2, PaO2, PaO2/FiO2 ratio, PaCO2, PH, SPO2 and OI), ventilator settings while patients on CMV (Vt, RR, plateau pressure and PEEP) and ventilator settings while patients on HFOV (frequency, mean airway pressure). Oxygenation Index (OI) is defined as 100 × mean airway pressure x (FiO2/PaO2).
Hemodynamic data (mean arterial blood pressure and heart rate) were continuously monitored and MABP was recorded hourly, and complications related to ventilation, also sedatives, vasoactive, neuromuscular-blocking drug dosing requirements and 30-day hospital mortality were measured for both groups in day 0, day 1, day 2, day 3 and day 4 and also after 3 h on HFOV in G2.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (± SD), median and range when appropriate. Comparison of numerical variables between the study groups was done using Student t test for independent samples. Within group comparisons between the different time points and the initial point were done using paired t test for paired (matched) samples. p values less than 0.05 was considered statistically significant. All statistical calculations were done using computer program SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) release 15 for Microsoft Windows (2006).
.1 Power analysis
Power analysis was done on comparison of FiO2, PEEP, PaO2/FiO2 and OI on day 4. Student’s t test for independent samples was chosen to perform the power analysis, the α-error level was fixed at 0.05 and the sample size was entered to be 60 participants divided equally into 2 groups. We considered that the reported difference is representative of the true population difference but we used the higher SD of the 2 groups. The final results are shown in the table below. Calculations were done using PS Power and Sample Size Calculations Software, version 3.0.11 for MS Windows (William D. Dupont and Walton D. Vanderbilt, USA).
Results
70 patients were submitted to the study. They were on LPS for the first 24 h. Then, patients were randomly divided into two groups, 35 patients each (Group 1 (CMV) and group 2 (HFOV)). Five patients were excluded from G1 because they died within the study period. Three of them died due to respiratory failure and the other 2 died because of profound hypotension. Also, 5 patients were excluded from G2 (two patients were shifted from HFOV to CMV after 3 h from HFOV due to refractory hypoxemia, one patient developed severe barotrauma and one patient developed hemodynamic instability). 60 patients remained in the two groups, 30 patients each ().
| #x2013; | Demographic data showed no statistically significant difference between both groups regarding age, sex and weight (). | ||||
| #x2013; | As regards CMV ventilator settings: in D0, there was no significant difference between both groups as regards PEEP and mPaw () (p > 0.05), and G1 patients were maintained on LPS in D1, 2, 3. In D4, there was a significant decrease in PEEP and mPaw in G2 than in G1 () (p < 0.05). | ||||
| #x2013; | As regards HFOV settings in G2: in D1, 2, 3: Bias flow 37 ± 2 L/min. Inspiratory time 38 ± 3%. Frequency 6 ± 1 Hz. mPaw 33.41 ± 2 cm H2O. | ||||
| #x2013; | As regards gas exchange parameters after 3 h from starting HFOV in G2, patients showed a statistically significant decrease of FiO2 from 0.94 ± 0.077 to 0.832 ± 0.1163 (p < 0.05), a significant increase of both PaO2 from 104.20 ± 10.3 to 119.70 ± 9.20 mmHg (p < 0.05) and PaO2/FiO2 from 111.43 ± 12.5 to 147.56 ± 28.80 mmHg (p < 0.05). | ||||
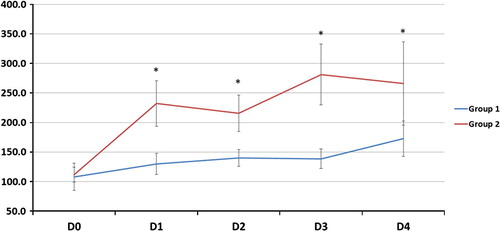
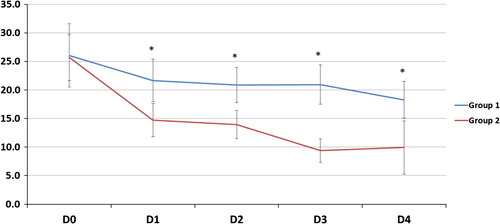
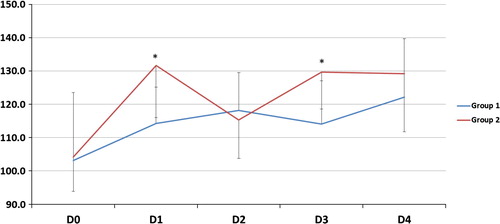
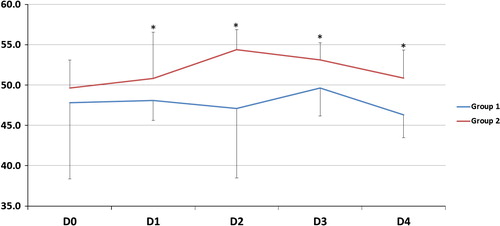
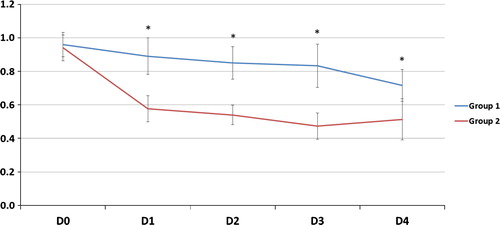
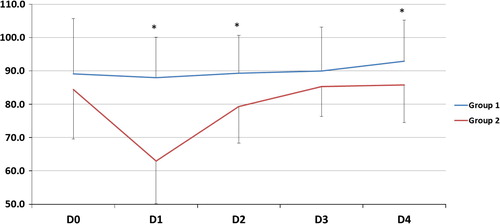
| #x2013; | As regards gas exchange parameters throughout the study period: there was no statistically significant difference between the two groups at D0 but at D1, 2, 3 and 4, there was a significant decrease of FiO2, OI and a significant increase of PaO2/FiO2, PaCO2 and PaO2 (except in D2) in G2 compared to G1 (). | ||||
| #x2013; | As regards MABP: patients in G2 developed hypotention with most statistically significant difference between both groups was found in D1 (). | ||||
| #x2013; | HR was ranging within clinically accepted levels 62–123 beats/min and from 66–127 beats/min in G1 and G2 respectively. | ||||
| #x2013; | As regards medication requirements: G2 patients showed statistically significant increase in doses and number of patients who required midazolam, atracurium and norepinephrine compared to G1 (). | ||||
| #x2013; | As regards barotraumas: pneumothorax developed in one patient (3.3%) in G1 versus 3 patients (10%) in G2. Intercostal tubes were inserted and patients continued the study. Three patients in G2 showed subcutaneous emphysema. | ||||
| #x2013; | PH was ranging within clinically accepted levels from 7.36 to 7.22 and from 7.34 to 7.20 in G1 and G2 respectively. SPO2 was ranging from 89% to 96% in G1 and from 89% to 95% in G2. | ||||
| #x2013; | Mortality: There was no statistically significant difference between the two groups as regards 30-day mortality. It was 16/30 (53.33%) in G1 versus 17/30 (56.66%) in G2. | ||||
able 1 Demographic data.
able 2 Ventilator settings in both groups in Day 0.
able 3 Ventilator settings in both groups in Day 4.
able 4 Medication requirements during D1, 2 and 3 in both groups.
Discussion
The use of HFOV in ARDS and the ideal timing for its use are still not decided. Selection of patients, timing of HFOV application, number of days of CMV before HFOV and patient condition before HFOV initiation may represent important factors that determine the outcome and modify HFOV application plan.
The present protocol considered the first 3 h on HFOV as a cutoff time at which the patient was considered non-responder and excluded if he didn’t show oxygenation improvement, which was suggested by Huang et al. [Citation29] who proved that if there is no improvement in gas exchange within the first 3 h on HFOV, patients should be considered to have failed HFOV and subjected to an alternative treatment as extracorporeal support.
The current study duration was designed to be 72 h which was supported by Camporota et al. [Citation30] who reported that PaO2/FiO2 improved significantly from baseline only in survivors in the first 72 h.
As regards selection of patients, identification of proper candidates who may benefit most from HFOV is important [Citation29]. Also, Dries et al. [Citation31] reported that there is no ideal respiratory support strategy for the patient with inhalation injury as pulmonary edema, bronchial edema, and secretions obstructing the airway leading to atelectasis and pneumonia. Moreover, it was concluded by Ryan et al. [Citation32] that smoke inhalational injury, together with age >60 years and >40% TBSA burned, is an independent factor for mortality. So, the present study excluded patients over 60 years and patients with inhalational injury. Bronchoscopy is highly effective in removing foreign particles and accumulated secretions [Citation33,Citation34].
The main findings of this study were as follows: G2 (HFOV) patients showed significant improvement of oxygenation parameters with no improvement of 30-day mortality rate, higher PaCO2 level, higher incidence of barotraumas, higher doses of sedatives, vasopressors and paralytic agents in comparison with G1 (CMV) patients.
The improvement of oxygenation parameters was denoted by a decrease in FiO2 and OI and an increase in PaO2 (except D2) and PaO2/FiO2 ratio in comparison with those on CMV. This improvement was observed early and sustained for the duration of HFOV period (days 1, 2, 3) and sustained after shifting patients to CMV (day 4). In spite of this oxygenation improvement, HFOV did not reduce 30-day mortality rate which could be explained by that oxygenation improvement does not indicate reduced lung injury or recovering from pathology, moreover by high liability of ARDS patients to multiple organ dysfunction syndrome (MOD) according to Meduri et al. [Citation35] but the authors failed to define pathophysiological relationship between ARDS and MOD.
These results were comparable to Huang et al. [Citation29] who studied five trials and found that HFOV significantly improved oxygenation on day one of therapy by 24%, but did not reduce mortality risk. It is also consistent with OSCAR trial [Citation26] that randomized 795 patients to HFOV or CMV, and they found no difference in rates of death between the HFOV and CMV group.
Conversely, many studies [Citation26,Citation27] did not support the use of HFOV as a salvage measure for refractory hypoxemia. Moreover, OSCILLATE trial [Citation27] that randomized 548 patients to either CMV or HFOV was terminated prematurely by the data safety management committee due to higher mortality in the HFOV group. Also, Salahuddin et al. [Citation28] concluded that it was not an ideal ‘rescue’ mode as it increased mortality (72.5%) in HFOV compared to (53%) in CMV.
On the other hand, G2 patients showed higher PaCO2 level in D1, 2, 3 than patients of G1. This is comparable to Cartotto et al. [Citation12] who reported that burned patients with ARDS and smoke inhalation injury did not achieve significant improvements in OI with higher rates of early HFOV failure due to severe hypercapnia due to the difficulties in delivery of nebulized medications during HFOV. The point of difference in the current study was improvement in OI which may be explained by exclusion of patients with smoke inhalational injury.
As regards barotraumas, the incidence was higher in HFOV. This incidence was less than late use of HFOV by Mehta et al. [Citation14] who reported the occurrence of pneumothorax in 21.8% of patients who received HFOV. A similar high percentage of patients suffered from gross barotrauma with HFOV was reported by Ferguson et al. [Citation38].
This low incidence was consistent with Greathouse et al. [Citation36], who concluded that earlier institution of HFOV improved oxygenation and reduced rates of barotraumas in severe burn pediatric patients and also consistent with Hodgson et al. [Citation37] who found the same findings in adult patients.
As regards hemodynamics, the present study showed decrease in MABP when HFOV was started. This hemodynamic instability was restorated by volume loading and vasopressors. This was consistent with David et al. [Citation39] who reported that during HFOV there were decreased cardiac index, stroke volume index, left-ventricular end-diastolic and end systolic area indices due to higher pleural pressure. In contrast to Mehta et al. [Citation15] who used HFOV in 24 ARDS patients after varying periods of CMV, they found no significant changes in systemic or pulmonary pressure associated with initiation and maintenance of HFOV.
In the current work, HFOV patients required higher doses of sedatives, vasopressors and paralytic agents than those on CMV. In consistent with the current study, Deem [Citation40] reported that HFOV patients needed more paralysis. In the current study, patients showed 88% SPO2 as the lowest accepted level as it was suggested by many authors [Citation41–Citation44] who reported targets of 94–98% saturation for healthy subjects and between 88% and 92% and a PaO2 > 55–60 mmHg for ARDS.
Conclusions
This study showed that early HFOV therapy (after 24 h of LPS) for a determined period (72 h) can support gas exchange and provide more effective lung recruitment than can be achieved with conventional ventilation in burn adults with ARDS (improved oxygenation), but increased incidence of hypercarbia and barotraumas. HFOV failed to reduce 30-day hospital mortality.
0 Limitations of the study and problems with HFOV
HFOV needs specific training and its circuit setup causes impaired clearance of pulmonary secretions. It also requires heavy sedation and neuromuscular blockade which are risk factors for intensive care-muscle weakness and required heavy sedation that may delay weaning. The present study didn’t follow up patients for long period after HFOV (only till D4) or measure organ specific failure scores, which may explain the high 30-day mortality in spite of oxygenation improvement.
1 Recommendations
HFOV as a lung-protective ventilation strategy remains attractive, but additional clinical trials are needed to determine whether this approach is superior to CMV.
For future studies, proper patients’ selection, determination of the proper time for its initiation, duration and determination of optimal oscillator settings are needed to gain benefit from using HFOV. Furthermore, Goffi [Citation45] reported that new monitoring technologies should be incorporated into future HFOV for optimizing the efficacy of the machine.
Conflict of interest
No conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- M.DavidN.WeilerW.HeinrichsHigh-frequency oscillatory ventilation in adult acute respiratory distress syndromeInten Care Med29200316561665
- V.M.RanieriG.D.RubenfeldB.T.ThompsonAcute respiratory distress syndrome: the Berlin DefinitionJAMA307201225262533
- K.R.HarbinT.E.NorrisAnesthetic management of patients with major burn injuryAANA J8062012430439
- P.EnkhbaatarD.L.TraberPathophysiology of acute lung injury in combined burn and smoke inhalation injuryClin Sci1072004137143
- R.L.SheridanRespiratory issues in Burns: a practical approach to immediate treatment and long-term care2012Mason Publishing LtdLondonp.48–54
- M.O.MeadeD.J.CookG.H.GuyattVentilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome; a randomized controlled trialJAMA2992008637645
- S.GrassoT.StripoliM.SacchiInhomogeneity of lung parenchyma during the open lung strategy: a computed tomography scan studyAm J Resp Crit Care Med1802009415423
- J.VillarJ.BlancoJ.M.AnonThe ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilationInten Care Med37201119321941
- C.Y.WangC.S.CalfeeD.W.PaulOne-year mortality and predictors of death among hospital survivors of acute respiratory distress syndromeInten Care Med4032014388396
- K.P1.ChanT.E.StewartS.MehtaHigh-frequency oscillatory ventilation for adult patients with ARDSChest1316200719071916
- P.FortC.FarmerJ.WestermanHigh-frequency oscillatory ventilation for adult respiratory distress syndrome – a pilot studyCrit Care Med251997937947
- R.CartottoG.WaliaS.EllisOscillation after inhalation: high frequency oscillatory ventilation in burn patients with the acute respiratory distress syndrome and co-existing smoke inhalation injuryJ Burn Care Res302009119127
- D.J.FunkE.LujanE.W.MorettiA brief report: the use of high-frequency oscillatory ventilation for severe pulmonary contusionJ Trauma652008390395
- S.MehtaS.E.LapinskyD.C.HallettProspective trial of high-frequency oscillation in adults with acute respiratory distress syndromeCrit Care Med29200113601369
- S.MehtaJ.GrantonR.J.MacDonaldHigh-frequency oscillatory ventilation in adults: the Toronto experienceChest1262004518527
- C.W.BollenG.T.van WellT.SherryHigh frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trialCrit Care942005430439
- S.DerdakS.MehtaT.E.StewartHigh-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trialAm J Resp Crit Care Med16662002801808
- D.DemoryP.MicheletJ.M.ArnalHigh-frequency oscillatory ventilation following prone positioning prevents a further impairment in oxygenationCrit Care Med3512007106111
- C.GuervillyJ.M.ForelS.HraiechRight ventricular function during high-frequency oscillatory ventilation in adults with acute respiratory distress syndromeCrit Care Med40201215391545
- M.BoussarsarG.ThierryS.JaberRelationship between ventilatory settings and barotrauma in the acute respiratory distress syndromeInten Care Med282002406413
- P.FortC.FarmerJ.WestermanHigh-frequency oscillatory ventilation for adult respiratory distress syndrome: a pilot studyCrit Care Med251997937947
- P.L.GrahamD.A.CookPrediction of risk of death using 30-day outcome: a practical end point for quality auditing in intensive careChest125200414581466
- L.C.CancioAirway management and smoke inhalation injury in the burn patientClin Plast Surg3642009555567
- M.B.AmatoC.S.BarbasD.M.MedeirosEffect of a protective-ventilation strategy on mortality in the acute respiratory distress syndromeNew Engl J Med3381998347354
- CareFusion C: 3100A® High frequency oscillatory ventilator – Operator’s manual. CareFusion 22745 Savi Ranch Parkway, Yorba Linda, CA; 1991. p. 92887–4668.
- D.YoungS.E.LambS.ShahHigh-frequency oscillation for acute respiratory distress syndromeNew Engl J Med3682013806813
- N.D.FergusonD.J.CookG.H.GuyattHigh-frequency oscillation in early acute respiratory distress syndromeNew Engl J Med2013 10.1056
- N.SalahuddinH.Al SaidiM.KherallahHigh frequency oscillatory ventilation may not rescue ARDS patients: an observational studyCrit Care Shock1620135864
- Chun-TaHuangHsien-HoLinSheng-YuanRuanEfficacy and adverse events of high-frequency oscillatory ventilation in adult patients with acute respiratory distress syndrome: a meta-analysisCrit Care182014R102
- L.CamporotaT.SherryJ.SmithPhysiological predictors of survival during high-frequency oscillatory ventilation in adults with acute respiratory distress syndromeCrit Care172013R40
- David J.DriesFrederick W.EndorfInhalation injury: epidemiology, pathology, treatment strategiesScand J Trauma Resuscitation Emerg Med21201331
- C.M.RyanD.A.SchoenfeldW.P.ThorpeObjective estimates of the probability of death from burn injuriesNew Engl J Med33861998362366
- A.ArakawaH.FukamizuI.HashizumeMacroscopic and histological findings in the healing process of inhalation injuryBurns332007855859
- M.J.MosierR.L.GamelliM.M.HalerzMicrobial contamination in burn patients undergoing urgent intubation as part of their early airway managementJ Burn Care Res292008304310
- G.U.MeduriD.AnnaneG.P.ChrousosActivation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapyChest1366200916311643
- S.T.GreathouseI.HadadM.ZiegerHigh-frequency oscillatory ventilators in burn patients: experience of Riley Hospital for ChildrenBurn Care Res3332012425435
- C.L.HodgsonG.CarteauxD.V.TuxenHypoxaemic rescue therapies in acute respiratory distress syndrome: why, when, what and which one?Injury Int J Care Injured20125209
- N.D.FergusonJ.D.ChicheR.M.KacmarekCombining high-frequency oscillatory ventilation and recruitment maneuvers in adults with early acute respiratory distress syndrome: the Treatment with Oscillation and an Open Lung Strategy (TOOLS) Trial pilot studyCrit Care Med3332005479486
- M.DavidR.S.von BardelebenN.WeilerCardiac function and haemodynamics during transition to high-frequency oscillatory ventilationEuro J Anaesthesiol21122004944952
- S.DeemIntensive-care-unit-acquired muscle weaknessRespir Care519200610421052 discussion 1052–1053
- M.AbdelsalamPermissive hypoxemia: is it time to change our approach?Chest12912006210211 32
- A.MercatJ.C.RichardB.ViellePositive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trialJ Am Med Assoc29962008646655
- L.PapazianJ.M.ForelA.GacouinNeuromuscular blockers in early acute respiratory distress syndromeNew Engl J Med363September (12)201011071116 34
- C.PutensenN.TheuerkaufJ.ZinserlingMeta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injuryAnn Intern Med151October (8)2009 2012; 33(3): 425–35
- A.GoffiN.D.FergusonHigh-frequency oscillatory ventilation for early acute respiratory distress syndrome in adultsCurr Opin Crit Care2020147785

