Abstract
Background and objectives
Sleeve gastrectomy has become a popular and effective treatment for morbidly obese patients. The aim of this prospective randomized study was to assess the efficacy of multimodal analgesia using pregabalin and dexmedetomidine in morbidly obese patients undergoing laparoscopic sleeve gastrectomy.
Materials and methods
After ethical approval 60 American Society of Anesthesiologists (ASA) physical status II patients were enrolled in this study and allocated randomly into 2 groups: group A received 75 mg oral pregabalin 2 h before surgery and dexmedetomidine infusion 0.4 μg/kg/h and group B (control group) received placebo capsule 2 h before surgery and saline infusion intraoperatively. Intraoperative fentanyl consumption, hemodynamics and postoperative opioid consumption, pain scores, level of sedation and any side effects were evaluated.
Results
There was a significant decrease in heart rate, mean arterial blood pressure, pain score, intraoperative fentanyl use, postoperative morphine consumption and nausea verbal rating scale in group A compared to group B. There was a significant increase in sedation score in group A compared to group B.
Conclusions
The combination of preoperative oral pregabalin and intraoperative dexmedetomidine infusion decreased intraoperative fentanyl use and ensured postoperative better pain control and less postoperative opioid consumption.
Introduction
This is the first study to investigate the effect of combination of oral pregabalin and dexmedetomidine infusion in morbidly obese patients undergoing sleeve gastrectomy. Multimodal analgesia is a technique which is achieved by combining different types of analgesics that act by different mechanisms of action and at different sites in the nervous system, resulting in additive or synergistic analgesia with lower incidence of adverse effects than the administration of individual analgesics [Citation1].
As the incidence of obesity is being increased, bariatric procedures are increasingly being performed all over the world. Bariatric surgery is an effective treatment for obesity, with a mean percentage of weight loss after 2 years of 68.2% for laparoscopic sleeve gastrectomy [Citation2]. Morbidly obese patients may be sensitive to the respiratory depressant effect of opioid analgesics and more likely to require postoperative ventilation to avoid postoperative hypoxia [Citation3].
Dexmedetomidine is a highly selective α2 adrenergic receptor agonist; it has anxiolytic, sedative, analgesic and sympatholytic properties without significant respiratory depressant effects [Citation4,Citation5]. The combination of sedative and analgesic effects with minimal respiratory depression of dexmedetomidine is of great value in the morbidly obese patient who may be at risk of postoperative obstructive apnea, respiratory depression and opioid-induced respiratory depression following laparoscopic bariatric surgery [Citation6,Citation7].
Pregabalin is a structural analogue of the inhibitory neurotransmitter γ-aminobutyric acid [Citation8]. It binds to the α-2-δ subunit of voltage-gated excitatory calcium channels, reducing the release of many excitatory neurotransmitters and preventing the development of hyperalgesia and sensitization of dorsal horn neurons [Citation9,Citation10]. Pregabalin has anticonvulsant, anti-hyperalgesic, and anxiolytic properties similar to gabapentin, but it has a more favorable pharmacokinetic profile, including high oral bioavailability [Citation11,Citation12]. It is also more potent than gabapentin with fewer side effects [Citation8]. Recently, pregabalin has been used in multimodal analgesia for acute postoperative pain [Citation13]. The gap in literature is the absence of a definite one technique to provide proper pain control in this risky group of patients without significant side effects and opioid sparing properties.
The aim of this study is to evaluate the effects of combining dexmedetomidine infusion and oral pregabalin on supplemental opioid requirements, pain scores and hemodynamic parameters during the first 24 h; in morbidly obese patients undergoing laparoscopic sleeve gastrectomy. This prospective randomized double blind placebo controlled study assigned patients to receive pregabalin plus dexmedetomidine or placebo, we expected that this combination of drugs administered would demonstrate additive and prolonged postoperative analgesia. The primary outcome was total amount of postoperative morphine consumption; secondary outcome measures were pain and sedation scores, intraoperative fentanyl consumption, postoperative nausea and vomiting, hemodynamic variables and time to ambulation.
Patients and methods
After obtaining informed written consents, and obtaining approval from Research Ethics Committee of anesthesiology department, this prospective randomized double blind controlled parallel-group with allocation ratio of (1:1) was conducted in Cairo university hospitals. A total of 60 morbidly obese patients with American Society of Anesthesiologists (ASA) physical status classification II, aged 18–50 years, of both sex, undergoing elective laparoscopic sleeve gastrectomy were enrolled in this study. Exclusion criteria included myocardial dysfunction (ejection fraction <40%), renal or hepatic dysfunction, inability to use the visual analogue scale (VAS), allergy to any of the study drugs, current treatment with narcotics, antihypertensives, benzodiazepines, α2 agonists, antiepileptics and antipsychotics.
Two hours before induction of anesthesia, patients were randomly allocated using computer generated randomization numbers, and sealed in closed opaque envelopes, into one of 2 groups: group A (n = 30) received 75 mg oral pregabalin (Lyrica®, Pfizer) and group B (n = 30) received placebo capsule.
On arrival to the preparation room intravenous cannula was inserted under local anesthesia, no sedation was given. In the operating theater standard monitors, non-invasive blood pressure, oxygen saturation and electrocardiogram were applied before induction of anesthesia, capnography after induction of anesthesia and baseline heart rate (HR), mean arterial blood pressure (MAP) and oxygen saturation (SpO2) were recorded. Then the patients of group A started dexmedetomidine (Precedex 100 μg/ml, Hospira®) infusion 0.4 μg/kg/h after a bolus of 0.5 μg/kg intravenously over 10 min and patients of group B received a bolus of saline then saline infusion identical to group A. Patients and investigators recording data in the operating room were blinded to the treatment given.
All patients in both groups received standardized anesthetic technique in the form of intravenous (i.v.) propofol 2 mg/kg, i.v. fentanyl 1–2 μg/kg and atracurium 0.5 mg/kg to facilitate endotracheal intubation, mechanical ventilation was adjusted to keep end tidal carbon dioxide (EtCO2) between 30 and 35 mmHg, and all drugs were based on ideal body weight. Sevoflurane 2% in 50% oxygen and air, and 0.15 mg/kg atracurium every 20 min were given for maintenance of anesthesia. Intraoperative hemodynamic variables and supplemental fentanyl requirements were recorded. Intraoperative HR, MAP, EtCO2 and SpO2 values were recorded at 5 min intervals till the end of operation. HR and MAP were maintained within ±20% of the baseline values. Hypotension (defined as MAP < 20% of the baseline value) treated by a bolus of 200 ml Ringer’s solution if not responding increments of 3–9 mg ephedrine was given. Hypertension (defined as MAP > 20% of the baseline value) and/or tachycardia (defined as HR > 20% of the baseline value) a supplemental dose (25–50 μg) fentanyl was given or increasing concentration of sevoflurane. Bradycardia (HR < 50 beat per minute) persisting for >2 min was treated with atropine, 0.4 mg i.v. boluses. Intraoperatively i.v. ondansetron, 4 mg (Zofran, GlaxoSmithKline) was given for prevention of postoperative nausea and vomiting. Before skin closure, all ports of laparoscope were infiltrated with 0.25% bupivacaine. By the end of surgery sevoflurane was discontinued and the residual neuromuscular block was antagonized with neostigmine 0.05 μg/kg, given with atropine 0.02 mg/kg, the endotracheal tube was removed after return of spontaneous breathing, and the patient was obeying commands in semi-sitting position, then the patient was transferred to the post-anesthesia care unit (PACU), where the patients were connected to a patient controlled analgesia (PCA) delivery system that was programmed to deliver morphine, 1 mg i.v. boluses on demand with a lockout interval of 20 min. MAP, SpO2 and HR were documented preoperatively as baseline, every 5 min; intraoperatively and in PACU then hourly in the first postoperative day (POD). Pain was evaluated using Visual Analogue Scale (VAS) for pain (0 = no pain to 10 = the worst pain); every 2 h in the first POD, if VAS > 4, supplemental dose of 2 mg morphine was given. Total amount of narcotics was recorded. Nausea levels were assessed using 11-point verbal rating scale (VRS) (0 = no nausea and 10 = the worst nausea ever). Nausea VRS was obtained every 30 min till discharge from PACU then every 2 h in the first POD. A protocol for antiemetic drugs was administrated according to nausea VRS scores: when the VRS score is 0–2 the patients received no antiemetic drugs, when the VRS score is 3–6 the patients received one dose of antiemetic drugs and when the VRS score is 7–10 the patients received two or more doses of antiemetic drugs to a target VRS of 0–2 [Citation14]. Rescue medications were the following in order of administration: metoclopramide 10 mg, ondansetron 4 mg and dexamethasone 8 mg.
Level of sedation was assessed every 2 h in the PACU using Ramsay sedation score (RSS) [Citation15]: 1 – Anxious, agitated or restless; 2 – Cooperative and oriented; 3 – Responsive to commands; 4 – Asleep, but response to light glabellar tap or loud auditory; 5 – Asleep, sluggish response to glabellar tab or auditory response; and 6 – Asleep, no response. Any episode of vomiting, visual disturbance and other side effects was documented. An independent, trained nurse blinded to the study drugs being received assessed pain, level of sedation, hemodynamic variables and nausea and/or vomiting.
Sample size was based on previous study [Citation16] and calculated by assuming that there was >50% reduction of the postoperative morphine consumption by combination of both dexmedetomidine and pregabalin and α error = 0.05 with power of 80% resulted in 30 patients in each arm.
Statistical analysis: Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Science) version 22. Data were summarized using mean and standard deviation in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between groups were done by using unpaired t test. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. P-values less than 0.05 were considered as statistically significant.
Results
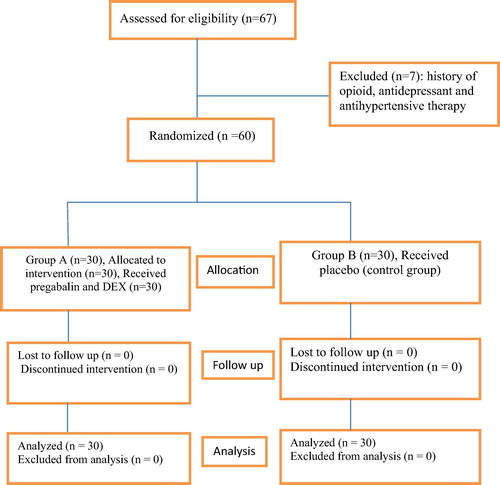
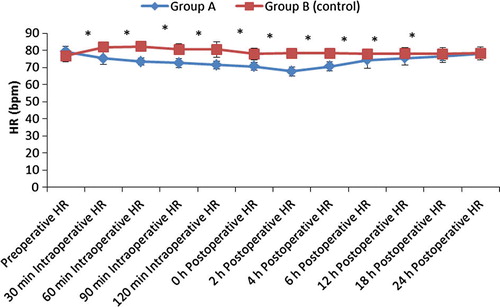
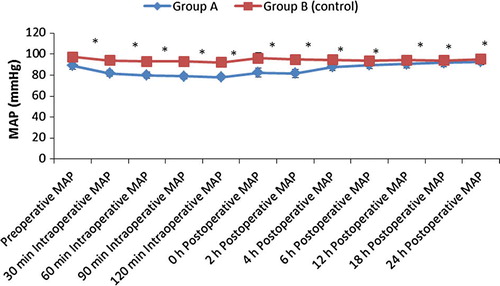
From August 2015 to January 2016, 67 consecutive morbidly obese patients undergoing laparoscopic sleeve gastrectomy were considered for study inclusion. Seven patients were excluded due to opioid, antihypertensive and antidepressant therapy and sixty patients were randomized for the study (). There were no differences in patient characteristics and operative time (). Also there was no significant difference between the groups regarding postoperative oxygen saturation. There was a statistically significant decrease in the mean heart rate and mean arterial blood pressure in group A compared to group B as shown in and .
able 1 Patients’ characteristics and duration of surgery.
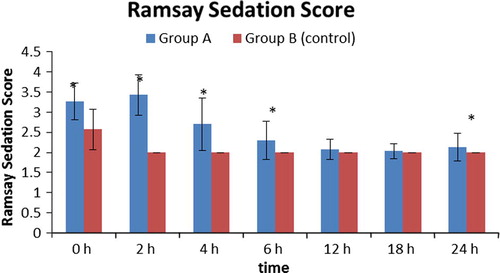
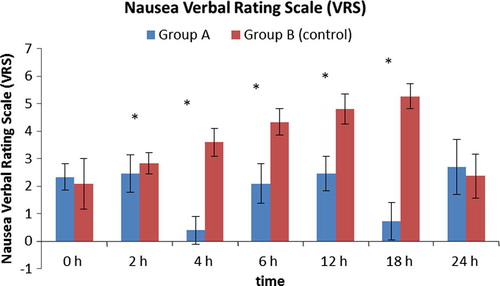
Sedation score was significantly higher in group A compared to group B during the first six hours and at 24th hours postoperatively as shown in ; this may be due to the extended effect of pregabalin.
As regards VAS score, there was significant decrease in group A as compared to group B in the first postoperative day (p < 0.001) ().
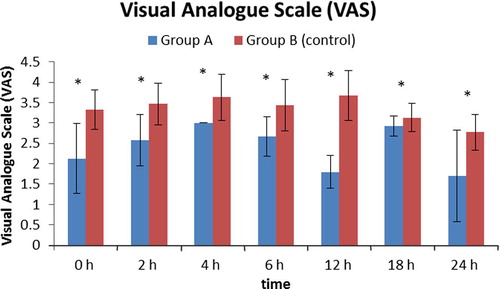
As regards nausea, group B showed significantly higher incidence of nausea than group A starting from the second hour to the 18th hour postoperatively (). Ten patients in group B experienced an attack of vomiting compared to one patient in group A (p < 0.001).
There was significant increase in fentanyl consumption intraoperatively and morphine postoperatively in group B compared to group A (p < 0.001) as shown in .
able 2 Narcotic consumption.
Discussion
The main finding of this study was significant reduction of intraoperative and postoperative narcotic consumption in patients received combination of dexmedetomidine and pregabalin, and also these patients showed better control of intraoperative and postoperative mean blood pressure and heart rate. In the postoperative period, pregabalin and dexmedetomidine decreased pain scores and PCA morphine use and showed better recovery profile compared with placebo.
The use of opioid drugs intraoperatively and postoperatively for pain management may increase the incidence of postoperative respiratory depression and hypoxia in morbidly obese patients undergoing laparoscopic sleeve gastrectomy [Citation17]. Therefore, the combination of two drugs with narcotic sparing effects could be very beneficial in morbidly obese patients with risk of obstructive sleep apnea.
Dexmedetomidine is a highly specific α2 agonist with sedative and analgesic effects, its use has been approved for sedation in intensive care unit [Citation18], but its use intraoperatively to decrease anesthetic and narcotic requirements is not fully established.
In a study done by Aho et al. [Citation19] in patients undergoing laparoscopic tubal ligation, dexmedetomidine had reduced pain scores and narcotic consumption and this is consistent with the results of the current study.
In a similar trial, Arian et al. [Citation4] reported that there was 66% reduction of postoperative morphine consumption when dexmedetomidine was used.
Feld et al. [Citation7] conducted a study in patients undergoing open gastric bypass and concluded that when dexmedetomidine used to substitute for fentanyl attenuates blood pressure and provides postoperative analgesia, and this is in line with the current study as regards narcotic consumption.
Tufanogullari et al. [Citation20] studied the effects of dexmedetomidine infusion during bariatric surgeries and concluded that adjunctive use of an intraoperative dexmedetomidine infusion reduced fentanyl use, antiemetic therapy, and the length of stay in the PACU, and they recommended using dexmedetomidine infusion to minimize the intraoperative cardiovascular adverse effects.
Pregabalin is a structural analogue of gamma-aminobutyric acid, and it is an attractive adjunct for perioperative analgesia as it can be taken on an empty stomach, does not lead to gastrointestinal upset, and is well tolerated [Citation21].
Agarwal et al. [Citation22] concluded that oral pregabalin 150 mg given before surgery was effective in reducing postoperative pain and the postoperative patient-controlled fentanyl analgesia in patients undergoing laparoscopic cholecystectomy.
The results of the current study are in line with the findings of Girija et al. [Citation23] who reported that the administration of oral pregabalin 150 or 300 mg as a single preoperative dose was effective in reduction of postoperative pain and total fentanyl requirements in patients undergoing lumbar disk surgeries.
In a meta-analysis done by Zhang et al. [Citation24], they concluded that the perioperative use of oral pregabalin has a significant opioid-sparing effect in the first 24 h after surgery with reduced incidence of postoperative nausea and vomiting, but visual disturbances were more common with pregabalin administration.
Limitations to the present study were the administration of a single dose of pregabalin, so its duration of action was time limited. Two different analgesic drugs were used simultaneously, and it was much better to use a single drug to show its precise effects. Also, sevoflurane consumption intraoperatively or length of hospital stay was not estimated. Other limitation was the patient satisfaction and antiemetic therapies were not documented. Further studies are recommended to compare different doses of pregabalin combined with dexmedetomidine.
Conclusions
The combination of preoperative oral pregabalin and intraoperative dexmedetomidine infusion decreased intraoperative fentanyl use and ensured postoperative better pain control and less postoperative opioid consumption.
Conflict of interest
None.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- R.ChouD.B.GordonO.A.de Leon-CasasolaManagement of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative CouncilJ Pain1722016131157
- H.BuchwaldY.AvidorE.BraunwaldBariatric surgery. A systematic review and meta-analysisJAMA292200417241737
- N.RawalU.SjostrandE.ChristoffersonB.DahlstromA.ArvillH.RydmanComparison of intramuscular and epidural morphine for postoperative analgesia in the grossly obese. Influence of postoperative ambulation and pulmonary functionAnesth Analg631984583592
- S.R.ArainR.M.RuehlowT.D.UhrichT.J.EbertThe efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgeryAnesth Analg982004153158
- H.IhmsenT.I.SaariDexmedetomidine. Pharmacokinetics and pharmacodynamicsAnaesthesist6112201210591066
- R.E.HoferJ.SprungM.G.SarrD.J.WedelAnesthesia for a patient with morbid obesity using dexmedetomidine without narcoticsCan J Anaesth522005176180
- J.M.FeldW.E.HoffmanM.M.StechertI.W.HoffmanR.C.AnandaFentanyl or dexmedetomidine combined with desflurane for bariatric surgeryJ Clin Anesth1820062428
- N.ManjushreeA.ChakrabortyK.ShashidharS.NarayanaswamyA review of the drug pregabalinInt J Basic Clin Pharmacol442015601605
- B.F.ShnekerJ.W.McAuleyPregabalin: a new neuromodulator with broad therapeutic indicationsAnn Pharmacother39200520292037
- B.A.ChizhM.GohringA.TrosterG.K.QuarteyM.SchmelzW.KoppertEffects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteersBr J Anaesth982007246254
- D.R.GuayPregabalin in neuropathic pain: a more ‘pharmaceutically elegant’ gabapentin?Am J Geriatr Pharmacother32005274287
- K.McKeageS.J.KeamPregabalin: in the treatment of postherpetic neuralgiaDrugs Aging26102009883892
- I.GilronGabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directionsCurr Opin Anaesthesiol202007456472
- S.Dabu-BondocN.VadiveluC.ShimonoA.EnglishB.KosarussavadiF.DaiK.ShelleyJ.FeinleibIntravenous dextrose administration reduces postoperative antiemetic rescue treatment requirements and postanesthesia care unit length of stayAnesth Analg11732013591596
- M.A.E.RamsayT.M.SavegeB.R.J.SimpsonR.GoodwinControlled sedation with alpaxalone–alphadoloneBMJ21974656659
- A.GurbetE.Basagan-MogolG.TurkerF.UgunF.N.KayaB.OzcanIntraoperative infusion of dexmedetomidine reduces perioperative analgesic requirementsCan J Anaesth532006646652
- J.HolmesJ.P.MayePostoperative respiratory depression and unresponsiveness following epidural opiate administration: a case reportAANA J722004126128
- T.KamibayashiM.MazeClinical use of alpha 2-adrenergic agonistsAnesthesiology93200013451349
- M.S.AhoO.A.ErkolaH.ScheininA.M.LehtinenK.T.KorttilaEffect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligationAnesth Analg731991112118
- B.TufanogullariP.F.WhiteM.P.PeixotoDexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variablesAnesth Analg106200817411748
- I.HindmarchL.TrickF.RidoutA double-blind, placebo- and positive-internal-controlled (alprazolam) investigation of the cognitive and psychomotor profile of pregabalin in healthy volunteersPsychopharmacology1832005133143
- A.AgarwalS.GautamD.GuptaS.AgarwalP.K.SinghU.SinghEvaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomyBr J Anaesth1012008700704
- P.R.GirijaY.RahulC.ArvindH.D.HariA63 effects of pregabalin on postoperative pain and preoperative anxiety in patients undergoing lumbar discectomyEur J Anaesth29Suppl. 492012S19
- J.ZhangK.Y.HoY.WangEfficacy of pregabalin in acute postoperative pain: a meta-analysisBr J Anaesth1062011454462