?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background and aim
Vasoplegic syndrome (VS) is a frequent complication following cardiopulmonary bypass (CPB) requiring escalating dose of vasopressor support. The guanylate cyclase inhibitor methylene blue (MB) could be an attractive alternative treatment in such cases. This study examines the efficacy and safety of using MB compared to the commonly used norepinephrine in VS in pediatric population following CPB.
Methods
Forty patients of pediatric age group who developed VS following CPB for elective corrective cardiac surgeries received 0.5 μg/kg/min norepinephrine intravenous infusion for 5 min without improvement (Time 1). Patients were randomly assigned to two equal groups. Group MB received 1.5 mg/kg methylene blue by intravenous infusion over 20 min. Group N did not receive MB. Norepinephrine infusion was continued in both groups and titrated according to the response of patients with a maximum dose of 2 μg/kg/min (Time 2). Heart rate, mean arterial pressure (MAP), central venous pressure (CVP), cardiac output (CO), cardiac index (CI), mean pulmonary artery pressure (MPAP), systemic vascular resistance (SVR) and systemic vascular resistance index (SVRI) were calculated in both groups. Side effects related to the study drug were recorded.
Results
Time 2 values of norepinephrine dose were significantly lower in MB group compared to N group. Time 2 values of MAP were significantly higher in MB group compared to N group with a significant decrease in HR in MB group compared to N group. No change in the rhythm was detected in the two groups. Time 2 values of CVP were higher in MB group compared to N group. Time 2 values of CO and CI were significantly lower in MB group compared to N group and SVR and SVRI were significantly higher in MB group compared to N group. Time 2 values of MPAP were comparable in both groups and showed no significant change. No side effects from using MB were recorded as pulmonary edema and respiratory distress.
Conclusion
In this study, MB showed superior efficacy and safety in managing VS in pediatrics following CPB compared to the conventionally used norepinephrine.
Keywords:
Introduction
Cardiopulmonary bypass (CPB) may be complicated by persistent hypotension due to low systemic vascular resistance, in 5–22% of patients [Citation1,Citation2]. Different causes have been associated with this situation, such as hypothermia, duration of CPB, total cardioplegic volume infused, reduced left ventricular function, preoperative treatment with angiotensin-converting enzyme inhibitors, and systemic inflammatory response syndrome (SIRS), or inappropriate low arginine-vasopressin secretion. An advanced form of this hypotension is the vasoplegic shock which is a life-threatening condition, intractable in the usual management with fluid administration, inotropes, and even vasopressor catecholamines [Citation3,Citation4].
Vasoplegic syndrome (VS) is generally defined as a mean arterial pressure (MAP) <50 mmHg, cardiac index (CI) >2.5 L/min/m2, right atrial pressure <5 mmHg, left atrial pressure <10 mmHg and low systemic vascular resistance (SVR) <800 dyne s/cm5 [Citation5].
Although largely effective in reestablishing minimally acceptable mean arterial pressures to maintain organ perfusion, catecholamines have important adverse effects and may even increase mortality rates. For example, norepinephrine, a potent and commonly used α-adrenergic agent in cases of septic shock, may decrease cardiac output (CO), oxygen delivery, and blood flow to vulnerable organs despite adequate perfusion pressure [Citation6].
Methylene blue (MB) or tetramethylthionine chloride is a well described alternative treatment for VS. It is believed to interfere with the nitric oxide (NO)-cyclic guanylate monophosphate (cGMP) pathway, inhibiting its vasorelaxant effect on smooth muscle. Methylene blue (MB) has been used to treat refractory hypotension in anaphylaxis, septic shock and after CPB in adults [Citation7–Citation9]. Methylene blue has been used in pediatrics in several limited trials to treat septic shock [Citation10] and methemoglobinemia [Citation11].
In this prospective randomized controlled trial, methylene blue is compared to norepinephrine use in pediatric patients who developed vasoplegic syndrome after CPB.
Patients and methods
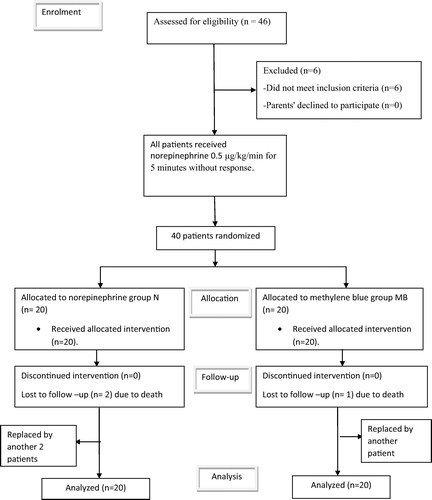
After approval of local ethics committee and informed written consent from patients’ parents, this randomized trial was done between March 2013 and April 2015. The study included forty patients between the age of 2 and 8 years old who developed systemic vasoplegic syndrome out of 1500 various elective pediatric corrective cardiac surgeries performed with CPB support at Atfal Misr Hospital.
Inclusion criteria were all patients who were diagnosed with vasoplegia after cardiopulmonary bypass. Vasoplegia was diagnosed by the following criteria: mean arterial pressure ⩽50 mmHg, systemic vascular resistance (SVR) <800 dyn s/cm5 or systemic vascular resistance index (SVRI) ⩽1500 dyn s/cm5/m2, cardiac index (CI) ⩾2.5 L/min/m2 and all patients received intravenous norepinephrine infusion of 0.5 μg/kg/min for 5 min without improvement (Time 1).
We assigned patients randomly into 2 groups by using a computer-generated random-number sequence and sealed envelopes; Group MB: 20 patients received the guanylate cyclase inhibitor MB (METHYLENE BLUE INJECTION, USP 1%, AMERICAN REGENT, INC.) in a dose of 1.5 mg/kg by intravenous infusion over 20 min in addition to norepinephrine infusion, the rate of which was adjusted for restoration of mean systemic blood pressure and SVRI (Time 2). Group N: 20 patients received norepinephrine intravenous infusion titrated according to the response of patients (Time 2). The maximum dose of norepinephrine was 2 μg/kg/min in both groups.
Exclusion criteria were previous cardiac surgery, metabolic, renal or hepatic disorders, ejection fraction (EF) lower than 0.50, with mean pulmonary arterial pressure (MPAP) more than 15 mmHg [both were determined by echocardiography], and patients with history of glucose-6-phosphate dehydrogenase (G6PD) deficiency. Also patients with abnormal valve area and patients with more than mild aortic or mitral regurge were excluded due to (echo) measurement difficulties. Patients not responding to the maximum norepinephrine dose of 2 μg/kg/min were excluded from the study and replaced by equal number of patients fulfilling the inclusion criteria.
Children were given 0.5 mg/kg of midazolam half an hour before induction of anesthesia as oral premedication. Anesthesia was induced by intravenous (IV) midazolam (0.05 mg/kg), propofol (1 mg/kg) and fentanyl (10 μg/kg). Anesthesia was maintained by inhalational isoflurane 0.5% supplemented with fentanyl increments of 1 μg/kg. Pancuronium bromide was used for neuromuscular blockade. Ventilation was pressure controlled (to ensure normal blood gases). Anticoagulation was accomplished with 400 IU/kg of heparin and further doses were added as required to maintain an activated clotting time longer than 400 s.
All CPB was primed by colloids and carried out at non-pulsatile, filtered arterial pump flow 2.5–3.0 L/min/m2 and gravity venous drainage. A hollow-fiber membrane oxygenator was used. Initial ante-grade warm hyperkalemic blood cardioplegia (20 ml/kg) was used and (10 ml/kg) was repeated every 25 min. The hematocrit was kept at 25% and the pH was managed by alpha-state strategy. Mean arterial pressure (MAP) was kept between 30 and 50 mmHg. Patients were cooled down to 28–30 °C. Nitroglycerin infusion (1–3 μg/kg/min) was started during CPB and titrated according to need. Once CPB re-warming was started, norepinephrine infusion (0.05–0.5 μg/kg/min) was added according to the hemodynamic parameters. Hypertension and hypotension were defined as an increase or a decrease in Mean Arterial Pressure (MAP) by ⩾20% from baseline, respectively. Hypertension was treated with increments of fentanyl. Hypotension was treated by intravenous administration of lactated Ringer’s solution (3–5 ml/kg). The reperfusion period before weaning from CPB was one-third of the aortic cross-clamping time. After weaning, the remaining blood volume of the CPB circuit was retransfused until the end of the operation. Heparin effects were reversed by protamine at a 1:1 ratio.
EF and MPAP were determined using trans-esophageal echocardiography. Left ventricular ejection fraction and MPAP were defined as normal when more than 0.50 and within 12–15 mmHg respectively.
For all patients, demographic data, past medical and drug history, pre-operative EF, type of surgical intervention, number of doses and total dose of cardioplegia, the aortic cross-clamp, and CPB times were recorded. The direct invasive MAP, central venous pressure (CVP), heart rate (HR), pulse oximetry (SpO2), end tidal CO2, and electrocardiogram were monitored continuously.
CO, CI, SVR and SVRI were assessed after CPB and one hour after finishing infusion of MB using trans-esophageal echo and calculation. The total dose of norepinephrine was recorded in each group. For all cases, the pre-operative diameter of aortic valve annulus (mm), aortic valve area (cm2), and aortic valve area index (cm2/m2) were recorded.
Calculation of SVR was carried out through measurement of time velocity integral (TVI) on the LVOT in the deep trans-gastric four chamber view using pulsed wave Doppler (PWD). This was achieved through adoption of the following equations:where TVI is the time velocity integral that represents distance of the RBC crossed in a single heart beat.
AVA is aortic valve area through which blood flows to systemic circulation.
Assuming that LVOT is a cylinder so blood volume ejected in a single heart beat will equal the volume of a cylinder = Base area × height or AVA × TVI = Stroke volume (SV)
This method has been clinically validated by Abbas and his colleagues [Citation12] who conducted that “Doppler echocardiography provides a reliable non invasive assessment of SVR”.
Pressure gradient is the difference between mean arterial blood pressure and central venous pressure MAP–CVP.
| #x2022; | So final equation will be | ||||
Systemic vascular resistance index will be calculated as followsNormal values:
| #x2022; | Stroke volume index 40–85 mL/m2/beat. | ||||
| #x2022; | Systemic vascular resistance index 1970–2390 dynes s/cm5/m2. | ||||
| #x2022; | Systemic vascular resistance 900–1600 dynes s/cm5. | ||||
| #x2022; | Normal value of TVI LVOT 16 ± 3 cm. | ||||
| #x2022; | Normal value of TVI aortic valve 22 ± 4 cm. | ||||
| #x2022; | Aortic valve area index 1.33 cm2/m2. | ||||
Sample size
A sample size of 18 patients per group achieves 83% power to detect a mean of paired differences of 300.0 in SVR with an estimated standard deviation of differences of 200.0 and with a significance level (alpha) of 0.05 using a two-sided paired t-test. We included 20 patients in each group for possible dropouts.
.1 Statistical analysis
The statistical analysis was performed using a standard SPSS software package version 17 (Chicago, IL). Normally distributed numerical data are presented as mean ± SD and differences between groups were compared using the independent Student’s t-test, and categorical variables were analyzed using the χ2 test or Fisher’s exact test as appropriate and are presented as number (%). p-value < 0.05 is considered statistically significant.
Results
There were no significant differences in patients’ characteristics between the two groups (). Cardiopulmonary bypass details showed no significant differences between the two groups ().
able 1 Patients’ Characteristics.
able 2 Cardiopulmonary bypass details.
Norepinephrine dose in (Time 2) was significantly decreased in MB group and significantly increased in N group compared to pre-infusion (Time 1) values in each group. Post-infusion (Time 2) norepinephrine dose was significantly lower in MB group compared to N group ().
able 3 Comparison between Time 1 and Time 2 values of the studied drugs in heart rate, MAP and CVP.
The Time 1 values of heart rate, MAP and CVP showed no significant differences between the two groups (). The MAP increased significantly in Time 2 compared to Time 1 in MB group while there was no significant increase in Time 2 compared to Time 1 in N group. The Time 2 MAP was significantly higher in MB group compared to N group. The HR decreased significantly in Time 2 compared to Time 1 in MB group while it showed no significant change in Time 2 compared to Time 1 in N group. This was accompanied with significant decrease in HR in MB group when compared to N group. No change was detected in the rhythm in the two groups (none of the patients showed major or prolonged dysrhythmia which needed defibrillation or pacing). In addition there was significant increase in CVP in Time 2 compared to Time 1 in MB group while N group showed no change in Time 2 compared to Time 1, and also in Time 2 CVP was higher in MB group compared to N group ().
The Time 1 values of CO, CI, MPAP, SVR and SVRI showed no significant differences between the two groups (). There was a significant decrease in CO and CI and significant increase in SVR and SVRI in Time 2 compared to Time 1 in MB group while in N group, there was no significant change comparing Time 2 to Time 1 in CO, CI, SVR and SVRI. Time 2 values of CO and CI were significantly lower in MB group compared to N group and SVR and SVRI were significantly higher in MB group compared to N group. Time 2 values of MPAP did not show significant change compared to Time 1 values in both groups. Time 2 values of MPAP were comparable in both groups ().
able 4 Comparison between Time 1 and Time 2 values of the studied drugs in CO, CI, MPAP, SVR and SVRI.
The mean readings of pulse oximetry after bypass were 98.6 ± 0.6% for MB group and 98.8 ± 0.7% for N group with no significant difference between both groups (p-value 0.875). All the 20 cases in MB group showed change of the color of urine to greenish blue. No side effects from using MB were recorded as arrhythmia, respiratory distress, and pulmonary edema (see ).
Discussion
In this prospective randomized controlled study, methylene blue significantly increased MAP, CVP, SVR, and SVRI while this effect was not observed in norepinephrine group. MB also decreased the dose requirements of norepinephrine used with no recorded side effects (except bluish discoloration of urine) thus improving the outcome in pediatric patients suffering from vasoplegic syndrome following CPB.
Vasoplegic syndrome is a state of endothelial dysregulation resulting in persistent hypotension and low systemic vascular resistance despite adequate fluid resuscitation and vasopressor administration [Citation13]. It is a form of severe systemic inflammatory response syndrome (SIRS) that occurs during the early postoperative period after cardiac surgery with cardiopulmonary bypass [Citation4] due to generation of pro-inflammatory mediators secondary to surgical stress, hypothermia, neutralization of heparin with protamine, the transfusion of blood products or occurrence of endotoxemia due to repeated episodes of hypotension from displacement and mobilization of the heart [Citation14]. Additional factors to the development of VS include preoperative chronic heart failure with low EF less than 35%, preoperative use of angiotensin converting enzyme inhibitors, Beta blockers, pre- and post-operative use of amiodarone and phosphodiesterase inhibitors [milrinone] [Citation15,Citation16].
It is reported that SIRS occurring after cardiac surgery is associated with a massive, unbalanced induction of inflammatory cytokines IL-6 and IL-8 [Citation17] and activation of inducible form of nitric oxide (NO) synthase [Citation18]. NO in turn stimulates the production of cGMP. Excessive formation of NO and cGMP is related to profound vasodilatation, myocardial depression, and decreased response to catecholamines [Citation7]. Methylene blue antagonizes the effect of NO and other nitrovasodilators in the endothelium and vascular smooth muscle by acting competitively with NO, binding to iron heme-moiety of soluble guanylylcyclase (sGC) and blocking sGC action in vascular smooth muscle [Citation19]. Regarding recovery and better control of blood pressure, it must be made clear that MB alone is not a vasoconstrictor. By blocking the cGMP, it “releases” the cAMP system in a kind of “crosstalk” between the two systems, and thus, norepinephrine exercises its vasoconstrictor effect [Citation20]. MB has chiefly been used to treat patients with septic shock and low peripheral vascular resistance [Citation21,Citation22].
In the present study, MB group showed a significant increase in MAP, CVP, SVR, and SVRI accompanied with a significant decrease in CO, CI and HR but with no arrhythmia detected which indicated an improvement in the signs of VS. This is consistent with a study done by Leyh et al. [Citation23] who used MB in a single dose (2 mg/kg) after CPB and recorded an increase in MAP, a decrease in the dose of norepinephrine, a significant decrease in CO and a decrease in the serum lactate within 24 h in the MB group.
Another study was done by Levin et al. [Citation24] who used MB in a dose of 1.5 mg/ kg over 1 h to treat VS after CPB and found that VS resolved completely in MB group with lower mortality compared to the control group. Also Maslow et al. [Citation25] used MB after CPB in patients who received ACEI in a dose of 3 mg/kg and recorded an increase in the MAP and SVR, decreased norepinephrine requirements and decreased serum lactate.
Regarding ethical aspects, it can be affirmed that MB can be used in clinical practice because it has been used since late 19th century for the treatment of malaria, which granted Paul Ehrlich the Medicine Nobel Prize in 1908. MB is the precursory molecule of the following: chemotherapy, antibiotics and neuroleptics (chlorpromazine), with long use as an urinary antiseptic, Schizophrenia treatment was an additive to stored blood bags, in order to neutralize microorganisms [Citation20].
In the current study, no statistically significant change was observed in MPAP as expected in the MB group (the same observation was recorded in the norepinephrine group); this is mainly attributed to the small dose of MB used. Several studies stated that unlike in the systemic circulation, MB induced dose-dependent increases in pulmonary arterial pressure and vascular resistance [Citation26,Citation27].
The current study is one of few studies that discussed the probability of using MB in pediatrics. There is no dose regimen for MB in children but using it in a dose less than 2 mg/kg appears to be safe (lethal dose is 40 mg/kg) [Citation28]. Thus the MB dose in this study was chosen to be 1.5 mg/kg. No side effects were observed other than change in the color of urine. MB was used in other studies on pediatric patients to treat refractory hypotension due to sepsis [Citation10,Citation29] and methemoglobinemia [Citation11,Citation30]. Another study used MB in a dose of 1 mg/kg on a 5 years old girl with hypoplastic left ventricle with a failed Fontan operation who underwent heart transplantation and developed VS and reported a successful recovery [Citation31]. Taylor and Holtby reported a child with vasoplegia who received 2 doses of MB at 2 mg/kg intravenously for hypotension in relation to bypass and recovered from VS [Citation32]. Lee and Ing also reported the successful use of MB in a 7 years old child with dilated cardiomyopathy supported by a left ventricular assisted device (VAD), a pulsatile extracorporeal device, and preoperatively anticoagulated with warfarin presented for orthotopic heart transplant. The course was complicated by persistent bleeding treated with prothrombin complex concentrate and refractory post bypass vasoplegia treated with methylene blue [Citation33].
Although this is the first prospective comparative controlled randomized study that used a single dose of MB comparing it to norepinephrine in VS in children, it did not follow the need of patients for a repeated dose and it did not include all pathological types of congenital heart diseases.
In conclusion, MB showed superior efficacy and safety in managing VS in pediatrics following CPB compared to norepinephrine. The present study is still a step toward establishing MB as a commonly used drug in VS even if it is not the first choice. More studies are needed to compare it to other commonly used drugs as vasopressin.
Conflict of interest
There is no conflict of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- T.CarrelL.EnglbergerP.MohacsiLow systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importanceJ Card Surg152000347353
- X.SunL.ZhangP.C.HillIs incidence of postoperative vasoplegic syndrome different between off-pump and on-pump coronary artery bypass grafting surgery?Eur J Cardiothorac Surg342008820825
- J.G.LaffeyJ.F.BoylanD.C.ChengThe systemic inflammatory response to cardiac surgery: implications for the anesthesiologistAnesthesiology972002215252
- W.J.GomesA.C.CarvalhoJ.H.PalmaVasoplegic syndrome: a new dilemmaJ Thorac Cardiovasc Surg1071994942943
- E.OzalE.KuralayV.YildirimPreoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgeryAnn Thorac Surg79200516151619
- J.A.RussellK.R.WalleyJ.SingerVasopressin versus norepinephrine infusion in patients with septic shockN Engl J Med3582008877887
- P.R.EvoraShould methylene blue be the drug of choice to treat vasoplegias caused by cardiopulmonary bypass and anaphylactic shock?J Thorac Cardiovasc Surg1192000632634
- P.R.EvoraR.L.LevinMethylene blue as drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypassJ Thorac Cardiovasc Surg12732004895896
- M.Y.KirovO.V.EvgenovN.V.EvgenovInfusion of methylene blue in human septic shock: a pilot, randomized, controlled studyCrit Care Med29200118601867
- W.DriscollS.ThurinV.CarrionEffect of methylene blue on refractory neonatal hypotensionJ Pediatr12961996904908
- R.PrasadR.SinghO.P.MishraDapsone induced methemoglobinemia: intermittent continuous intravenous methylene blue therapyIndian J Pediatr7532008245247
- A.E.AbbasF.D.FortuinB.PatelNoninvasive measurement of systemic vascular resistance using Doppler echocardiographyJ Am Soc Echocardiogr172004834838
- W.J.GomesA.C.CarvahloJ.H.PalmsVasoplegic syndrome after open heart surgeryJ Cardiovasc Surg (Torino)391998619623
- W.J.GomesM.R.EvlichmanM.L.Batista-FilhoVasoplegic syndrome after off-pump coronary artery bypass surgeryEur J Cardiothorac Surg232003165169
- S.G.RajaG.D.DreyfusVasoplegic syndrome after off-pump coronary artery bypass surgeryTexas Heart Inst J312004421424
- L.HosseinianM.WeinerM.A.LevinG.W.FischerMethylene blue: magic bullet for vasoplegia?Anesth Analg12212016194201
- S.HiraiSystemic inflammatory response syndrome after cardiac surgery under cardiopulmonary bypassAnn Thorac Cardiovasc Surg92003365370
- U.IkedaK.KurosakiK.OhyaK.ShimadaAdenosine stimulates nitric oxide synthesis in vascular smooth muscle cellCardiovasc Res351997168174
- A.M.OliveiraN.M.QuarteW.V.VicenteMethylene blue: an effective treatment for contrast medium induced anaphylaxisMed Sci Monit9CS2003102106
- P.R.EvoraL.J.AlvesC.A.FerreiraTwenty years of treatment of vasoplegic syndrome in heart surgery. Methylene blue revisedRev Bras Cir Cardiovasc30120158492
- J.C.PreiserP.LejeuneA.RomanMethylene blue administration in septic shock: a clinical trialCrit Care Med231995259264
- Y.GaliliY.KlugerZ.MiamskiMethylene blue: a promising treatment modality in sepsis induced by bowel perforationEur Surg Res291997390395
- R.G.LeyhT.KofidisM.StrüberMethylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypassJ Thorac Cardiovasc Surg125200314261431
- R.L.LevinM.A.DegrangeG.F.BrunoMethylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgeryAnn Thorac Surg7722004496499
- A.D.MaslowG.StearnsP.ButalaThe hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary by-passAnesth Analg1031200628
- J.R.FinemanM.R.CrowleyM.A.HeymannIn vivo attenuation of endothelium-dependent pulmonary vasodilation by methylene blueJ Appl Physiol711991735741
- Z.HaiboR.PeterP.Jean-CharlesEffects of methylene blue on oxygen availability and regional blood flow during endotoxic shockCrit Care Med23199517111721
- A.C.MenardiF.ViaroW.V.VicenteHemodynamic and vascular endothelium functions studies in healthy pigs after intravenous bolus infusion of methylene blueArq Bras Cardiol8742006525532
- C.RutledgeB.BrownK.BennerA novel use of methylene blue in the pediatricPediatrics13642015e1030e1034
- K.AllegaertM.MiserezT.LerutMethemoglobinemia and hemolysis after enteral administration of methylene blue in a preterm infant: relevance for pediatric surgeonsJ Pediatr Surg3912004e35e37
- T.BhallaA.SawardekarH.RussellJ.D.TobiasThe role of methylene blue in the pediatric patient with vasoplegic syndromeWorld J Pediatr Congenit Heart Surg22011652655
- K.TaylorH.HoltbyMethylene blue revisited: management of hypotension in a pediatric patient with bacterial endocarditisJ Thorac Cardiovasc Surg13022005566
- J.K.LeeC.IngProthrombin complex concentrate and methylene blue for treatment of coagulopathy and vasoplegia in a pediatric heart transplant patientA Case Rep652016127129