Abstract
Objective
We designed this study to test whether dexmedetomidine 1 μg/kg can be an alternative to epinephrine 5 μg/ml (1/200.000) as an adjuvant to lidocaine 2% for fastening the extension of labor epidural analgesia into an adequate block for emergency cesarean section (CS).
Methods
Sixty patients having epidural analgesia for normal delivery who required emergency CS were assigned to either lidocaine–epinephrine (LE) group (n = 30) received 19 ml of lidocaine 2% and 1 ml containing 5 μg epinephrine or lidocaine–dexmedetomidine (LD) group (n = 30) received 19 ml of lidocaine 2% and 1 ml containing 1 μg/kg dexmedetomidine. If the patient feels any discomfort (VAS >3) during surgery, intravenous fentanyl 25–50 μg was given. Sedation level was assessed using five points numerical scale.
Results
Both groups were comparable regarding the onset time and time to maximum block height, p value >0.05. The number of patients required intraoperative fentanyl was higher in LE group compared to LD group, p value <0.05. The mean total fentanyl supplementation was more in LE group compared to LD group, p value <0.001. Overall sedation score was higher in LD group than in LE group (p value <0.001), and more patients had bradycardia in LD group compared to LE group (p value <0.001). The mean time to two segment regression, mean time to regression to Bromage 0 and mean time to first analgesic requirement were significantly longer in LD group compared to LE group, p value <0.001.
Conclusion
Epidural dexmedetomidine is comparable to epinephrine as an adjuvant to epidural lidocaine in fastening the onset of surgical anesthesia and resulted in better intraoperative analgesia and in longer duration of sensory and motor block in the settings of converting labor epidural analgesia for emergency CS.
Introduction
Epidural analgesia is commonly used to provide pain relief during labor. To extend the epidural analgesia in labor for emergency cesarean section (CS), a fast onset solution of local anesthetic is required aiming to achieve rapid and good quality of epidural anesthesia. Different solutions of local anesthetics are used for this reason. The optimum choice of local anesthetic solution for achieving rapid and reliable epidural anesthesia for CS is still not clarified [Citation1]. The solution used is selected according to local policies.
Addition of other drugs to the local anesthetic as adrenaline, bicarbonate and fentanyl has been described [Citation2–Citation4].
Hillyard et al. [Citation5] reported in meta-analysis that the combination of 2% lidocaine with epinephrine for epidural top-up provided the fastest onset of surgical anesthesia.
Dexmedetomidine is α-2 adrenergic agonist with analgesic properties that augment local anesthetic effects when given by epidural route [Citation6,Citation7]. It was demonstrated that dexmedetomidine enhances the local anesthetic action of lidocaine either by causing vasoconstriction around the site of injection delaying lidocaine absorption and hence prolonging its action or by causing direct inhibition of the peripheral neuronal activity [Citation8].
This study was designed to test whether dexmedetomidine 1 μg/kg can be an alternative to epinephrine 5 μg/ml (1/200.000) as an adjuvant to lidocaine 2% for speeding the onset to fasten the extension of epidural analgesia into an adequate block for emergency CS in patients having epidural analgesia for normal delivery who required CS.
Our primary outcome was the time for T4 loss of sensation to cold (onset time). Secondary outcomes were maximum block height, block duration, need for intravenous fentanyl supplementations, side effects, and neonatal outcomes.
Materials and methods
After obtaining institutional ethical approval, an informed written consent was signed by each patient included in the study; mothers were instructed and agreed not to breast-feed their babies for 24 h. Sixty patients between 20 and 40 years old, American Society of Anesthesiology (ASA) I–II, were included in the study at Saad Specialist Hospital, Alkhobar, Saudi Arabia, in the period between January 2014 and July 2015. Inclusion criteria were as follows: emergency CS in the absence of maternal or fetal compromise (category 2, 3) () [Citation9], well-functioning epidural catheter (required 2 or less intra-partum supplementation with bupivacaine 0.125%), and uncomplicated pregnancy with singleton pregnancy ⩾36 weeks of gestation. Exclusion criteria included emergency CS with maternal or fetal compromise (category 1) () [Citation9], a poorly functioning epidural catheter during the labor, if narcotics or alpha 2 agonists rather than our regimen was given within the previous 4 h, last intrapartum supplementation of epidural catheter less than 2 h and if the parturient had pre-eclampsia or eclampsia, bleeding, liver impairment, renal impairment, diabetes mellitus or cardiac disease.
able 1 Categorization of urgency of cesarean section [Citation9].
The routine method used for epidural labor analgesia in our hospital is the administration of a bolus of 10 ml 0.125% bupivacaine with 50 μg fentanyl followed by a continuous infusion at a constant rate (10–12 ml/h) of 0.125% bupivacaine with 2 μg/ml fentanyl. An additional bolus of 5 ml 0.125% bupivacaine was supplemented when required aiming to have adequate analgesia for labor up to T10 level.
Patients were randomly assigned by computer-generated random numbers using sealed envelopes to one of two groups. Group lidocaine–epinephrine (LE) (n = 30) received 19 ml of lidocaine 2% and 1 ml containing 5 μg (1:200.000) epinephrine ((Daihan Pharm Co., Seoul, Korea) and group lidocaine–dexmedetomidine (LD) (n = 30) received 19 ml of lidocaine and 1 ml containing 1 μg/kg dexmedetomidine (Precedex®, Hospira, Lake Forest, IL, USA). Both solutions were identical and labeled as test medication and freshly prepared by a pharmacist not involved in the study who allocated the patients according to the number in the sealed envelope. The solution was injected over 5 min using stopwatch (4 ml/min) through epidural catheter after negative aspiration to both blood and CSF.
To convert the epidural analgesia to epidural anesthesia for emergency CS patients were shifted to the operating room, and electrocardiography, pulse oximetry, and non-invasive blood pressure monitoring were applied. An intravenous infusion of 500 ml Lactated Ringer’s solution started. The patient was positioned in the supine position with a left lateral tilt using a wedge. Baseline blood pressure and heart rate were recorded, immediately before the top-up, assessment of both the sensory level (cold) and the degree of motor block (modified Bromage scale score [Citation10]: 0, patient can raise extended leg; 1, can bend knees; 2, can bend ankles; 3, unable to bend knees or ankles).
After injecting the study medication the block was assessed every 2 min. Sensory block was assessed in both sides in the midclavicular line using ice (cold sensation) and motor block assessed by using modified Bromage Scale.
The highest sensory level was recorded; time to highest sensory level and time from skin incision to delivery were recorded.
Systolic blood pressure (SBP) and heart rate (HR) were measured every 2 min, ephedrine 5 mg boluses were given if SBP dropped <100 mmHg or >20% from baseline and repeated every 5 min if needed, and atropine 0.5 mg boluses were given iv if the HR was less than 55 beats/min and repeated after 5 min if needed (maximum 2 mg).
If the patient had any discomfort during surgery (VAS > 3), analgesic supplementation was provided by intravenous fentanyl 25–50 μg over 5 min. If the patient was not satisfied general anesthesia was provided.
Recovery from sensory block was defined as the time from injection of our test medications till the patient requested analgesia (VAS ⩾ 4), and recovery from motor block was defined as time from injection of our test medications till Bromage score = 0 (tested each ½ h postoperatively).
Our primary outcome was the time to loss of cold sensation to T4 on both sides (compared to cold sensation on the shoulder), tested every 2 min from the end of the epidural injection of the test solution. Secondary outcomes were maximum block height, block duration, need for intravenous fentanyl supplementations, side effects, and neonatal outcomes.
Sedation level was assessed every 20 min using five points numerical scale (0 means fully awake, 1 means calm, 2 means awake on verbal commands, 3 means awake on gentle tactile stimulation, 4 means awake on vigorous stimulation and 5 means unarousable).
The surgeons, who were blinded to the given medication, were asked to qualify the operative condition by the following scale: 1 = unsuccessful, 2 = poor, 3 = acceptable and 4 = perfect.
Sample size was calculated depending on previous study done by Lucas et al. [Citation2]; they reported that 30% reduction in the time for loss of cold sensation at T4 is required between groups to be clinically significant. Hence, 30 patients per group were calculated with an alpha value of 0.05 and power of 80%.
.1 Statistical analysis
Data were described in terms of mean ± standard deviation (±SD), median and range, or number of cases (frequency) and percentages when appropriate. Comparison of numerical variables between the study groups was done using Student’s t test for independent samples in comparing normally distributed data and Mann–Whitney U test for independent samples when data were not normally distributed. For comparing categorical data, Chi square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. p values less than 0.05 were considered statistically significant. All statistical calculations were done using computer program SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) release 15 for Microsoft Windows (2006).
Results
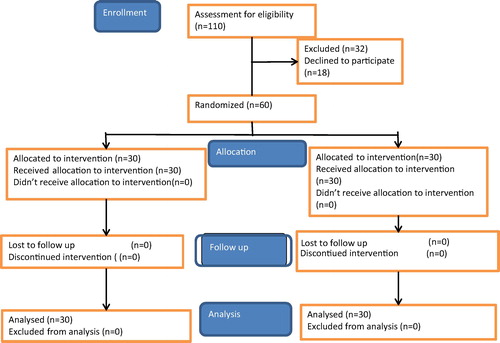
A total of 110 parturients were surveyed for their eligibility. Thirty-two of them did not meet the inclusion criteria and eighteen patients refused to participate. The sixty remaining patients were randomized into two equal groups (). Both groups had similar characteristics as regards age, height, weight, gestational age and parity ().
able 2 Demographic and intraoperative data.
Time to T4, duration of operation, intraoperative fluid requirement, maximum height of the block, time to maximum block, time to incision and time to delivery were comparable in both groups ().
Only 4 patients in group LD required fentanyl supplementation (25 μg) compared to 11 patients in group LE (7 patients required 25 μg and 4 patients required 50 μg); p value <0.05, .
Also mean total fentanyl requirement was significantly less in group LD (25 ± 0 μg) compared to group LE (34 ± 13 μg); p value <0.001, .
Apgar scores at 1 min and 5 min were comparable in both groups as shown in . Surgeon’s satisfaction was also comparable in both groups (p value >0.05).
Data of the pre-existing epidural are represented in , and there was no significant difference between both groups regarding duration of analgesia, pre-existing sensory and motor levels, pre-existing VAS and the time elapsed since the last top-up, p value >0.05.
able 3 Data of pre-existing epidural analgesia.
There was a significant difference in the overall degree of sedation between both groups , p value <0.001. Higher number of patients in group LE were in grade 0 (19 patients), while in group LD higher number of patients were in grade 3 (14 patients).
able 4 Degree of sedation.
There was no statistically significant difference between groups regarding the number of patients who had hypotension, nausea and vomiting, dizziness and respiratory depression, p value >0.05 ().
able 5 Incidence of side effects.
The number of patients who had bradycardia were significantly more in group LD (9 patients) compared to group LE (none of the patients had bradycardia), p value <0.001 ().
The postoperative characteristics regarding mean time to two segment regression, mean time to regression to Bromage score 1 and mean time for 1st analgesic requirement were significantly longer in group LD than in group LE, p value (<0.001) .
able 6 Postoperative characteristics.
Discussion
Our results showed that using dexmedetomidine 1 μg/kg as adjuvant to lidocaine 2% for converting epidural analgesia to anesthesia for emergency CS in parturient with satisfactory epidural catheter was comparable to epinephrine 1/200.000 (5 μg/ml) as regards the fast onset time of surgical anesthesia and resulted in better intraoperative analgesia and in longer duration of sensory and motor block and better sedation without adverse events on neonatal outcome.
Converting labor epidural analgesia to surgical anesthesia for emergency CS is frequently needed and is considered one of the advantages of labor epidural analgesia.
Pan et al. [Citation11] found that 41% of pre-existing labor epidural catheters were used for CS, showing the common practice of extending epidural block in the presence of a well-functioning epidural catheter.
The fast speed of onset of epidural anesthesia after top-up doses is very important in emergency cesarean section in certain situations as was emphasized in the report of “Saving Mother’s lives” [Citation12].
Previous studies reported that lidocaine–epinephrine combination with or without fentanyl has the fastest onset when comparing with other local anesthetic solutions [Citation5,Citation13,Citation14]. The vasoconstrictor effect of epinephrine reduces the absorption of lidocaine into the bloodstream, consequently reduces its systemic toxicity and prolongs its duration of action [Citation15]. However, epinephrine use can result in hypertension, tachycardia and arrhythmia and should be used cautiously in hypertensive, hyperthyroid and cardiac patients [Citation16].
We compared dexmedetomidine with epinephrine in our study as epinephrine is used as a standard adjunct to local anesthetic due to its vasoconstrictor property [Citation16]. We chose the concentration of epinephrine 1:200.000, as it is the amount generally used to produce vasoconstriction when added to local anesthesia [Citation16].
Previous researches have shown that single injection (over 3–5 min) of 20 ml epidural lidocaine 2% with adrenaline was safe [Citation17].
The maximum block heights reached in both groups were (T1–T4) in lidocaine epinephrine group and T2–T4 in lidocaine dexmedetomidine group.
Although Price et al. reported extension of the block up to C7 with no serious complications [Citation17], others reported that, this is unlikely to happen if top-up is injected after a previously well-functioning epidural block [Citation18] and can happen occasionally, if the epidural catheter is misplaced in the subdural space, a large top-up can lead to tearing of the arachnoid and leads excessively high block [Citation19].
In our study patients received lidocaine–epinephrine were ready for surgery after 10 ± 1 min. And patients in lidocaine–dexmedetomidine group were ready after 10 ± 0 min. This coincides with the results of Price et al. [Citation17], who reported that 92% of patients were ready for surgery within 10 min of using lidocaine 2% with 1/200.000 epinephrine for epidural top-up.
A previous trial reported the enhancement effect of dexmedetomidine on the local anesthetic effect of lidocaine through α2-A adrenoceptor, which is caused either by vasoconstriction around the site of injection resulting in reduced absorption of local anesthetic and hence prolonging its duration of action or by direct inhibition of peripheral neuronal activity [Citation8].
Our results support the previous studies in which dexmedetomidine was used as an additive to local anesthetics; Bajwa et al. [Citation20] reported that the use of epidural dexmedetomidine 1.5 μg/kg as adjuvant to epidural ropivacaine resulted in early sensory and motor block and prolonged postoperative analgesia in patients undergoing vaginal hysterectomy and found that it is a better adjuvant than clonidine in terms of intra-operative and postoperative analgesia, cardiorespiratory side effects and patient’s satisfaction. Also, Honoura et al. [Citation21], concluded that adding dexmedetomidine to bupivacaine and fentanyl improved intraoperative condition and quality of postoperative analgesia with no significant maternal or neonatal side effects. Also, El-Hennawy et al. [Citation22] compared dexmedetomidine and clonidine as additive to caudal bupivacaine in children undergoing lower abdominal surgeries and reported that both drugs significantly improved postoperative analgesia.
Apgar score was satisfactory in both groups at 1 min and 5 min, and this coincides with the results of Selim et al. [Citation23] who used dexmedetomidine along with LA for epidural analgesia during labor and reported good maternal satisfaction without deleterious side effects on uteroplacental circulation and neonatal outcome. This is explained by the unique pharmacokinetics of dexmedetomidine as it does not cross placenta significantly and it is highly lipophilic and consequently retained in placental tissue [Citation24].
Patients in lidocaine–dexmedetomidine group required less intravenous fentanyl supplementation than in lidocaine–epinephrine group, and this could be explained by the anti-nociceptive effect of dexmedetomidine as it is highly lipophilic and hence rapidly distributed in neural tissues binding to alpha 2 receptors in the spinal dorsal horn cells when administered neuroaxially [Citation25].
Dexmedetomidine can cause systemic effects as sympatholytic effect, sedation, anxiolysis as well as complications such as hypotension and bradycardia [Citation26].
Our study clearly shows the sedative effect of epidural dexmedetomidine as it caused sedation score grade 3 in 46.6% of cases who were awakened by gentle tactile stimulation and 30% of the patients were sedated with grade 2 sedation score who were awakened by verbal commands. In the lidocaine–epinephrine group 63.3% of patients were not sedated, only 10% of patients were grade 2 and 26.7% of the patients were grade 1; most probably the sedation was related to fentanyl supplementation.
The overall sedation scores were statistically significant with administration of dexmedetomidine.
30% of patients received lidocaine–dexmedetomidine had bradycardia which is one of the side effects of dexmedetomidine. The ɑ-2 agonist induced bradycardia is explained by their central effect on reducing sympathetic outflow [Citation26].
Although there was tendency to hypotension in LD group, the incidence of hypotension was statistically not significant between groups, 10% in LE group and 16.6% in LD group. This incidence coincides with the reported incidence of hypotension under epidural anesthesia for CS which ranges between 6.7% and 50% [Citation27]. The incidence of nausea and vomiting was also comparable in both groups 10% in LE group and 13.3% in LD group: hypotension could be one of the factors that induce nausea and vomiting [Citation28].
Both groups were comparable as regards the pre-existing block data; this excludes the presence of unequal blockade relating to previous labor analgesia.
Song et al., compared dexmedetomidine and epinephrine as an adjuvant to 1% mepivacaine in brachial plexus block and concluded that dexmedetomidine is good alternative as an adjuvant to local anesthesia in patients who are contraindicated to use epinephrine [Citation29].
There have been controversies regarding problems of breast feeding after epidural anesthesia in parturients [Citation30]. No studies were done to prove the safety of breast feeding after the use of dexmedetomidine; hence, we requested our patients to avoid breast feeding for 24 h after delivery [Citation30].
Limitations to our study: we mixed two drugs in each group and this can lead to errors and delay during emergency situation [Citation31], but we couldn’t use pre-mixed solutions as epinephrine degrades by 24 h when stored at 20 °C [Citation32].
Further studies are needed to find whether smaller doses of dexmedetomidine can be helpful in achieving fast onset time for surgical readiness without side effects.
Conclusion
Epidural dexmedetomidine is comparable to epinephrine as an adjuvant to epidural lidocaine in fastening the onset of surgical anesthesia and resulted in better intraoperative analgesia and in longer duration of sensory and motor block in the settings of converting labor epidural analgesia for emergency cesarean section.
Conflict of Interest
The authors declare that there are no conflict of interests.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- K.J.ReganG.O’SullivanThe extension of epidural blockade for emergency Caesarean section: a survey of current UK practiceAnaesthesia632008136142
- D.N.LucasG.K.CicconeS.M.YentisExtending low-dose epidural analgesia for emergency Caesarean section. A comparison of three solutionsAnaesthesia54199911731177
- D.T.C.LamK.S.KhawW.D.Ngan KeeExtension of epidural blockade in labour for emergency Caesarean section using 2% lidocaine with epinephrine and fentanyl, with or without alkalinisationAnaesthesia562001790794
- J.Goring-MorrisI.F.RussellRandomised comparison of 0.5% bupivacaine with a lidocaine/epinephrine/fentanyl mixture for epidural top-up for emergency caesarean section after ‘low dose’ epidural for labourInt J Obstet Anesth152006109114
- S.G.HillyardT.E.BateT.B.CorcoranM.J.PaehG.O.O’SullivanExtending epidural analgesia for emergency Caesarean section: a meta-analysisBr J Anaeth1072011668678
- D.MemisA.TuranB.Karamanlioğ luZ.PamukuI.KurtAdding dexmedetomidine to lidocaine for intravenous regional anesthesiaAnesth Analg982004835840
- G.E.KanaziM.T.AouadS.I.Jabbour KhouryAl.JazzarM.M.AlameddineR.Al-YamanEffect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal blockActa Anaesthesiol Scand502006222227
- T.YoshitomiA.KohijitaniS.MaedaH.HiguchiM.ShimadaT.MiyawakiDexmedetomidine enhances the local anesthetic action of lidocaine via an α-2A adrenoceptorAnesth Analg107962008101
- D.N.LucasS.M.YentisS.M.KinsellaA.HoldcroftA.E.MayM.WeeP.N.RobinsonUrgency of caesarean section: a new classificationJ R Soc Med932000346350
- A.C.GrahamJ.H.McClureQuantitative assessment of motor block in labouring women receiving epidural analgesiaAnaesthesia5652001470476
- P.H.PanT.D.BogardM.D.OwenIncidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: a retrospective analysis of 19,259 deliveriesInt J Obstet Anesth132004227233
- J.H.McClureG.M.CooperT.H.Clutton-BrockSaving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006–8: a reviewBr J Anaesth1072011127132
- V.ClarkE.McGradyC.SugdenJ.DicksonG.McLeodSpeed of onset of sensory block for elective extradural Caesarean section: choice of agent and temperature of injectateBr J Anaesth721994221223
- A.C.NortonA.G.DavisR.J.SpicerLignocaine 2% with adrenaline for epidural Caesarean section: a comparison with 0.5% bupivacaineAnaesthesia431988425430
- E.M.LauranceT.T.GregoryProperties, absorption and disposition of local anesthetic agentsM.J.CousinsP.O.BridenbaughNeural blockadein clinical anesthesia and management of pain medicine4th ed.2009Lippincott Williams & WilkinsPhiladelphia70
- J.F.ButterworthClinical pharmacology of local anesthetic agentsM.J.CousinsP.O.BridenbaughNeural blockade in clinical anesthesia and management of pain medicine2009Lippincott Williams & WilkinsPhiladelphia96113
- M.L.PriceF.ReynoldsB.M.MorganExtending epidural blockade for emergency caesarean sectionInt J Obstet Anesth119911318
- B.MorganUnexpectedly extensive conduction blocks in obstetric epidural analgesiaAnaesthesia451990148152
- F.ReynoldsH.M.SpeedyThe subdural space: the third place to go astrayAnaesthesia451990120123
- S.J.S.BajwaS.K.BajwaJ.KaurG.SinghV.AroraS.GuptaDexmedetomidine and clonidine in epidural anaesthesia: a comparative evaluationIndian J Anesth5522011116121
- S.E.HanouraR.HassaninR.SinghIntraoperative condition and quality of postoperative analgesia after adding dexmedetomidine to epidural bupivacaine and fentanyl in elective cesarean section using combined spinal-epidural anesthesiaAnesthesia72013168172
- A.M.El-HennawyA.M.Abd-ElwahabA.M.Abd-ElmaksoudH.S.El-OzairyS.R.BoulisAddition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in childrenBr J Anaesth10322009268274
- M.F.SelimA.M.ElnabtityA.M.HasanComparative evaluation of epidural bupivacaine – dexmedetomidine and bupivacaine -fentanyl on Doppler velocimetry of uterine and umbilical arteries during laborJ Prenat Med6320124754
- A.S.NairK.SriprakashDexmedetomidine in pregnancy: review of literature and possible useJ Obstet Anaesth Crit Care3201336
- A.PertovaaraAntinociception induced by alpha-2-adrenoceptor agonists, with special emphasis on medetomidine studiesProg Neurobiol401993691709
- P.TalkeE.LoboR.BrownSystemically administered alpha2-agonist-induced peripheral vasoconstriction in humansAnesthesiology9920036570
- H.L.SunQ.D.LingW.Z.SunR.S.WuT.J.WuS.C.WangLower limb wrapping prevents hypotension, but not hypothermia or shivering, after the introduction of epidural anesthesia for cesarean deliveryAnesth Analg992004241244
- C.C.RoutD.A.RockePrevention of hypotension following spinal anesthesia for cesarean sectionInt Anesthesiol Clin321994117135
- J.H.SongH.Y.ShimT.J.LeeJ.K.JungY.D.ChaD.I.LeeJ.W.KimJ.U.HanComparison of dexmedetomidine and epinephrine as an adjuvant to 1% mepivacaine in brachial plexus blockKorean J Anesthesiol6642014283289
- S.J.BajwaS.K.BajwaImpact of epidural analgesia on breast feeding: a possible relation and the existing controversiesJ Obstet Anaesth Crit Care220125759
- S.M.YentisK.RandallDrug errors in obstetric anaesthesia: a national surveyInt J Obstet Anesth122003246249
- J.RobinsonR.FernandoW.Y.S.Sun WaiF.ReynoldsChemical stability of bupivacaine, lidocaine and epinephrine in pH adjusted solutionsAnaesthesia552000853858