Abstract
Background
Adequate pain management has a great importance for smooth postoperative recovery, early hospital discharge and early rehabilitation. In this study is we compare between the analgesic effect and possible side effects of different routs of magnesium sulphate administration in cases of spinal anesthesia for knee arthroscopy.
Methods
120 patients scheduled for knee arthroscopy 4 groups (30 patients each): group C received only Bupivacaine intrathecally. group Mg-Sp received 50 mg Mg sulphate with Bupivacaine intrathecally, group Mg-Iv 10 min after intrathecal injection, received intravenous injection of 30 mg/kg Mg sulphate in 100 ml saline over 10 min followed by 10 mg/kg intravenous infusion over one hour and group Mg-Art received intra-articular injection of 800 mg Mg sulphate diluted in 12 ml normal saline (0.9% NaCl) 10 min before the end of surgery. Operative time in minutes, VAS at rest and at passive movement, time to be able to perform knee flexion, time from end of surgery until first requirement of analgesics, analgesic consumption, hemodynamic changes and any possible side effects were recorded.
Results
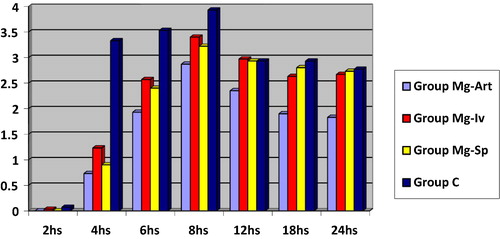
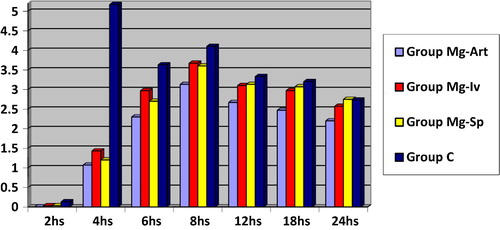
Regarding Time to be able to perform knee flexion, time taken from end of surgery until first analgesic dose and amount of pethidine consumption in first postoperative 24 h, group Mg-Art is significantly better than other groups (P = 0.000) (P = 0.000) (P = 0.000) respectivly. Group Mg-Iv is significantly better than group Mg-Sp and group C as regards time taken from end of surgery until first analgesic dose (P = 0.000) and as regards amount of pethidine consumption in first postoperative 24 h (P = 0.000). VAS at rest and with passive movement in group Mg-Art is significantly lower other groups (P = 0.000).
Conclusion
Intraarticular administration of magnesium sulphate is found to be better than Intravenous magnesium and intrathecal in postoperative analgesia.
1 Introduction
Adequate pain management has a great importance for smooth postoperative recovery, early hospital discharge and early rehabilitation [Citation1]. Preemptive and preventive analgesia decrease pain transmission in the central nervous system, prevent central excitability and therefore decrease pain after surgery or injury [Citation2].
N-methyl-D-aspartate (NMDA) receptors are involved in processes of central nociceptive transmission, modulation and sensitization of acute pain states [Citation3].
It is supposed that administration of NMDA receptor antagonists before surgical injury of the tissues leads to enhancement of preemptive analgesia as it attenuates central sensitization from peripheral nociceptive stimulation [Citation4].
Magnesium is a physiological and pharmacological blocker of (NMDA) receptors in neuronal tissue, so it is widely used now for the management of both acute and chronic pain [Citation5]. Various routes of administration, as intravenous, intrathecal, and epidural, were studied in giving magnesium as adjuvant in different analgesic regimens [Citation2,Citation4,Citation6].
Intraoperative administration of intravenous magnesium reduces postoperative opioid requirements in association with better quality of sleep and improved comfort [Citation7]. Intra-articular magnesium was also used and demonstrated to be effective in postoperative analgesia [Citation8].
The aim of this study is to compare between the analgesic effect and possible side effects of different routs of magnesium sulphate administration in cases of spinal anesthesia for knee arthroscopy.
2 Patient and methods
This study was an experimental prospective randomized controlled study conducted in Zagazig University Hospitals. After obtaining approval from the hospital ethics committee, written informed consents were taken from one hundred twenty patients ASA I and II who were scheduled for diagnostic or therapeutic knee arthroscopy. These patients were all patients scheduled for knee arthroscopy in Saturday and Tuesday for four consecutive months from January to April 2016. These two days were selected by the simple random sampling technique method. Patients were randomly allocated to one of four groups using a computer-generated randomization list (n = 30 for each group). Age, height and weight were documented. Exclusion criteria were patient refusal and contraindications of spinal anesthesia, age under 18 years, ASA class III, IV and V patients, sever hepatic or renal disease, abnormal blood electrolytes level, allergy to the test drug and patients who had received any analgesic drugs in the preceding 6 h. When the operative time exceeded 2 h, the cases were excluded. A day before surgery, patients were learnt on visual analogue pain scales (VAS): 1–10 with 10 = worst pain imaginable and 0 = no pain.
Half an hour before operation, all patients received 0.01 mg/kg atropine i.m.
After explanation of the technique and on arrival to the anesthetic room, i.v. access and routine monitoring in the form of electrocardiography, non invasive arterial blood pressure and oxygen saturation were established in all the patients. All Patients received 2 mg midzolam, 500 mL of lactated Ringer’s solution i.v. placed in the sitting position. 15 mg Bupivacaine 0.5% was injected intrathecally by 22-gauge, 119-mm Whitacre spinal needle placed by median or paramedian approach through L3-L4 intervertebral space.
Control group “group C” (30 patients) received only Bupivacaine intrathecally and did not receive Mg sulphate by any route of administration
Group Mg-Sp (30 patients) received 50 mg Mg sulphate (0.5 ml of 10% Mg sulphate) with Bupivacaine intrathecally. All Patients were immediately turned to the supine horizontal position.
Ten minutes after intrathecal injection, group Mg-Iv (30 patients) received intravenous injection of 30 mg/kg Mg sulphate in 100 ml saline over 10 min followed by 10 mg/kg intravenous infusion over one hour.
Group Mg-Art (30 patients) received intra-articular injection of 800 mg Mg sulphate (8 ml of 10% Mg sulphate) diluted in 12 ml normal saline (0.9% NaCl) 10 min before the end of surgery.
The following parameters were assessed:
| • | Operative time in minutes. | ||||
| • | Pain score measured by visual analogue scale (VAS) from zero to ten, with zero was no pain and 10 was the maximum pain. It was taken at rest as well as with passive movement at 2, 4, 6, 8, 12, 18 and 24 h postoperatively. | ||||
A rescue dose of pethidine 50 mg intravenously (cannot be repeated within 6 h) was available as a postoperative analgesia at the patient’s request or when the VAS value was higher than 4.
| • | Time to be able to perform knee flexion in minutes. | ||||
| • | Time from end of surgery until first requirement of analgesics (rescue dose). | ||||
| • | Amount of pethidine consumption in first postoperative 24 h. | ||||
| • | Recording of any side effects according to patient complaint within the postoperative 24 h (if founded) such as:
| ||||||||||||||||||||||||||||||||||
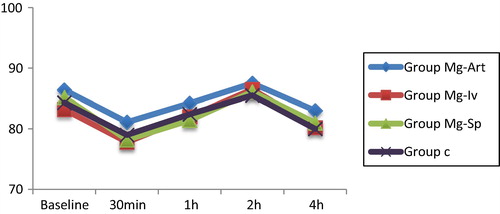
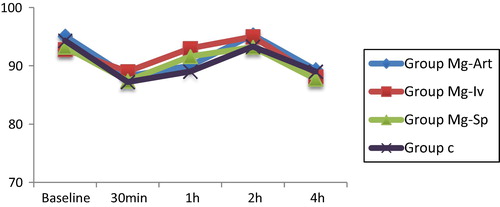
| • | Hemodynamics: Hypotension or bradycardia was considered with decrease in mean blood pressure or heart rate more than 20% from the base line. Rescue measures for hypotension and bradycardia were intravenous giving of 5 mg ephedrine and 0.5 mg atropine respectively. 5 reading were considered: basal, 30 min after anesthesia, 1 h after anesthesia, 2 h after anesthesia and 4 h after anesthesia. | ||||||||||||||||||||||||||||||||||
2.1 Statistical analysis
Collected data were handled using a data base software program (version 14.0, SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± SD, median or as number and percentage. ANOVA, chi square and Kruskal Wallis test were performed. P < 0.05 was considered as statistically significant.
3 Results
| • | The four groups were comparable regarding the patient characteristics in the term of demographic data of patients (). | ||||
| • | There was no statistically significant difference between groups regarding operative time (). | ||||
| • | Regarding Time to be able to perform knee flexion: Group Mg-Art was significantly lower than other groups. There were no statistically significant differences between group Mg-Iv and group Mg-Sp, but they were both significantly lower than Group C (). | ||||
| • | As regards time taken from end of surgery until first analgesic dose: Group Mg-Art was significantly higher than group Mg-Iv, group Mg-Sp and group C. Group Mg-Iv was significantly higher than group Mg-Sp and group C. Group Mg-Sp was significantly higher than group C (). | ||||
| • | illustrates the results of the amount of pethidine consumption in first postoperative 24 h: Group Mg-Art was significantly lower than the other groups. Group Mg-Iv was significantly lower than group Mg-Sp and group C. Group Mg-Sp was significantly lower than group C. | ||||
| • | illustrates VAS at rest at 2, 4, 6, 8, 12, 18 and 24 h postoperatively in the four studied groups:
| ||||||||||||||||||||||||||||
| • | illustrates VAS with passive movement at 2, 4, 6, 8, 12, 18 and 24 h postoperatively in the four studied groups:
| ||||||||||||||||||||||||||||
| • | There were no statistically significant differences in heart rate among groups (). There were no statistically significant differences in mean arterial pressure among groups (). There were no significant changes in heart rate or mean arterial pressure among time intervals within each group ( and ). | ||||||||||||||||||||||||||||
Table 1 Patients characteristics (mean ± SD).
Table 2 Operative time in minutes (mean ± SD).
Table 3 Time to be able to perform knee flexion in minutes.
Table 4 Time from end of surgery until first analgesic dose in minutes.
Table 5 Amount of analgesia (Pethidine) taken in first 24 h (mgs).
Side effects ():
| • | The incidence of nausea was 6.66% in group Mg-Art and group Mg-Iv, 10% in group Mg-Sp and it was 13.33 in % group C. There was no cases of vomiting except a single case in group C The incidence of shivering was 3.33% in group Mg-Art, 0% in group Mg-Iv, 3.33% in group Mg-Sp and 6.66% in group C. No patients had developed pruritis or respiratory depression in the four studied groups. | ||||
| • | There were no statistically significant differences between all groups regarding nausea, vomiting, pruritis, shivering or respiratory depression. | ||||
Table 6 Frequency of side effect (%).
4 Discussion
Although there are many studies on the analgesic effect of each route of magnesium administration, there is lack of researches comparing these different routes. This study is designed to compare the analgesic effect of intraarticular, intravenous and intrathecal magnesium sulphate administration in cases of knee arthroscopy receiving spinal anesthesia and to discuss their possible side effects.
The main finding of this study is that intraarticular administration of magnesium is found to be better than other routs of administration in cases of spinal anesthesia for knee arthroscopy.
Elsharnouby and co-workers [Citation9] found that intraarticular combining of magnesium sulphate with bupivacaine is more effective than using bupivacaine alone as regards reduction of postoperative pain.
In this study, we used the same intraarticular dose used by Radwan and co-workers [Citation10] who found that intra-articular magnesium sulphate is effective in increasing duration of analgesia, decreasing postoperative intake of analgesics and reducing postoperative pain.
Liu and co-workers [Citation11] emphasized on the fact of the peripheral antinociceptive effect of NMDA antagonists and demonstrated that NMDA antagonists inhibits excitation of nociceptive input terminals of C-fibers and this affects and limits central processing of pain. It is still possible that the action is achieved by both central (through systemic absorption of magnesium) and peripheral mechanisms.
Banwait and co-workers [Citation6] states that with any route of administration (intravenous, intrathecal, or epidural) the actual site of magnesium action is the spinal cord NMDA receptors. This may be the cause that results of this study shows that intravenous and intrathecal magnesium administration are comparable, with preference to intravenous rout in amount of analgesics needed in first postoperative 24 h and the time needed to first analgesic dose.
Pascual-Ramirez and co-workers [Citation12] supposed that addition of intrathecal magnesium sulphate to spinal anesthesia offers a longer period of postoperative analgesia and a longer time needed to first analgesic requirement as well as less amount of postoperatve consumed analgesics, but we did not recognize these effects clearly. The explanation of this may be introduced by Unlugenc and co-workers [Citation13] who suggested that changing pH of the injectate by adding magnesium sulphate to the local anesthetic may be the cause.
The results of previous studies on analgesic effects of intravenous and intrathecal magnesium sulphate are conflicting.
On the contrary of results of this study Ko and co-workers [Citation14] and Peach and co-workers [Citation15] found that perioperative administration of intravenous magnesium sulphate does not affect postoperative pain or increase duration of analgesia.
On the other hand, many previous studies [Citation16–Citation19] proved the effect of perioperatve infusion of magnesium sulphate on decreasing postoperative pain and decreasing analgesic requirements. Taheri and co-workers [Citation20] considered single intravenous magnesium sulphate dose (50 mg/kg) effective in decreasing postoperative pain and analgesic consumption.
Meta-analysis of Pascual-Ramirez and co-workers [Citation12] declared that addition of 50–100 mg of magnesium sulphate to intrathecal anesthetics will decrease postoperative anesthetic requirements without affecting the block or increasing adverse effects, but there is no evidence in its advantages over other available adjutants thus far.
However, Kherazi and co-workers [Citation4] found that intrathecal magnesium failed to prolong the time to rescue analgesic dose although it decreased the total analgesic requirements.
The contradiction between the results of this study and studies done by Ko and co-workers, peach and co-workers and Kherazi and co-workers may be attributed to the different types of surgical procedures. Patients of this study underwent knee arthroscopy while patient of the other studies underwent abdominal hysterectomy, cesarean section and lower limb surgeries respectively.
There are limitations in this study. Firstly, we used fixed doses of intrathecal (50 mg) and intravenous (30 mg/kg followed by 10 mg/kg) magnesium administration. Although these doses had been proved to be effective by previous studies [Citation4,Citation7,Citation16,Citation21,Citation22], but we assume that comparing larger doses may produce different results.
Secondly, this study did not measure the effect of intrathecal magnesium on the onset of both sensory and motor blockade.
As regard hemodynamic changes and side effects, statistically there were no significant differences between groups.
In conclusion, this study concluded that in cases of spinal anesthesia for knee arthroscopy, intraarticular administration of magnesium sulphate is better than itravenous or intrathecal administration regarding postoperative VAS, time needed to rescue dose of analgesics, amount of used postoperative analgesics and time to perform knee flexion. Intravenous magnesium is better than intrathecal as regards amount of analgesics needed in first postoperative 24 h and the time needed to first analgesic dose.
Our recommendation is to perform future studies to determine the least effective intraarticular magnesium dose for postoperative analgesia of knee arthroscopy cases.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
References
- M.A.DaabissA.KandilEvaluation of the effect of magnesium vs. midazolam as adjunct to epidural bupivacaine in patients undergoing total knee replacementBrit J Med Pract612013a610
- A.OlapourM.R.GousheM.SoltanzadehComparison of intravenous Magnesium and placepo administration on postoperative pain and analgesic consumption during spinal anesthesia for inguinal hernia repairJ Pharm Scient Innov (JPSI)2320131619
- R.S.BondokA.M.Abd El-HadyIntra-articular magnesium is effective for postoperative analgesia in arthroscopic knee surgeryBJA9732006389392
- M.-B.KhezriS.YaghobiM.HajikhaniComparison of postoperative analgesic effect of intrathecal magnesium and fentanyl added to bupivacaine in patients undergoing lower limb orthopedic surgeryActa Anaesthesiol Taiwanica5020121924
- M.F.M.JamesMagnesium: an emerging drug in anaesthesia. Editorial IBJA10342009465467
- S.BanwaitS.SharmaM.PawarR.GargEvaluation of single epidural bolus dose of magnesium as an adjuvant to epidural fentanyl for postoperative analgesia: a prospective, randomized, double-blind studySaudi J Anaesth632012273278
- A.BuvanendranR.J.McCarthyJ.S.KroinIntrathecal magnesium prolongs fentanyl analgesia: a prospective, randomized, controlled trialAnesth Analg952002661666
- T.B.SaritasH.BorazanS.OkesliIs intra-articular magnesium effective for postoperative analgesia in arthroscopic shoulder surgery?Pain Res Manag20120153538
- N.M.ElsharnoubyH.E.EidN.F.AbouElezzIntraarticular injection of magnesium sulphate and/or bupivacaine for postoperative analgesia after arthroscopic knee surgeryAnesth Analg1065200815481552
- Y.A.RadwanA.A.AlfekyM.F.FaramawiAnalgesic effect of intra-articular magnesium sulphate compared with bupivacaine after knee arthroscopic menisectomyJ Adv Res42013355360
- H.-T.LiuM.W.HollmanW.-H.LiuC.W.HoenemannM.E.DurieuxModulation of NMDA receptor function by ketamine and magnesium: Part IAnesth Analg92200111731181
- J.Pascual-RamirezS.Gil-TrujilloC.AlcantarillaIntrathecal magnesium as analgesic adjuvant for spinal anesthesia: a meta-analysis of randomized trialsMinerva Anestesiol792013667678
- H.UnlugencM.OzalevliM.GunduzS.GunastiComparison of intrathecal magnesium, fentanyl, or placebo combined with bupivacaine 0.5% for parturients undergoing elective cesarean deliveryActa Anaesthesiol Scand532009346353
- S.H.KoH.R.LimD.C.KimMagnesium sulfate does not reduce postoperative analgesic requirementsAnesthesiology952001640646
- M.J.PeachE.F.MagannD.A.DohertyDoes magnesium sulfate reduce the short- and long-term requirements for pain relief after caesarean delivery? A double-blind placebocontrolledAm J Obstet Gynecol194200615961602
- S.KaurN.BaghlaEvaluation of intravenous magnesium sulphate for postoperative analgesia in upper limb orthopaedic surgery under general anaesthesia: a comparative studyInter J Anesthesiol3022012
- E.M.ManaaA.F.AlhabibEffect of magnesium sulfate on the total anesthetic and analgesic requirements in neurosurgeryJ Neurol Neurophysiol201210.4172/2155-9562.S11-001 [S11-001]
- J.Y.HwangH.S.NaY.T.JeonIV infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesiaBJA104120108993
- J.H.RyuM.H.KangK.S.ParkEffects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesiaBJA10032008397403
- A.TaheriK.HaryalchiM.M.GhanaieEffect of low-dose (single-dose) magnesium sulfate on postoperative analgesia in hysterectomy patients receiving balanced general anesthesiaAnesthesiol. Res. Pract.20152015 Article ID 30614510.1155/2015/306145 6 pages
- Jabalameli M, Pakzadmoghadam SH. Adding different doses of intrathecal magnesium sulfate for spinal anesthesia in the cesarean section: a prospective double blind randomized trial. Adv Biomed Res 1: 7. Published online 2012 May 11. http://doi:10.4103/2277-9175.94430.
- S.D.GuptaK.MitraM.MukherjeeEffect of magnesium infusion on thoracic epidural analgesiaSaudi J Anaesth5120115561