Abstract
In this study, the ancient potteries from Tamilnadu (Nedungur: Lat.10° 57′N; Long.77°51′E) were collected and investigated for their chemical and mineralogical characteristics to estimate their firing temperature and firing atmosphere. The physical and chemical state of iron (Fe2+/Fe3+) and iron oxide phases obtained using Mössbauer spectra were used to establish the temperature and atmosphere of firing. The clay mineral type and its structural deformation due to firing were established using X-Ray Diffraction (XRD), X-Ray Fluorescence (XRF) and Fourier Transform Infrared (FTIR) Spectroscopy. The data collected from these techniques show that quartz was the predominant mineral, followed by traces of albite, orthoclase and Fe-bearing minerals (hematite and magnetite) and suggested that the firing temperature of potteries lies in the range of 600–800 °C in oxidizing atmosphere. The mineral types and domain states of the constituent magnetic fine particles were elucidated using the variation of susceptibility at various frequencies and temperature under low field. An attempt has also been made to correlate the magnetic parameters from the percentage of Fe2+/Fe3+ and iron oxides. The information obtained paves a way for a better understanding of the technological development that took place in the ancient past and also the suitability of the samples for the determination of reliable ancient geomagnetic field intensity values.
1 Introduction
Ceramics represent one of the earliest and most significant innovations of mankind. Their first appearance marks the beginning of a new period in man's evolution during which important technological developments rapidly succeeded one another. Pottery is considered to be the prime important factor of archaeology [Citation1,Citation2].
Providing rich data for an understanding of the past culture, it is a source for the study of ancient society and history, it occupies a unique position by its distinguishing and characteristic features as well as by its distribution in different areas and different periods. Since pottery differs in style, size, color, etc., and changes from region to region and age to age, it serves as an important criterion for identifying cultures possessing different traits and tracing extraneous influences.
Clay, the chief ingredient used in pottery, is a fine grained material that develops some ductile behavior when mixed with water. On firing clay to get the final products, clay minerals undergo a sequence of chemical and structural modifications (dehydration, oxidation, dehydroxylation, decomposition and formation of new phases) that generally improve mechanical strength, durability, etc. The temperature at which changes occur depends on the chemical and mineralogical compositions of the original clay, duration of heating and the atmosphere under which heating has been carried out. Various spectroscopic techniques have been adopted by researchers to establish the type of clay used, firing temperature and firing atmosphere. Venkatachalapathy et al. [Citation3,Citation4]; Manoharan et al. [Citation5,Citation6]; Dhanapandian et al. [Citation7] have carried out extensive studies on Indian archaeological artefacts.
The place chosen for the present (Fig. 1) study is Nedungur [Lat. 10° 57′N; Long. 77°57′E], situated on the ancient trade route, i.e. Kongu Peruvazhi, in the present Aravakkuruchi taluk of Karur District and located about 15 km from Karur town. This ancient trade route passed from Kerala, through Palaghat pass, via., Vellaur, Sulur, Kangeyam, Karur, Uraiyur and led to Kaveripumpattinam on the eastern coast. Today though it is a small sleepy village, based on the style, shape, texture, color of the pottery and other archaeological remains from the habitation, archaeologists of Tamilnadu state have assigned two periods that is 3rd century BC and 4th century AD. Approximately a variety of 18 pottery specimens were collected. Based on their macroscopic features (color and fabric), 9 sherds were selected from both periods for mineralogical, chemical and rock magnetic studies. The characteristics of all the above mentioned potsherds are given in .
Table 1 Description of potsherds collected from Nedungur, Tamilnadu, India.
In the present study, attention has been focused on the characterization of pottery samples collected from Nedungur by using Fourier Transform Infrared (FTIR) spectra, X-ray Diffraction (XRD), Mössbauer and Rock magnetic studies.
Bricks and potteries are the baked clay. When the kiln is hot, the particles of oxidized iron present in the clay freely oriented themselves and get frozen in particular position, along the ambient geomagnetic field, when they get cooled. They have not responded to the subsequent variation of the earth magnetic field, both in magnitude and direction. Therefore, they can be considered to be the best fossilized magnetic record of the state of the magnetic field of the earth in the past. The remanent magnetization is characteristic of baked clays. The fossil magnetism in the archaeological sample possessing remanent magnetization is proportional to the intensity of the ambient geomagnetic field [e.g., [Citation8,Citation9]]. Archaeomagnetic study is primarily based on the thermoremanent magnetization acquired by the baked clay materials at the time and in the place of its last firing. Main limitation of archaeomagnetic studies is the fact that most of burned archaeological artefacts are displaced and thus no primary geomagnetic directions may be retrieved. However, archaeointensity studies have the great advantage that no oriented material is required. The type of magnetic minerals and their concentration and domain states are important factors in determining the reliability of the results possessed by the artefacts. To establish the above, the samples have been subjected to rock magnetic studies.
2 Experimental details
The FTIR absorption spectra were recorded in the frequency region of 4000–400 cm−1 using model paragon 500, Perkin–Elmer spectrophotometer with 16 scan mode by using standard KBr pellet technique. The room temperature Mössbauer measurements have been performed with a conventional constant acceleration spectrometer (M/s. WISSEL, Germany) equipped with a 57Co (Rh) source. The spectra have been fitted on a PC with the least squares minimization procedure assuming Lorentzian line shapes. XRD spectra have been recorded on X-pert MPD from Philips using CuKα radiation in the 2θ range from 10° to 70° in steps of 0.02°. X-ray fluorescence spectrometry (Bruker model S4 Pioneer sequential wavelength-dispersive X-ray spectrometer) was performed to determine chemical composition by pressed pellet technique. Mass-specific magnetic susceptibility is measured with the Bartington MS2B dual frequency meter at two frequencies (χLF at 0.47 kHz and χHF at 4.7 kHz) for specimen of disk shaped (2.2 cm in diameter) were cut from each selected sample. Percentage frequency-dependent magnetic susceptibility χFD%=(χLF − χHF) × 100/χLF as well as mass specific frequency dependent susceptibility χFD = χLF − χHF are then calculated. Temperature dependence of magnetic susceptibility χ–T (in air atmosphere) is then measured using Bartington MS2WFB with high temperature furnace by heating samples up to 700 °C and cooling down to 100 °C in steps of 5 °C.
3 Results and discussion
3.1 FT-IR analysis
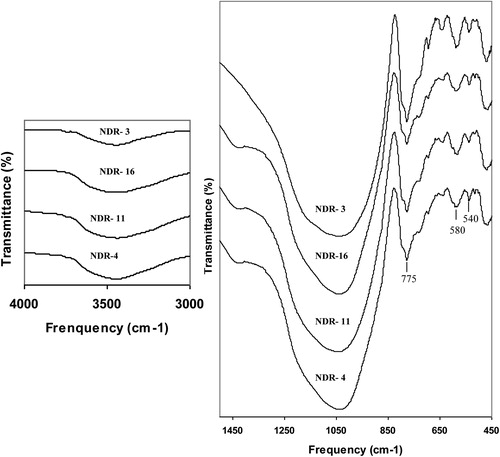
Infrared spectroscopy is a sensitive technique to monitor dehydroxylation and dehydration of clay minerals. In the present study the FTIR spectra were recorded for the local clay (NDR-Clay) in as received state (ARS) and fired to higher temperatures in steps of 100–800 °C in laboratory conditions are shown in Fig. 2. Kaolinite with mostly Al in the octahedral positions has four absorption bands at 3700, 3670, 3655, and 3622 cm−1 in the OH stretching region [Citation10]. In the disordered kaolinite structure, the two weak bands 3670 and 3655 are replaced by a single band at 3655. In NDR-clay, the distinct band at 3700 and 3620 cm−1 along with weak shoulder at 3650 cm−1 in as received state indicates that the kaolinite is of disordered type. No peaks of kaolinite at 3700 cm−1 region were identified at and above 500 °C since it decomposes at 500–550 °C [Citation11]. The presence of broad bands at 3440 cm−1 and 1640 cm−1 are attributed to adsorbed water molecules that should disappear on firing clay above 100 °C [Citation12,Citation3].
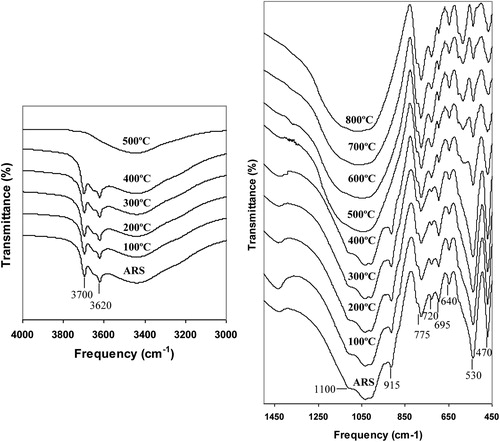
The ARS spectrum of clay shows a broad band centered around 1030 cm−1 with asymmetry around 1100, 935 and 915 cm−1 which are attributed to Si–O–Si, [Al–O–(OH)]6, O–H deformation and Al–OH, respectively. On firing the clay, intensity of the band 1100, 935 and 915 cm−1 decreases and disappears above 500 °C, due to dehydroxylation. Above 600 °C, a broad symmetry band is observed at 1030 cm−1, due to the destruction of octahedral sheet structure. The broad band observed at 1030 cm−1 reveals that the type of clay is red [Citation13]. The 1030 cm−1 absorption band in the range of asymmetric silicate tetrahedral vibrations are broadened and become less intensive at 800 °C [Citation11].
The sharp bands appeared at 795 and 775 cm−1 along with 695 cm−1 are due to quartz [Citation3]. The band at 640 cm−1 is attributed to Al–O co-ordinate vibration. The Si–O–Al bending vibration observed at 530 cm−1 is the most sensitive band to the presence of residual Al in the octahedral sheet [Citation14,Citation15]. On firing, no perceptible changes are observed in this region upto 500 °C and above this temperature as iron replaces aluminum, the intensity of the band at 530 cm−1 decreases with appearance of the band at 540 cm−1 along with weak shoulder at 580 cm−1 attributed to iron oxides. Above 500 °C, the intensity of the band at 540 and 580 cm−1 increases with further oxidation and the formation of crystalline hematite [Citation16,Citation17]. The band at 470 cm−1 due to Si–O–Si bending is free from any temperature effects. From the above details, it is concluded that the local clay is of disordered kaolinite type and structural changes due to temperature effects are well established.
The absence of hydroxyl bands (Si–O–Si) in the region 3700 cm−1 and the presence of a broad symmetry band centered around 1030 cm−1 in the spectra of samples NDR-1, 3, 4, 11, 16 and 18 indicate that all the samples have been fired above 600 °C and are made up of red clay [Citation13,Citation18,Citation6,Citation11]. The well resolved and distinct peaks at 540 and 580 cm−1 in the spectra of all samples reveal the presence of iron oxides [Citation16,Citation17], which also confirms the firing temperature as above 600 °C. The sharp and increased intensity of the absorption peak 580 cm−1 along with 540 cm−1 is due to the formation of crystalline iron oxides, which forms normally above 700 °C [Citation19]. From the above statements, one can conclude that samples NDR-3, 10 and 17 might have been fired above 700 °C and other samples in between 600 and 700 °C. While establishing the firing temperature of the samples, the spectra were compared with the spectra of clay samples fired to different temperatures. The FTIR spectra of representative samples recorded in ARS and the region of interest are shown in Fig. 3.
3.2 XRD and Mössbauer analysis
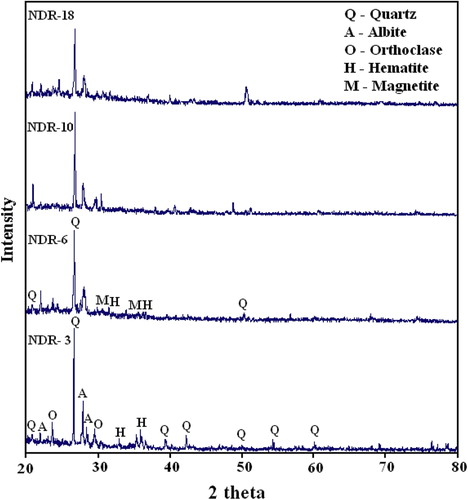
The effect of X-ray diffraction by mineral structure constitutes the basis for identifying minerals and their crystalline substances. For the present investigation, the XRD spectra have been recorded at room temperature and the minerals associated with the samples are identified using JCPDS file [Citation20]. From the XRD pattern of the samples, the minerals like quartz (3.33, 4.23, 1.81, 1.53 and 2.27 Å), albite (3.19, 4.03, and 3.21 Å), orthoclase (3.31, 3.77 and 2.99 Å), hematite (2.69 and 2.51 Å) and magnetite (2.53 and 2.96 Å) are identified.
XRD reflection spectra for the representative samples are shown in Fig. 4. Quartz is predominant, might have been added to make the clay self tempered. Information like firing temperature and atmosphere can be obtained from Mössbauer parameters, viz., isomer shift, quadrupole splitting and magnetic hyperfine interaction which specify the presence of Fe2+/Fe3+ and iron oxides [Citation21]. Mössbauer spectra of Nedungur pottery samples have been recorded and the representative sample spectra are shown in Fig. 5 and the Mössbauer parameters derived from the peak position are given in . From the presence or absence of paramagnetic Fe2+ and Fe3+, the firing condition, firing temperature and coloring mechanisms of the archaeological potteries have been deduced. The decrease or disappearance of Fe2+ ion indicates that oxygen is rich in original firing atmosphere. The pottery fired under oxidizing atmosphere, at temperatures above 500 °C show no Fe2+ in their Mössbauer spectrum. Coey [Citation22] pointed out that the isomer shift (δ) value ranging from 0.8 to 1.5 mm/s and quadrupole splitting (Δ) value from 1 to 3.5 mm/s are attributed to Fe2+. The isomer shift value from 0.2 to 0.6 mm/s and quadrupole splitting value from 0 to 1.8 mm/s are attributed to Fe3+. From , it is evident that Mössbauer parameters for magnetite and hematite are in good agreement with the reported values [Citation23,Citation19,Citation3,Citation11].
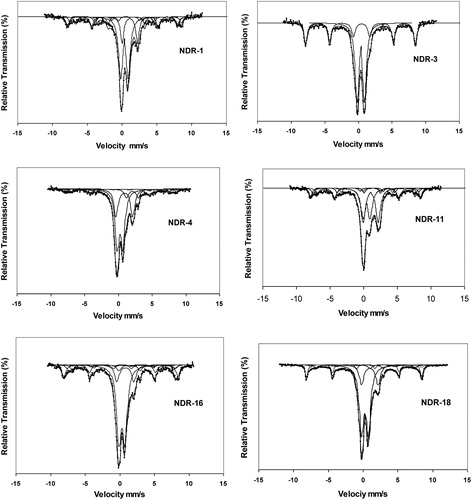
Table 2 Mössbauer parameters of Nedungur pottery samples in the as-received state.
On firing sample under reducing atmosphere, the reduction of Fe3+ in clay begins at 400 °C and the Fe2+ content raises until on firing about 800 °C. A small amount of Fe3+ is left between 600 and 650 °C under reducing condition [Citation19]. The above notification leads to conclude that the samples NDR-1, 4, 6, 11, 16 and 18 have been fired under reduced atmosphere above 400 °C, but the presence of Fe3+ indicates that the sample is not fired under strong reduced atmosphere upto 800 °C or the sample is fired under reduced atmosphere and air is admitted during cooling which might have enabled the conversion of Fe2+ to Fe3+. Admission of air during cooling is either to achieve the red color of the ceramics, or due to the fact they inadvertently open the kiln before it has cooled sufficiently.
The structural Fe2+ in the clay begins to be oxidized to Fe3+, a process that is concluded at about 450 °C, hence Fe2+ is absent in the case of clay fired under oxidizing atmosphere above this temperature. The presence of Fe2+ and Fe3+ in the case of samples NDR-1, 4, 6, 11, 16 and 18 reveals that samples are fired under changing conditions established from two different colors of the materials [Citation19].
When previously reduced material is heated again in air at an increasing temperature, the Fe2+ is reoxidized to Fe3+ between about 450 and 600 °C. Since Fe3+ formed during reoxidation is no longer structural iron in dehydroxylated sheet silicates but mainly superparamagnetic hematite, one does not observe the high quadrupole splitting of non magnetic Fe3+ as observed when fresh clays are fired in air. The presence of more percentage of non paramagnetic Fe2+ and Fe3+ indicates that well ordered iron oxides are not formed, i.e., the samples are not fired to higher temperature or iron oxide particles are less than 100 nm. It leads to the behavior as superparamagnetic (SP) which blocks the formation of well ordered sextet in the spectra [Citation21].
In the present study, the absence of well ordered sextet in the spectra indicates that the samples are not fired to higher temperature also due to the presence of SP grains. The presence of poor sextet is due to the presence of grains with sizes greater than SP like SD/PSD. The above conclusions are also confirmed through rock magnetic studies of the above samples. From the above notion it can be concluded that the samples NDR-1, 4, 6, 11, 16 and 18 are fired under changing conditions (reduced followed by oxidation). The red outer surface and inner black core also confirm the changing atmosphere followed by artisans during manufacturing of the artifacts in the archaeological past.
The Mössbauer spectra of the pottery sample NDR-3, 10, and 17 show the presence of paramagnetic Fe3+ and iron oxide, namely, hematite. The absence of Fe2+ indicates that the sample is fired in a strong oxidizing atmosphere. The larger percentage of non magnetic Fe3+ and the presence of poor crystalline hematite in the spectra reveal that the sample is not fired above 900 °C (at which the entire structural iron forms hematite also due to the smaller grain size (superparamagnetic hematite particles)). For this above reason, it may be concluded that the samples are fired under oxidizing atmosphere, the same is also revealed from the reddish brown color of the outer surface. The light brown color may be due to carbon deposition. The observed quadrupole splitting value of Fe 3+ (1.28 mm/s) confirms the firing temperature of this sample as around 700 °C during manufacturing [Citation19].
Poor oxygen diffusion can be noticed from the presence of Fe2+ percentage which is higher in the case of samples NDR-4, 6 and 11 [Citation24]. Presence of Fe2+ in the case of samples NDR-1, 4, 6, 11, 16 and 18 shows the changing atmosphere followed during manufacturing. The black-and-red and brown-and-black ware of the samples also reveal the changing conditions adopted during firing cycle. The black color corresponds to carbon formed from dense smoke during reduced atmospheric firing using charcoal and wood. The outer red color of the sherds is due to the blow of air at higher temperatures during the end of the firing cycle (during cooling). The admission of air at lower temperature during cooling leads to gray (or) brown outer surface and inner black color. Red slip is prepared from ochre and the application of slip and robbing process completely change the appearance of the pot and fill up the minute cavities [Citation1]. The presence of hematite in the case of red slipped ware NDR-11 is confirmed through chemical and Mossbauer analyses.
The above discussion reveals that all the samples have been fired under changing conditions except NDR-3, 10 and 17, which is fired under oxidizing condition.
3.3 Chemical analysis
The physico-chemical properties of ceramic materials depend mainly on the raw clay composition and firing features, indicative of the local technology available at that time [Citation25]. Firing techniques have been assessed through FTIR and Mossbauer analysis. Chemical analysis has been carried out to measure the composition of clay and baked clay. gives the chemical composition of archaeological pottery sherds collected from Nedungur. It is noticed that the pottery sherds present a typical composition and they are constituted mainly by silica and alumina and minor contents of Ca, K, Mg, Ti and Na oxides. The significant amounts of Fe2O3 and Fe3O4 are responsible for the reddish and black color of the pottery sherds respectively [Citation26–Citation[27]Citation28]. In the present study, the CaO content in the above samples is less than 6% indicative of the non-calcareous clay used for the production of pottery [Citation29]. The loss on ignition (6–10%) obtained for the pottery sherds is within the usual range and is associated mainly with volatile components and organic matter. These results are in agreement with Mossbauer and XRD results. Especially the presence of iron oxides established in chemical analysis is in good agreement with the presence of sextets belonging to hematite and magnetite.
Table 3 Chemical composition of archaeological pottery sherds of Nedungur site by XRF (wt.%).
3.4 Mineral magnetic studies
Magnetic susceptibility (χ) describes the magnetic response of a sample when exposed to a (generally weak) magnetic field and χ is mainly a function of the concentration and mineralogy of the ferrimagnetic (magnetite, maghemite, Fe-sulphides) minerals present, but it can also depend on the strength of the applied magnetic field and the particle size distribution of the magnetic grains. In the absence of ferrimagnetic minerals, χ can be found due to antiferromagnetic, paramagnetic and diamagnetic minerals. Magnetic susceptibility χ is also dependent on sample size. Therefore, it is customary to present susceptibility as mass or volume normalized susceptibility χ [Citation30].
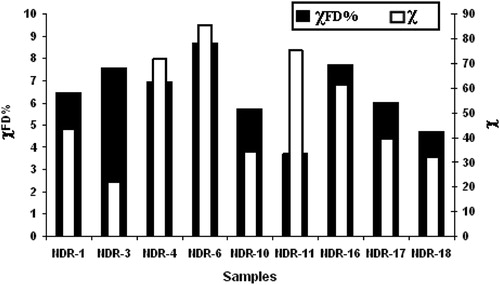
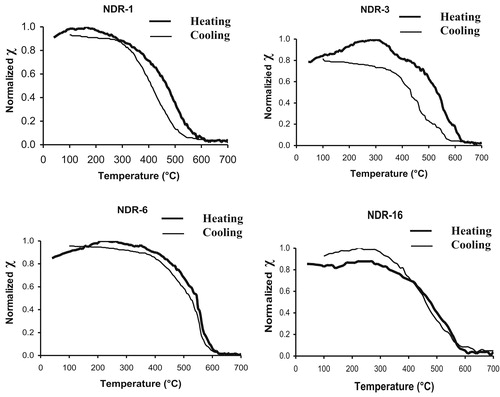
Magnetic susceptibility and its frequency dependence are widely used in studying magnetic enhancement and are also useful in the detection of fine magnetite/maghemite grains [Citation31]. The frequency dependent susceptibility is a non-destructive indicator to decipher the nature of magnetic carriers that are very much suitable for archaeomagnetic studies. Kostadinova and Kovacheva (2008) [Citation32] have reported that burnt clay samples with χFD% > 4 have detectable concentration of superparamagnetic (SP) grains and this indicates that magnetite grains in superparamagnetic boundary. Frequency dependent magnetic susceptibility of Nedungur pottery samples is shown in Fig. 6. The magnetic susceptibility χLF of pottery samples is more evenly spread over a wide interval (22–85 × 10−7 m3/kg) pointing to higher magnetic enhancement. High values of χFD% indicate the presence of very fine grained metastable magnetic grains spanning the superparamagnetic (SP)–stable single domain (SSD) boundary [Citation33]. All the samples show χFD% >2 but most of the samples fall in between 4 and 10% suggesting the presence of significant amount of the superparamagnetic magnetite grains. Dearing et al. [Citation34] have reported that addition of increasing amount of MD – magnetite to soil containing predominately SP magnetite grains (χFD% = 10.5) causes χFD% to decrease to >2, while χLF increases with concentration. In the present study, the absence of multi domain nature of magnetite (χFD > 2) reveals the suitability of the sample for archaeomagnetic studies also. The results suggest that the pottery samples under investigation are magnetically enhanced materials in terms of concentration and degree of crystallinity of magnetite. Higher values of susceptibility are obtained for samples with larger percentage of ferrous ion or Fe2+/Fe3+ ratio. Similar results have been obtained for ancient Quatari pottery samples by Ref. [Citation35]. The temperature–susceptibility measurements are to monitor the variations of the susceptibility in order to determine the Curie temperature and to identify magnetic minerals which are responsible for acquiring ancient geomagnetic field and nature of mineral transformation, if any. The temperature dependent susceptibility curves for four representative samples are shown in Fig. 7. In the present investigation, the reversibility of the heating and cooling curves of all the NDR samples (except NDR-3) reveal that there is no distinct mineralogical changes and that magnetite, the main magnetic mineral; with Curie temperature around 580–590 °C is found. The NDR-3 in (Fig. 7) samples has irreversible behavior during heating and cooling due to mineralogical alteration and these samples are not suitable for archaeointensity measurements. The irreversibility behavior of sample may be due to alteration of lower curie phase mineral maghaemite to magnetite curie phase during laboratory heating [Citation36]. Thermomagnetic curves are useful indicators for the thermal stability of magnetic minerals upon temperature in baked materials [Citation37]. The reversibility between the heating and cooling susceptibility curves used here for testing the stability of the magnetic mineralogy upon temperature. The above observations are coinciding well with the chemical and Mossbauer analysis.
4 Conclusion
FTIR results indicate that the shreds are fired above 600 °C under changing atmospheric conditions to achieve different coloration. The ratio of Fe2+/Fe3+ and iron oxides using Mössbauer spectra helps to understand the probable firing temperature and firing atmosphere in supportive of the FTIR studies. XRF analysis confirms that the samples are rich in fluxes more significantly the amount of iron contents hematite and magnetite. It is obvious that the iron oxides are the main colorants. The artisans of Nedungur have used only non-calcareous clays for their household utilities. Higher mass specific susceptibility obtained using magnetic studies reveals the possibility of either the iron from the parent unbaked clay (or) due to higher firing temperature during baking. The susceptibility values are found to be higher with a higher percentage of Fe2+. The mineral magnetic studies reveal that ferrimagnetic mineral magnetite is the main contributor with superparamagnetic/single domain in nature and hence it is suitable for archaeointensity measurements except NDR-3. The present investigation also gives the clue that the people, who lived in between the 3rd BC century to 3rd century AD in Nedungur, India were well aware of producing single and multi colored pottery for different domestic uses like storing food grains, water and cooking purposes.
Acknowledgment
The authors are grateful to The Director, State Archaeological Department, Tamilnadu, for providing sufficient samples for this study. The authors gratefully acknowledged. Dr. T. Rathakrishna, Scientist G and Head, Geosciences Division, National Centre for Earth Science Studies (NCEES), Kerala for giving permission to carry out the magnetic measurements and his valuable discussions. The authors wish to extend their sincere thanks to the Director, Dr. Dipankar Dass (Scientist) IUC, Calcutta for recording Mössbauer spectra and for their fruitful discussion. Finally, corresponding author of the manuscript is highly indebted to the anonyms reviewer for giving useful comments and suggestions.
Notes
Peer review under responsibility of Mansoura University.
References
- S.GurumurthyCeramic Traditions in South India1981University of Madras155
- Y.ManiatisA.SimopoulosA.KostikasAn investigation of ancient ceramic technologies by Mössbauer spectroscopyJ.S.OlinA.D.FranklinArchaeological ceramicsvol. 11982Smithsonian Institution PressWashington, DC97108
- R.VenkatachalapathyT.SridharanS.DhanapandianC.ManoharanDetermination of firing temperature of ancient potteries by means of infrared and Mossbauer studiesSpectrosc Lett3562002769779
- R.VenkatachalapathyT.BakasN.BasavaiahK.DeenadayalanMössbauer and mineral magnetic studies on archaeological potteries from Adhichanalur Tamilnadu, IndiaHyperfine Interact18620088998
- C.ManoharanR.VenkatachalapathyS.DhanapandianK.DeenadayalanmFTIR and Mössbauer spectroscopy applied to study of archaeological artifacts from Maligaimedu, Tamil Nadu, IndiaIndian J Pure Appl Phys452007860865
- C.ManoharanK.VeeramuthuR.VenkatachalapathyT.RadhakrishnaR.IlangoSpectroscopic and ancient geomagnetic field intensity studies on archaeological pottery samples, IndiaLithunian J Phys4822008195202
- S.DhanapandianC.ManoharanP.SutharsanApplications of FTIR and 57Fe Mössbauer techniques in studies of recently excavated Indian archaeological potteryActa Phys Pol A1212012592598
- N.JordanovaE.PetrovskyM.KovachevaD.JordanovaFactors determining magnetic enhancement of burnt clay from archaeological sitesJ Archaeol Sci28200111371148
- M.KovachevaN.JordanovaV.KarloukovskiGeomagnetic field variations as determined from Bulgarian archaeomagnetic data. Part II: the last 8000 yearsSurv Geophys191998431460
- J.L.BantigniesC.Cartier Dit MoulinH.DexpertWettability contrasts in kaolinite and illite clays: characterization by infrared and X-ray absorption spectroscopiesClays Clay Miner4521997184193
- V.ValancieneR.SiauciunasJ.BaltusnikaiteThe influence of mineralogical compositions on the colour of clay bodyJ Eur Ceram Soc30201016091617
- R.G.WolffInfrared absorption patterns (OH region) of several clay mineralsAm Mineral501965240244
- S.N.GhoshInfra-red spectra of some selected minerals, rocks and productsJ Mater Sci131978 1877–1866
- P.KomadelChemically modified smectitesClay Miner382003127138
- J.MadejovaFTIR techniques in clay mineral studiesVib Spectrosc312003110
- A.KostikasA.SimopoulosN.H.GangasMössbauer study of ancient potteryJ Phys Colloq351974107115
- U.WagnerR.GebhardG.GrosseT.HutzelmannE.MuradJ.Riedereret alClay: an important raw material for prehisteric manHyperfine Interact1771998325335
- R.VenkatachalapathyD.GournisC.ManoharanS.DhanapandianK.DeenadayalanFTIR and Mössbauer spectroscopic studies of archaeological potteries from Nathikudi TamilnaduIndian J Phys7812200413711375
- U.WagnerF.E.WagnerW.HauslerI.ShimadaThe use of Mössbauer spectroscopy in studies of archaeological ceramicsD.C.CreaghD.A.BradleyRadiation in Art and Archaeometry2000417443
- JCPDSJoint Committee on Powder Diffraction Standards, Mineral Powder Diffraction File1999
- F.E.WagnerU.WagnerMössbauer spectra of clays and ceramicsHyperfine Interact15420043582
- J.M.D.CoeyClay minerals and their transformations studied with nuclear techniquesAt Energy Rev18198073124
- T.TomingaM.TakedaJ.F.MabuchiJ.PalqciosCharacterization of ancient Japanese roofing tiles by 57Fe Mössbauer spectroscopyArchaeometry201978135146
- L.NodariL.MaritanC.MazzoliU.RussoSandwich structures in the Etruscan-Paden type potteryAppl Clay Sci272004119128
- V.TudiscaC.CasieriF.DemmaM.DiazL.PinolC.Terenziet alFiring technique characterization of black-slipped pottery in Prasnesete by low field 2D NMR relaxometryJ Archaeol Sci382011352359
- G.E.De BenedettoR.LavianoL.SabbatiniP.G.ZamboninInfrared spectroscopy in the mineralogical characterization of ancient potteryJ Cult Herit332002177186
- S.N.MonterioC.M.F.VieiraM.M.RibeiroF.A.N.SilvaRed ceramic industrial products incorporated with oily wastesConstr Build Mater2111200720072011
- C.ManoharanP.SutharsanS.DhanapandianR.VenkatachalapathyR.Mohamed AsanullaAnalysis of temperature effect on ceramic brick production from alluvial deposits, Tamilnadu, IndiaAppl Clay Sci5420112025
- Y.ManiatisM.S.TiteTechnological examination of Neolithic–Bronze age pottery from Central and Southeast Europe and from the Near EastJ Archaeol Sci819815976
- S.D.MooneyC.GeissM.A.SmithThe use of mineral magnetic parameters to characterize archaeological oresJ Archaeol Sci3052003511523
- C.MullinsM.TiteMagnetic viscosity, quadrature susceptibility, and frequency dependence of susceptibility in single-domain Assemblies of magnetite and maghemiteJ Geophys Res781973804809
- M.KostadinovaM.KovachevaCase study of the Bulgarian Neolithic archaeological site of Piperkov Chiflik and its archaeomagnetic datingPhys Chem Earth332008511522
- J.EyreFrequency dependence of magnetic susceptibility for populations of single-domain-grainGeophys J Int1291997209211
- J.A.DearingP.M.BirdR.J.L.DannS.F.BenjaminSecondary ferrimagnetic minerals in Welsh soils: a comparison of mineral magnetic detection methods and implications for mineral formationGeophys J Int1301997727736
- N.A.EissaH.A.SallamA.M.SanadA.F.MiraMössbauer effect study of the origin of ancient Qatari potterIndian J. Pure Appl. Phys.171979731733
- L.M.Alva-ValdiviaComprehensive paleomagnetic study of a succession of Holocene olivine-basalt flow: Xitle Volcano (Mexico) revisitedEarth Planets Space572005839853
- S.SpassovJ.HusEstimating baking temperatures in a Roman pottery kiln by rock magnetic properties: implications of thermochemical alteration for archaeointensity determinationsGeophys J Int1672006592604