 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study determined the in-vitro and in-vivo activities of Voriconazole (VOR, 1 µg), Nystatin (NYS, 25 µg), Fluconazole (FLC, 100 µg), red force® (RFC) [Copper (I) oxide, 60% + Metalaxyl-M 6% WP], ultimax plus® (UPL) [Metalaxyl 12% WP + Copper (I) oxide 60% WP], and forcelet® (FCE) [Carbendazim 50% WP] and Acalypha wilkesiana (Red Acalypha) on fungal deteriogens obtained from deteriorated sweet orange fruits. The 22 fungal deteriogens tested had in-vitro susceptibility of 13.0 ± 2.6–28.7 ± 1.2 mm inhibition zone for VOR (n = 14), 9.3 ± 1.2–28.0 ± 3.0 mm for NYS (n = 21), 7.7 ± 1.5–21.7 ± 1.5 mm for FLC (n = 4), 13.3 ± 1.2–31.7 ± 2.1 mm for RFC (n = 5), 15.3 ± 4.2–30.7 ± 1.2 mm for UPL (n = 5), 15.7 ± 1.2–31.7 ± 1.5 mm for FCE (n = 19) and 0.0 ± 0.0 mm for Red Acalypha (n = 22). Fifteen of the fungal deteriogens including the yeast, Candida glabrata were able to initiate deterioration in-vivo with 40–100% severity within the incubation period of 11 days. FCE biocide significantly (p = 0.001) prevented or reduced deterioration in-vivo. There was no deterioration sign in uninoculated control (Control 1) and uninoculated control with FCE (Control 2). Further search for active agents against fungal deteriogens especially from natural sources is required for longer preservation of Citrus sinensis.
1 Introduction
Citrus is one of the world’s fruit crops of high economic importance with annual worldwide production estimated at over 123 million metric tons as at 2010 [Citation1]. They are the highest valued fruit crop regarding international trade [Citation2]. The commonly cultivated species include sweet orange (Citrus sinensis), grapefruit (Citrus paradise), lime (Citrus aurantifolia), lemon (Citrus limon), tangerine (Citrus reticulate) and sour orange (Citrus aurantium). Oranges constitute the bulk of citrus fruit production, accounting for approximately 55% of global citrus production.
Although believed to have originated from certain parts of Southeast Asia [Citation3,Citation4] , tropical Africa is greatly endowed with varied ecological and climatic conditions suitable for the production of citrus in commercial quantity. For instance, Nigeria has an estimated annual citrus production of about 930,000 metric tons [Citation5] which mostly comprised oranges [Citation2,Citation6] . Developing nations, therefore, have the immense comparative advantage and the potential to lead the world in citrus production and trade.
Sweet orange (Citrus sinensis) which belongs to the family Rutaceae are globose to ovoid shaped, berry-like edible fruits that vary in their sizes, colours, shapes and fruit quality [Citation7]. Early acceptance of sweet orange in international trade despite their being highly perishable might be due to their high nutritional and therapeutic values [Citation8]. They contain high vitamin C content, appreciable amount of vitamin A, folate, and fibre which have implication in stimulation of white blood cell function in the immune system, bone formation, eye health, DNA production, reduction in cardiovascular disease risk, etc. They, however, do not contain fat, sodium, cholesterol and has low energy level value [Citation9].
Commercial cultivation of this important fruit crop for large-scale processing and international export is still at infancy in most developing countries. For instance, most of the existing plantations are in mixed cropping system in the remote part of Nigeria’s rainforest and guinea savannah where there is no good transportation system. Only a few large plantations are in existence [Citation2]. According to NIHORT [Citation10], about 45% of citrus currently produced in Nigeria is consumed fresh, 25% is processed while as much as 30% is wasted due to postharvest spoilage mostly caused by fungal pathogens. Infection of oranges with fungal species may occur before harvesting, at the time of harvesting and during postharvest handling, storage or distribution [Citation11].
Fungi have been extensively studied as important plant pathogens especially in crops. Several species were implicated in various plant diseases such as Alternaria brown spot, greasy spot, melanose, scab disease, black spot, post bloom fruit drop, Pseudocercospora fruit and leaf spot, etc. [Citation12Citation[13]Citation[14]Citation[15]–Citation16] . In the study of Embaby et al. [Citation17], the five species of fungal pathogens, Alternaria citri, Botryodiplodia theobromae, Fusarium sp., Penicillium digitatum and Penicillium italicum isolated from rotten orange fruits were pathogenic and increase considerably the percentage of disease incidence and disease severity as well as significant decrease in shelf life.
Also, fungi associated with spoilage of sweet orange (Citrus sinensis) have been reported to produce toxins [Citation18] and have been reported to be pathogenic [Citation19]. They produce aflatoxins which cause cancer of the liver, aflatoxicosis and acute hepatitis in humans [Citation20]. A single infected orange can be a source of infection to other oranges during storage and in transit [Citation21]. Isolation and identification of the pathogens are desirable to strategize the control measures to (or “intending to”) reducing losses due to spoilage or infections [Citation22].
There has been an increased need to identify and isolate fungi associated with orange spoilage as a proactive measure towards a drastic reduction in economic loss due to fruit decay especially in developing nations where there is no access to improved postharvest storage and transport. The effort may lead to the discovery of previously unknown pathogens that must be isolated and studied.
Previous studies on the fungal postharvest spoilage of sweet orange fruits in Nigeria were limited to isolation, characterization and pathogenicity of the associated fungi [Citation23Citation[24]Citation[25]Citation[26]Citation[27]–Citation28] . Such information may only be considered meagre considering the huge potential for sweet orange production by the citrus producing states in the country. Limited information, however, exists on the susceptibility of the indigenous spoilage-associated fungi to both chemical and biological controls.
Red Acalypha are known to possess antimicrobial activities with extensive usage in the treatment of bacterial and fungal infections [Citation29]. However, its use in prevention of deterioration in sweet orange fruits has not been investigated. Also, there is no report on the comparative evaluation of its effectiveness with chemical biocides in prevention of the activities of fungal deteriogens in sweet orange fruits.
This study, therefore, focused on the characterization of fungi associated with deteriorating sweet orange fruits. In-vitro and in-vivo susceptibility of the fungal deteriogens to chemical biocides and leaf extracts of Red Acalypha were compared with the view to developing a more sustainable biological control for sweet orange deterioration in developing nations.
2 Materials and methods
2.1 Study location and sampling
The study was conducted in the Microbiology Laboratory of Osun State University, Osogbo, Nigeria. Deteriorated sweet orange (Citrus sinensis) fruit samples were obtained from fresh fruit stalls in a local market in Osogbo (7.7667° N, 4.5667° E) during peak citrus fruits production season (August–November 2015). Apparently-healthy ripe, sweet orange fruit samples were obtained from the horticultural garden within the University main campus.
Deteriorated sweet orange fruit samples (10 fruit samples) were randomly collected in sterile polythene bags. As indication of deterioration, sweet orange fruits showing obvious mechanical wound or bruise, with purplish to dark brown rot, blue rot, green rot or black lesions were selectively obtained [Citation30]. Samples were transported immediately to the laboratory and stored refrigerated (4 ± 2 °C) until analysed. All apparently-healthy ripe, sweet orange fruit samples (125 fruit samples) were obtained from the same sweet orange tree over a period of two months for the in-vivo assays.
2.2 Isolation and characterization of fungal deteriogens
Deteriorated sweet orange fruit samples were surface-sterilized with cotton wool soaked in 70% ethanol and then rinsed in two different changes of sterile distilled water. Each of the infected sweet orange fruit tissue was cut out into small segments using sterile scalpel. One gram of the excised portion was aseptically weighed into a tube of 9 ml of phosphate buffered saline (1X PBS) and shaken vigorously. The tube content then served as stock for 10-fold serial dilution. A 100 µl inoculum each of the 10−2 and 10−3 dilutions was aseptically spread-plated onto sterile solidified Potato Dextrose Agar (PDA, Oxoid®) medium containing Chloramphenicol (30 µg/ml) in duplicates.
All the inoculated plates were incubated at 28 ± 2 °C for seven days. Fungal isolates were further purified on fresh PDA plates. Pure strains were then maintained on PDA slants and stored in the refrigerator at 4 ± 2 °C for further analysis. Purified fungal colonies were identified based on their morphological and microscopic appearances. Growth on PDA plates was observed for appearance and growth pattern, colony texture, colony colour and pigmentation, presence or absence of aerial mycelium, and presence or absence of wrinkles and furrows [Citation31,Citation32] .
A drop of lactophenol cotton blue stain was placed on a clean grease-free glass slide after which a sterile inoculating wire loop was used to pick the mycelium from the edge and the centre of each growing fungal colony onto the glass slide [Citation33,Citation34] . The mycelium was then spread evenly on the slide. Teasing was done to separate the mycelium to get a homogenous mixture, and the mixture was then gently covered with coverslips and then allowed to stay for few seconds before observing with the microscope under X40 magnification.
The microscope examination of actively growing mould was by their vegetative and reproductive structures such as hyphal colour and structures, shape and size of conidia, conidiophores, and microsclerotia. Isolates’ macroscopic and microscopic features were then compared with standard keys and published literature [Citation34Citation[35]Citation[36]Citation[37]–Citation38] .
2.3 Fungicide preparation
The antifungal susceptibility testing discs of Voriconazole (VOR, 1 µg); Nystatin (NYS, 100 µg); Fluconazole (FLC, 25 µg) were obtained directly from the manufacturer (Oxoid, England). Powdered fungicide formulations such as red force® (RFC) [Copper (I) oxide, 60% + Metalaxyl-M 6% WP], ultimax plus® (UPL) [Metalaxyl 12% WP + Copper (I) oxide 60% WP], and forcelet® (FCE) [Carbendazim 50% WP] were obtained from local agrochemical stores in Osogbo, Nigeria.
The fresh leaves of Red Acalypha (A. wilkesiana), the extract of which was used in this comparative study, were obtained within the premises of Osun State University, Nigeria. The taxonomy was authenticated by a Plant Biologist in the Department. Extract of this Red Acalypha was prepared in hot water as previously described [Citation39,Citation40] .
For comparability, the three powdered fungicide formulations and the Red Acalypha extract were made into discs to simulate the commercial antifungal susceptibility testing discs. Concentrations of 1.25 mg, 0.6 mg and 0.6 mg of RFC, UPL and FCE respectively as well as 1% w/v, 0.5% w/v, 0.25% w/v and 0.125% w/v of Red Acalypha leaf extract respectively were prepared. A 100 µl of each solution was impregnated on sterile perforated Whatman filter paper (6 mm) and allowed to dry. All the discs were preserved at 4 °C until used.
2.4 In-vitro susceptibility test of isolated fungal deteriogens
The disk-diffusion method was used to determine the in-vitro susceptibility of the isolated fungal deteriogens to the six commercial fungicides (chemical biocides) and the Red Acalypha leaf extract [Citation41]. A 100 µl standardised spore suspension (106 spores/ml) of each isolated fungal deteriogens was swabbed on the surface of PDA plates. The fungicide discs were aseptically placed on the inoculated plates and incubated at 28 ± 2 °C for 48 h. The experiment was done in duplicate, and the test was conducted twice. The zone of inhibition was measured with a scale in mm.
2.5 In-vivo susceptibility test of isolated fungal deteriogens
Based on the results of in-vitro susceptibility test, only the FCE was used in the subsequent in-vivo susceptibility assay. Apparently healthy sweet orange fruits were used for this assay. The fruits were fresh and slightly ripe (referred to as fresh orange fruits in this study). The fresh orange fruits were surface-sterilized by dipping in 0.4% freshly prepared chlorine for 2 min and subsequently rinsed in three different changes of sterile distilled water [Citation34].
Two different Groups of fresh orange fruits containing two oranges each were used (A and B). Similar experiment was also set up for two different Controls (1 and 2). Two different treatments were administered to each Group and Control experiments as shown in .
Table 1 Experimental design for the In-vivo susceptibility test.
For each Control (1 and 2) and Group (A and B), treatment one was done aseptically by inoculating sterile distilled water or fungal spores of each isolated fungal deteriogens into two fresh orange fruits using different syringes and needles. For the Control experiments, treatment two was done on each by soaking in sterile distilled water (Control 1) or filter-sterilized FCE (Control 2). However, for the Groups experiments, different containers were used for soaking of oranges for each isolated fungal deteriogen in sterile distilled water (Group A) or filter-sterilized FCE (Group B).
The treated fresh orange fruits were then placed in clean perforated plastic plates, each moistened with wet balls of absorbent cotton wool to create a humid environment, and incubated at room temperature (28 ± 2 °C) for 11 days. Treated samples were left undisturbed for the first four days for visible deterioration signs. Measurement of the diameter of rot was done at days 5, 6, 7, 8, 10 and 11. The percentage of deterioration was then calculated as the percentage of the diameter of the external rotted portion to the total diameter of the sweet orange fruit according to the following formula: where n = diameter of the external rotted portion and N = total diameter of the orange [Citation42].
Visual observation of the treated fresh orange fruits was also made each day for deterioration signs and severity of deterioration was based on the extent of spoilage observed on the 11th day of incubation and scored according to the following scales: 0 = no spoilage sign; 1 = spongy appearance or rot; 2 = spongy appearance + rot; 3 = spongy appearance + rot + watery exudate; 4 = spongy appearance + rot + mycelial growth and 5 = spongy appearance + rot + watery exudate + mycelial growth. The percentage deterioration severity was calculated by adapting and modifying the formula of Townsend and Heuberger [Citation43] as follows:where D.S. = disease severity; n = number of sweet orange fruits used; r1….r5 = category number and N = total examined fruits multiplied by the maximum numerical disease grade, i.e. 5. The causal agents (i.e. deteriogens) were re-isolated from the infected orange fruits and compared with the original fungal isolates at the end of the experiment.
2.6 Statistical analysis
The results of the study are presented with descriptive statistics [mean ± SD]. Inferential statistics to establish the presence or absence of significant difference in mean values were done using ANOVA. A p value of ≤0.05 was used as indicator of significant difference in a two-tail hypothesis. The analyses were done using SPSS 17.0 software (USA).
3 Results
3.1 Fungal isolates from deteriorated sweet orange fruits
The presumptive identity of the twenty-two (22) fungal species isolated from the deteriorated sweet orange fruits is presented in . They include 20 mould and two yeast groups belonging to 9 different genera namely: Alternaria (1), Arthroderma (1), Aspergillus (6), Candida (1), Fusarium (5), Gliocladium (1), Paecilomyces (1), Penicillium (5) and Trichosporon (1).
Fig. 1 Presumptive identity and % occurrence of fungal species isolated from deteriorated sweet orange fruits.
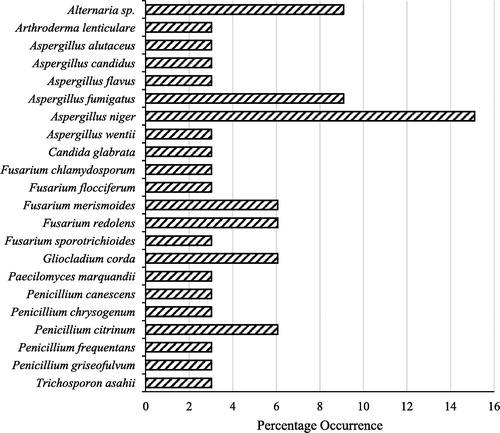
Aspergillus niger had the highest occurrence of 15.1% in the deteriorated sweet orange fruits followed by Alternaria sp. and Aspergillus fumigatus with 9.1%. Fusarium merismoides, Fusarium redolens, Gliocladium corda and Penicillium citrinum, occurred in similar proportion of about 6.1% in the deteriorated sweet orange fruits. Arthroderma lenticulare, Aspergillus alutaceus, A. candidus, A. flavus, A. wentii, Fusarium chlamydosporum, F. sporotrichioides, F. flocciferum, Paecilomyces marquandii, Penicillium griseofulvin, P. canescens, P. chrysogenum, P. frequentans as well as the yeasts Candida glabrata and Trichosporon asahii had the least occurrence 3.0% respectively ().
3.2 In-vitro response of fungal deteriogens to chemical biocides and Red Acalypha extract
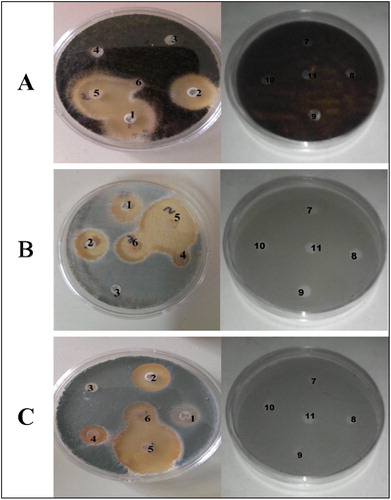
Results of the in-vitro exposure of fungal isolates from deteriorated sweet orange fruits to the six commercial fungicides (chemical biocides) and the Red Acalypha leaf extract are presented in while their appearance on plates are also presented in .
Table 2 Results of the in-vitro susceptibility test of isolated fungal deteriogens.
Plate 1 Susceptibility of the selected fungal deteriogens to the tested biocides [Zone of inhibition in mm]. A – Aspergillus niger, B – Penicillium canescens; C – Penicillium citrinum; 1 – VOR; 2 – NYS; 3 – FLC; 4 – RFC; 5 – FCE; 6 – UPL; 7 – 1% w/v Acalypha; 8 – 0.5 w/v Acalypha; 9 – 0.25 w/v Acalypha; 10 – 0.125 w/v Acalypha; 11 – sterile distilled water (control).
In-vitro susceptibility of fungal deteriogens from deteriorated sweet orange fruits to VOR ranged between 13.0 ± 2.6 mm and 28.7 ± 1.2 mm. The largest zone of inhibition by VOR was obtained in Penicillium griseofulvum (28.7 ± 1.2 mm) and the least in Penicillium canescens (13.0 ± 2.6 mm). No zone of inhibition (0.0 ± 0.0 mm) was observed for Fusarium chlamydosporum, Fusarium flocciferum, Fusarium merismoides, Gliocladium corda, Paecilomyces marquandii, Penicillium chrysogenum, Penicillium citrinum and Penicillium frequentans. VOR was active against 63.6% fungal deteriogens (n = 14) including the two yeast species. It was active against 100% of the Aspergillus sp. (n = 6), 40% of the Penicillium sp. (n = 5), 40% of the Fusarium sp. (n = 5), and the Alternaria sp. (n = 1).
NYS was the most effective antifungal agent inhibiting 21 (95.5%) out of the 22 fungal deteriogens. It was active against 100% of the yeasts species (n = 2) and 95% of the mould species (n = 20) except Paecilomyces marquandii. NYS showed the highest activity against Penicillium chrysogenum (28.0 ± 3.0 mm) and the least activity against Fusarium sporotrichioides (9.3 ± 1.2 mm).
FLC was the least effective among the commercially available fungicide. It was active against only four out of the 22 fungal deteriogens tested (18.18%). The sensitive species included the moulds Alternaria sp. and Fusarium sporotrichioides as well as the yeasts Candida glabrata and Trichosporon asahii. FLC had the highest activity against Alternaria sp. (21.7 ± 1.5 mm) and the lowest activity against Fusarium sporotrichioides (7.7 ± 1.5 mm).
Only five out of the 22 fungal deteriogens (22.73%) isolated from the deteriorated sweet orange fruits were sensitive to RFC and UPL powdered biocides. The yeast species, Candida glabrata, and Trichosporon asahii showed resistance to the two fungal biocides. Up to 80% of the Penicillium sp. (n = 5) and 16.67% of the Aspergillus sp. (n = 6) were susceptible to RFC with Penicillium chrysogenum and Aspergillus wentii showing the highest and lowest sensitivity of 31.7 ± 2.1 mm and 13.3 ± 1.2 mm respectively. Conversely, UPL activity was observed against 80% of the Penicillium sp. (n = 5) and 20% of the Fusarium sp. (n = 5) with Penicillium chrysogenum and Penicillium frequentans showing the largest (30.7 ± 1.2 mm) and the lowest (15.3 ± 4.2 mm) zones of inhibition respectively.
Out of the 22 fungal deteriogens of the sweet orange fruits tested, 19 which represent about 86.4% were susceptible to the FCE, making it the most effect powdered fungal biocide with field application potential. The FCE was active against 100% of the Arthroderma sp. (n = 1), Aspergillus sp. (n = 6), Fusarium sp. (n = 5), Gliocladium sp. (n = 1), Paecilomyces sp. (n = 1) and Penicillium sp. (n = 5). However, Alternaria sp. and the two yeast isolates, Candida glabrata and Trichosporon asahii, were resistant to FCE. Aspergillus alutaceus (31.7 ± 1.5 mm) had the highest zone of inhibition while, and the least zone of inhibition was observed in Arthroderma lenticulare (15.7 ± 1.2 mm).
The zone of inhibition for all the fungal deteriogens exposed to various concentrations of Red Acalypha extract was 0.0 ± 0.0 mm. The result showed that Red Acalypha did not show or possess any bioactivities against moulds and yeasts.
3.3 In-vivo pathogenicity of fungi isolated from deteriorated sweet orange fruits
Ability of the 22 fungal deteriogens to initiate visible deterioration in fresh orange fruits and uninoculated control was observed over a period of 11 days in a pathogenicity test ().
Fig. 2 % Deterioration incidence of fresh orange fruits inoculated with fungal deteriogens recorded in days 5, 6, 7, 8, 10 and 11 [Data represents mean of duplicate samples].
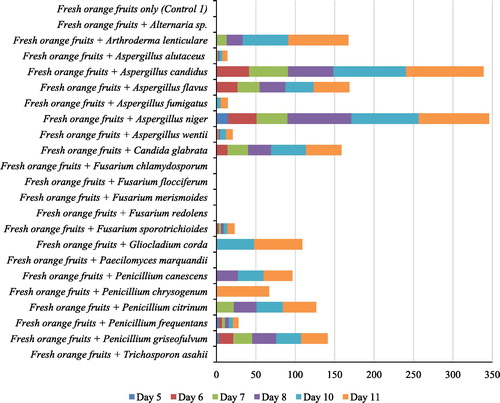
Fresh orange fruits inoculated with fungal deteriogens that were capable of causing infection started exhibiting visible deterioration signs measurable in % deterioration (mean value) of rot on different days: Aspergillus flavus (0.6%), Aspergillus niger (14.8%), Aspergillus wentii (0.8%), Fusarium sporotrichioides (1.2%), Penicillium frequentans (3.3%) and Penicillium griseofulvum (3.4%) in day five; Aspergillus candidus (41.4%) and Candida glabrata (14.1%) in day six; Arthroderma lenticulare (13.1%), Aspergillus alutaceus (1.9%) and Penicillium citrinum (22.2%) in day seven; Penicillium canescens (27.3%) in day eight; Aspergillus fumigatus (5.5%) and Gliocladium corda (47.8%) in day 10; and Penicillium chrysogenum (66.9%) in day 11. However, Alternaria sp., Fusarium chlamydosporum, Fusarium flocciferum, Fusarium merismoides, Fusarium redolens, Paecilomyces marquandii, Trichosporon asahii and uninoculated fresh orange fruits (Control 1) did not show any sign of deterioration within the incubation period of 11 days.
The mean % deterioration of inoculated sweet orange fruits ranged from 6.4 ± 0.0% [mean ± SD] for Aspergillus alutaceus to 98.4 ± 0.1% for Aspergillus candidus. Overall comparison of the mean % deterioration i.e. the indicator of fungal pathogenicity induced by the deteriogens showed highly significant difference (p = 0.001, n = 15). Pairwise comparison of the mean% deterioration revealed that Aspergillus candidus and Aspergillus niger induced significantly higher (p = 0.001) deterioration compared to each of the remaining 13 fungal deteriogens. However, there was no significant difference (p = 0.35; 0.82, 0.77 and 0.45 respectively) when in-vivo pathogenicity of Aspergillus wentii was compared with those of Aspergillus alutaceus, Aspergillus fumigatus, Fusarium sporotrichioides and Penicillium frequentans.
3.4 In-vivo susceptibility of fungal deteriogens to the FCE biocide [Carbendazim 50% WP]
The in-vivo response of the isolated fungal deteriogens inoculated into fresh orange fruits to the FCE biocide is presented as % deterioration and severity on day 11 after inoculation and treatment (). Representative fresh orange fruit samples inoculated with fungal deteriogens and treated with FCE biocide are also presented in .
Table 3 % Deterioration and severity of treated and untreated fresh orange fruits on day 11 of incubation#.
Plate 2 Representative fresh orange fruits showing deterioration signs after 11 days of incubation. Group A – fresh orange fruits inoculated with fungal spores only; Group B – fresh orange fruits inoculated with fungal spores and treated with FCE; 1 – Control 1 (fresh orange fruits inoculated with sterile water); 2 – Control 2 (fresh orange fruits inoculated with sterile water and treated with FCE); 3 – Aspergillus flavus; 4 – Aspergillus niger; 5 – Candida glabrata; 6 – Penicillium chrysogenum.
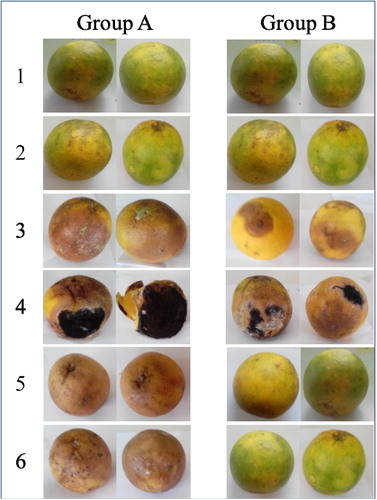
Antifungal effect of FCE biocide revealed wide differences in susceptibility of each deteriogen. The susceptibility of the fungal deteriogens ranged from 100% for Arthroderma lenticulare, Aspergillus fumigatus, Candida glabrata, Fusarium sporotrichioides, Gliocladium corda and Penicillium griseofulvum to 37.3% for Aspergillus niger.
Compared to Group A, the FCE biocide significantly (p = 0.001) prevented deterioration in fresh orange fruits inoculated with Arthroderma lenticulare, Aspergillus fumigatus, Candida glabrata, Fusarium sporotrichioides, Gliocladium corda and Penicillium griseofulvum while the FCE biocide significantly (p = 0.001) reduced deterioration in fresh orange fruits inoculated with Aspergillus alutaceus, Aspergillus candidus, Aspergillus flavus, Aspergillus niger, Aspergillus wentii, Penicillium canescens, Penicillium chrysogenum, Penicillium citrinum and Penicillium frequentans.
Deterioration severity was highest (100%) in Aspergillus candidus, Aspergillus niger, and Penicillium griseofulvum inoculated fresh orange fruits and was reduced to 20% in Aspergillus candidus, and 0% in Penicillium griseofulvum inoculated fresh orange fruits when treated with FCE biocide. However, there was no reduction in deterioration severity of FCE biocide treated fresh orange fruits inoculated with Aspergillus niger.
Deterioration severity of 80% was reported for fresh orange fruits inoculated with Aspergillus flavus and Penicillium canescens while 40% was reported for Aspergillus alutaceus and Penicillium frequentans. Deterioration severity was 60% in fresh orange fruits inoculated with Candida glabrata, 40% in Arthroderma lenticulare, F. sporotrichioides, G. corda and 0% when the fresh orange fruits were treated with FCE solution. Deterioration severity of fresh orange fruits inoculated with Penicillium citrinum (40%) was reduced to 20% when treated with FCE biocide solution.
There was comparable (p = 1.00) absence of deterioration in the fresh orange fruits + Arthroderma lenticulare + FCE (Group B), fresh orange fruits + sterile distilled water (Control 1) and fresh orange fruits + FCE (Control 2). Group B showed significant (p = 0.001) reduction in deterioration compared to Group A ().
4 Discussion
Postharvest loss of sweet orange (Citrus sinensis) fruits due to the activities of deteriogens can be up to 25% of a single harvest in the developing countries [Citation42]. In developing countries, percentage loss may increase as a direct consequence of the handling, transport and storage practices which increase the physical damage and introduce more microbial deteriogens into the orange fruits. The intrinsic properties of the sweet orange fruits including low pH as well as high sugar and nutrient contents make them more susceptible to fungal attack.
The 22 fungal deteriogens isolated from deteriorated orange fruit samples belonged to the nine genera Alternaria, Arthroderma, Aspergillus, Candida, Fusarium, Gliocladium, Paecilomyces, Penicillium, and Trichosporon. The fungal genera have been found to be associated with spoilage of fruits as previously reported [Citation23,Citation24,Citation27,Citation42,Citation44Citation[45]–Citation46] . Fungal species isolated from this study have been implicated in fruit infection commonly referred to as blue mould, green mould, brown rot, black spot, black rot, black pit, etc. [Citation26].
Aspergillus sp, Penicillium sp. and Fusarium sp. which were prevalent in this study are known to be the most important deteriogens affecting fruits worldwide [Citation46]. The high prevalence of Aspergillus niger in the fungal isolates obtained in this study is similar to the works of Akintobi et al. [Citation25] and Onuorah et al. [Citation28]. Khan et al. [Citation46] stated that Aspergillus niger is the most predominant fungal isolate associated with the deterioration of citrus fruit.
Voriconazole (VOR), Nystatin (NYS) and Fluconazole (FLC) are chemical biocides (synthetic antifungal agents) commonly used for treatment of fungal infections. The in-vitro exposure of the 22 fungal deteriogens to these antifungal agents revealed their broad spectrum nature and therefore confirmed their efficacy as antifungal drugs for treatment of mild to serious invasive fungal infections. More of the fungal deteriogens used in this study were more susceptible to NYS and VOR compared with FLC. VOR is one of the new generation triazoles reported to have a wide range of activity, since they are active against Candida, Aspergillus, Fusarium, Penicillium, Scedosporium, Acremonium, and Trichosporon, and dimorphic fungi, dermatophytes, and Cryptococcus neoformans [Citation47Citation[48]–Citation49] .
Red force® (RFC), ultimax plus® (UPL) and forcelet® (FCE) are also chemical biocide brands with antifungal activities usually in the form of powdered fungicide formulations. In-vitro exposure of the 22 fungal deteriogens also revealed that the three chemical powdered formulations have a wide range of antifungal activities with FCE as the most effective. FCE contains Carbendazim, a systemic benzimidazole, commonly used to control a broad range of fungal attacks in crops, fruits and vegetables as well as in their postharvest storage [Citation50]. It has a widespread usage in postharvest preservation of sweet orange fruits, especially in developed countries.
Antimicrobial activities of crude leaf extracts of Acalypha wilkesiana (Red Acalypha) is well documented [Citation51Citation[52]–Citation53] . For instance, Alade and Irobi [Citation51] showed that the water and ethanol extracts of Red Acalypha leaf inhibited the growth of standard and local strains of bacteria and fungi including the yeast Candida albicans and mould Aspergillus flavus. The present study, however, showed that all the 22 fungal deteriogens tested including the yeasts Candida glabrata and Trichosporon asahii were not inhibited in-vitro by the crude extract of Red Acalypha leaf. It, therefore, suggests that extracts of Red Acalypha may not be a suitable biocontrol alternative for prevention of fungal deteriogens in postharvest storage of sweet orange fruits.
The in-vivo pathogenicity data () suggests that a break in the lines of defence of the sweet orange fruits (i.e. skin, flavedo, and albedo) precedes early appearance of deterioration signs and eventual spoilage. Nune et al. [Citation54] stated that wound infection in crop produce is essential to the establishment of deterioration. Production of toxins and important digestive enzymes by the growing fungal deteriogens in the sweet orange fruits might be responsible for the observed deterioration by 15 of the 22 study fungal deteriogens [Citation26]. The result also showed that not all the fungal isolates were capable of initiating deterioration in healthy fresh orange fruits. The possible reasons for their non-pathogenicity is subject to further studies.
Deterioration signs were not noticed in the fresh orange fruits inoculated with spores of the fungal deteriogens until five days of incubation, and where visible deterioration occurred, the rot diameter increased daily until the experiment was terminated on day 11. The reason for the five-day incubation period for the visible development of deterioration was that adequate time is required for the germination of conidia as well as the elongation of fungal hyphae especially of slowly growing fungi [Citation55]. The uninoculated fresh orange fruits used as control in the pathogenicity test remained wholesome throughout the incubation period.
Aspergillus niger was the most pathogenic among the 22 fungal isolates. Similar observation was made by Akintobi et al., [Citation25]. FCE biocide was capable of controlling the development of deterioration caused by fungal isolates in almost all the citrus fruit studied. Abano and Sam-Amoah [Citation56] stated that dips of biocides were effective in controlling post-harvest rot caused by fungal species.
The in-vivo experiment involving FCE biocide treatment of the fresh orange fruits inoculated with various fungal deteriogens confirmed the broad spectrum nature of the powdered biocide (). The FCE significantly prevented or reduced deterioration in healthy fresh orange fruits. Use of FCE biocide for preservation of sweet orange fruits is therefore recommended to extend shelf life of sweet oranges. It will thereby reduce postharvest loss of this important seasonal and perishable fruit.
However, no deterioration signs were observed in fresh orange fruits + sterile distilled water (control 1) and fresh orange fruits + FCE (control 2) within the incubation period of 11 days. Absence of deterioration in the two controls confirmed the need for good agricultural practices that will reduce physical damage and contamination of fresh orange fruits, especially during harvest, transport and storage.
5 Conclusion
Sweet orange (Citrus sinensis) fruits is a perishable agricultural produce which is available in large quantity especially during its season. Preservation for a longer period is a major challenge especially in developing countries where farmers do not have access to high-tech harvesting and storage facilities. This study compared the effectiveness of selected chemical biocides and Acalypha wilkesiana leaf extract against postharvest fungal deteriogens of sweet orange (Citrus sinensis) fruits for sustainable application.
Fungal species are associated with deterioration of sweet orange fruits, and physical damage is a precondition for initiation of biological decay. Although VOR antibiotic possessed highly effective activity against most of the fungal deteriogens of sweet orange fruits, their use in sweet orange fruits preservation is however limited to avoid early fungal resistance. Forcelet® (FCE) powdered biocide, a brand of Carbendazim (50% WP), also possessed a broad spectrum activity (in-vitro and in-vivo) against fungal deteriogens of sweet orange fruits. In an attempt to provide a cost-effective and sustainable preservation option, Acalypha wilkesiana (Red Acalypha), a suggested biocontrol alternative, did not show any activity against fungal deteriogens including yeasts.
Although FCE biocide can reduce deterioration in healthy fresh orange fruits inoculated with fungal deteriogens, maintaining the integrity of the sweet orange fruits especially during harvest, transport and storage is important to the prolonged shelf life and reduction of postharvest loss. Future research will be aimed at testing other non-chemical preservation alternatives for a more sustainable solution.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However, the authors wish to thank the Department of Biological Sciences, Osun State University, Osogbo, Nigeria for material support. We are particularly grateful to Dr W.F. Sule for proof reading the article.
References
- UNCTAD. Market information in the commodities area: information on citrus fruit. United Nations Conference on Trade and Development. Available from: http://www.unctad.info/en/Infocomm/Agricultural_Products/Citrus-fruit/market/Production/. Accessed 2015 November; 2013.
- I.C.OlifeO.A.IbeaghaA.P.OnwualuCitrus fruits value chain development in NigeriaJ Biol Agric Health5420153647
- F.G.GmitterX.L.HuThe possible role of Yunnan, China, in the origin of contemporary citrus species (Rutaceae)Econ Bot441990267277
- Y.LiuE.HeyingS.A.TanumihardjoHistory, global distribution, and nutritional importance of citrus fruitsComp Rev Food Sci Food Safety1162012530545
- FAOHigh-Level Conference on World Food Security: The Challenges of Climates Change and Bioenergy2008FAOItaly
- Bates RP, Morris JR, Crandall PG. Principles and practices of small- and medium-scale fruit juice processing. FAO Agricultural Services Bulletin 146. Rome, Italy: Food and Agricultural Organization of the United Nations; 2001, pp. 226, ISBN 92-5-104661-1.
- M.ParleD.ChaturvediOrange: range of benefitsInt Res J Pharm3720125963
- S.GorinsteinO.Martin-BellosoY.ParkR.HaruenkitA.LojekM.Cizet al.Comparison of some biochemical characteristics of different citrus fruitsFood Chem7432001309315
- T.TurnerB.J.BurriPotential nutritional benefits of current citrus consumptionAgriculture32013170187
- NIHORT25 Years of Research into Horticultural Crops Development in Nigeria (1975–2000)O.A.DentonK.O.AlasiriM.A.Adejoro2000National Horticultural Research Institute
- M.BarthT.R.HankinsonH.ZhuangF.BreidtMicrobiological spoilage of fruits and vegetablesW.H.SperberM.P.DoyleCompendium of the Microbiological Spoilage of Foods and Beverages, Food Microbiology and Food Safety2009LLCSpringer Science Business Media135183
- C.R.WellingsPathogenicity of fungi associated with a citrus greasy spot in New South WalesTrans Br Mycol Soc7631981495499
- V.SinghB.J.DeverallBacillus subtilis as a control agent against fungal pathogens of citrus fruitTrans Br Mycol Soc8331984487490
- E.ChalutzC.L.WilsonPostharvest biocontrol of green and blue mould and sour rot of citrus fruit by Debaryomyces hanseniiPlant Dis7421990134137
- H.W.BrowningR.J.McGrovernL.K.JacksonD.V.CalvetW.F.WardawskiFlorida Citrus diagnostic guide on citrus and control green and blue moulds of citrusBiol Control819958188
- A.El-GhaouthC.L.WilsonM.WisniewskiS.DrobyJ.L.SmilanickL.KorstenBiological control of postharvest disease of citrus fruitsS.S.GnanamanickamBiological control of crop diseases2002New YorkMared Dekker Inc289312
- E.M.EmbabyM.HazaaL.F.HagagT.E.IbrahimF.S.Abd El-AzemThe decay of some citrus fruit quality caused by fungiJ Appl Sci Res911201359205929
- R.R.Al-HindiA.R.Al-NajadaS.A.MohammedIsolation and identification of some fruit spoilage fungi: screening of plant cell wall degrading enzymesAfr J Microbiol Res542011443448
- E.M.MonsoOccupational asthma in greenhouse workersCurr Opin Pulm Med1022004147150
- R.A.BaiyewuN.A.AmusaO.A.AyoolaO.O.BabalolaSurvey of the postharvest diseases and aflatoxin contamination of marketed pawpaw fruit (Carica papaya L.) in South Western NigeriaAfr J Agric Res242007178181
- J.M.JayMicrobial spoilage of food4th ed.Modern food microbiology2003Chapman and Hall Inc.New York187195
- L.L.SingletonJ.D.MihailC.M.RushMethods for research on soil-borne phytopathogenic fungi1992American Phytopathological Society PressSt Paul, MN, USA264
- A.BukarM.O.MukhtarS.AdamuIsolation and identification of postharvest spoilage fungi associated with sweet orange (Citrus sinensis) traded in Kano metropolisBayero J Pure Appl Sci212009122124
- O.O.AkinmusireFungal species associated with the spoilage of some edible fruits in Maiduguri northern eastern NigeriaAdv Environ Biol512011157161
- A.O.AkintobiI.O.OkonkwoS.O.AgunbiadeO.R.AkanoO.OnianwaIsolation and identification of fungi associated with the spoilage of some selected fruits in Ibadan, Southwestern NigeriaAcademic Arena3112011110
- S.S.D.MohammedN.KumarD.DamisaE.BalaI.L.MuhammadR.G.Muhammadet al.Fungi associated with decayed sweet oranges (Citrus sinensis) collected from Lapal, Niger State, NigeriaIndian J Life Sci222013123131
- I.Y.TafintaK.ShehuH.AbdulganiyyuA.M.RabeA.UsmanIsolation and identification of fungi associated with the spoilage of sweet orange (Citrus Sinensis) fruit in Sokoto StateNigerian J Basic Appl Sci2132013193196
- S.OnuorahI.ObikaU.OkaforFilamentous fungi associated with the spoilage of post-harvest sweet orange fruits (citrus sinensis) sold in Awka major markets, NigeriaBioeng Biosci3320154449
- M.K.OladunmoyeComparative evaluation of antimicrobial activities and phytochemical screening of two varieties of Acalypha wilkesianaInt J Trop Med13200613481356
- G.R.BalaliS.M.NeateE.S.ScottD.L.WhissonT.J.WicksAnastomosis group and pathogenicity of isolates of Rhizoctonia solani from potato crops in South AustraliaPlant Pathol44199510501057
- I.PromputthaR.JeewonS.LumyongE.H.C.McKenzieK.D.HydeRibosomal DNA fingerprinting in the identification of non-sporulating endophytes from Magnolia liliifera (Magnoliaceae)Fungal Divers202005167186
- B.ThiyamSharma GD Isolation and identification of fungi associated with local fruits of Barak Valley, AssamCurr World Environ822013319322
- M.O.FawoleB.A.OsoLaboratory Manual of Microbiology1st ed.1995Spectrum Books LtdIbadan, Nigeria3435
- Samson RA, Hoekstra ES, Frisvad JC. Introduction to food borne fungi. 6th ed. American Society Microbiology; 2001, pp. 396, ISBN-10: 9070351420.
- K.DomsdhW.GamsT.AndersonCompendium of soil fungi1980Academic PressLondon859
- R.A.HumberFungi: IdentificationL.A.LaceyManual of Techniques in Insect Pathology1997Academic PressSan Diego153185 5–1
- E.-J.ScholteB.G.J.KnolsR.A.SamsonW.TakkenEntomopathogenic fungi for mosquito control: a reviewJ Insect Sci4192004124
- S.GuyS.RichardIdentifying Fungi, A Clinical Laboratory Handbook2nd ed.2011Star Publishing Inc.377
- A.O.JekayinfaA.O.GeorgeK.T.JaiyeobaAcalypha wilkesiana: preliminary in vitro microbiological and a clinical trial on dermatitisAfr J Health Sci4119963942
- R.K.P.GuptaM.H.PradeepaIn vitro antioxidant and H+, K+-ATPase inhibition activities of A. wilkesiana foliage extractJ Pharm Bioall Sci532013214
- A.W.BaeurW.M.KirbyJ.C.SherrisM.JurckAntibiotic susceptibility testing by a standard single disc methodAm J Clin Pathol4541966493496
- M.E.El-SayedM.HazaaF.H.LailaE.I.TalaatS.A.FatenThe decay of some citrus fruit quality caused by fungiJ Appl Sci Res911201359205929
- G.R.TownsendJ.W.HeubergerMethods for estimating losses caused by diseases in fungicide experimentsPlant Dis Rep27171943340343
- N.A.AmusaJ.A.KehindeO.A.AshayeBiodeterioration of breadfruit (Artocarpus communis) in storage and its effects on the nutrient compositionAfr J Biotechnol1220025760
- S.SangwanichS.SangchoteW.LeelasuphakulBiocontrol of citrus green mould and postharvest quality parametersInt Food Res J206201333813386
- A.KhanS.IramA.RasoolPathogens identification and characterisation that compromised citrus fruit quality in selected orchards of SargodhaInt J Environ Sci Toxicol Res3420155459
- C.ChiouA.GrollT.WalshNew drugs and novel targets for treatment of invasive fungal infections in patients with cancerOncology522000120135
- J.A.SaboS.M.Abdel-RahmanVoriconazole: a new triazole antifungalAnn Pharmacother349200010321043
- P.VandeputteS.FerrariA.T.CosteAntifungal resistance and new strategies to control fungal infectionsInt J Microbiol20122012126
- B.Gilbert-LópezJ.F.García-ReyesM.MezcuaA.Molina-DíazA.R.Fernández-AlbaDetermination of postharvest fungicides in fruit juices by solid-phase extraction followed by liquid chromatography electrospray time-of-flight mass spectrometryJ Agric Food Chem552620071054810556
- P.I.AladeO.N.IrobiAntimicrobial activities of crude leaf extracts of Acalypha wilkesianaJ Ethnopharmacol3931993171174
- S.K.AdesinaO.IdowuA.O.OgundainiH.OladimejiT.A.OlugbadeG.O.Onawunmiet al.Antimicrobial constituents of the leaves of Acalypha wilkesiana and Acalypha hispidaPhytother Res1452000371374
- R.SeebaluckA.Gurib-FakimF.MahomoodallyMedicinal plants from the genus Acalypha (Euphorbiaceae) – a review of their ethnopharmacology and phytochemistryJ Ethnopharmacol1592015137157
- C.NunesA.DuarteT.MansoJ.GarcíaJ.CayuelaK.Yousfiet al.The relationship between post-harvest diseases resistance and mineral composition of citrus fruitActa Hort8682010
- J.MeletiadisJ.F.MeisJ.W.MoutonP.E.VerweijAnalysis of growth characteristics of filamentous fungi in different nutrient mediaJ Clin Microbiol3922001478484
- E.E.AbanoL.B.Sam-AmoahApplication of antagonistic microorganisms for the control of postharvest decays in fruits and vegetablesInt J Adv Biol Res21201218
