Abstract
This work was aimed to develop a rapid, simple, selective, and precise spectrophotometric method for the estimation of salicylhydroxamic acid found in both capsules and raw materials. Spectrophotometric detection was conducted at maximum absorption of 294 nm with the aids of methanol: water (1:99, v/v) as solvent. The figures of merit of the newly developed method were validated for linearity, specificity, accuracy, precision, robustness, ruggedness, limit of detection (LOD), and limit of quantification (LOQ). The detector response for the salicylhydroxamic acid was linear over the concentration range studied 0.1–50 μg mL−1 with a correlation coefficient (R2) of 0.9999. Accuracy was between 99.0% and 101.7% with a mean value of 100.07%. The intra- and inter-day precisions, expressed as relative standard deviation (R.S.D.), were less than 0.0.322 and 0.421%, respectively. LOD and LOQ were 0.031 and 0.098 μg mL−1, respectively. Results confirmed that the excipients in the commercial capsules did not interfere with the method and can be employed for routine quality control analysis of salicylhydroxamic acid whether in capsules or raw materials.
1 Introduction
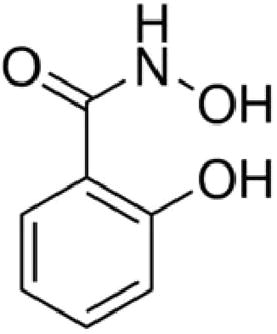
Salicylhydroxamic acid (SHAM) (C7H7NO3), which is a phenolic compound [Citation1Citation[2]–Citation3] , is considered an intense and irreversible inhibitor used against bacteria. Additionally plant urease generally utilized for urinary tract diseases and known for its pharmaceutical applications (). This structure of SHAM is similar to urea, however, is not hydrolyzable by the urease [Citation3Citation[4]Citation[5]Citation[6]Citation[7]–Citation8] . It prevents the development/formation of calcium oxalate stones in kidneys [Citation9,Citation10] . At the point when administered orally, it is metablolized to salicylamide which applies pain relieving, antipyretic and anti-inflammatory effects. SHAM is additionally viewed as a typical ligand that utilized as a part of the combination of metal-crowns [Citation4]. The mechanism of action of SHAM to stop the development of phosphate stones is by back off urease catalyst action [Citation9]. The change procedure of urea to carbon dioxide and ammonia is then catalyzed by the impact of urease enzyme in the case of occurrence of urinary tract disease. When urease action is restrained, SHAM suppress ammonia formation and holds urea acidic [Citation9]. Moreover, it diminishes serum uric acid and the rate of uric stones and ureate [Citation11].
Fig. 1 The structure of salicylhydroxamic acid (pKa 7.40 and 9.70) [Citation12].
Literature search uncover that few methods for the estimation of SHAM have been accounted for whether the kinetics of the acid and base hydrolysis of SHAM and O-acetyl-salicylhydroxamic acid (OAc-SHAM) at various parameters, for example, time, temperature and pH, utilizing reversed phase performance liquid chromatography with UV detector (RP-HPLC/UV) [Citation9], or for assessing SHAM in entire blood utilizing HPLC/UV [Citation13].
SHAM was additionally evaluated by measuring the absorbance of its V(V) complex at 620 nm and limit of detection was 50 μM [Citation14]. Moreover, potentiometric method for the estimation of SHAM in light of the inhibition of urease action was likewise conducted [Citation15]. A linear calibration curve between 0.5 and 7 μg mL−1 with a limit of detection of 0.1 μg mL−1 was achieved. Shetty et al. utilized HPLC for measuring SHAM and its metabolites in the urine of rat [Citation16]. Cu(II) can chelate some active ligands, for example, salicylamide, salicylhydroxamic and gallic acid which were likewise analyzed by electron spin resonance (ESR) [Citation17]. Besides, Capitan et al. have reported the utilization of SHAM in spectrophotometric methods for determination of Ti(IV) in aluminum alloys and mineral specimens as well. The complex Ti(IV)-SHAM and its blended ligand namely, Ti(IV)-SHAM – thiocyanate complex, were estimated [Citation18]. On the other hand, Salem created a few methods for the estimation of SHAM utilizing atomic absorption spectrophotometry (AAS) and spectrophotometry methods. The AAS technique depends on precipitating the [Cu (NH3)4]2+-SHAM complex with the aid of excess of [Cu(NH3)4]2+ to SHAM solution. The Cu2+ ion in the supernatant layer was determined by AAS. The spectrophotometric technique is relies on upon measuring the green color produced by adding [Cu (NH3)4]2+ to SHAM solution consisted of 50% dioxane: water solution [Citation19].
Spectrophotometry is notable for its ease of use, minimal effort, low cost, and availability in all research centers. In addition, the pretreatment steps prior sample preparation is at times essential prior the application of such technique in order to overcome sample interferences and to preconcentrate the analyte before subjected to analysis [Citation19Citation[20]–Citation21] .
The target of the present work is to create simple, fast, specific and accurate UV spectrophotometric method for the estimation of SHAM in raw materials and capsules. This method was additionally validated for the accompanying parameters, for example, linearity, accuracy, precision, sensitivity, ruggedness, and robustness. The limits of detection (LOD), as well as quantification (LOQ), were also determined. Also, the experimental parameters for the developed method were validated in accordance with the International Conference on Harmonization (ICH) rules Q2 (R1) (ICH, 2005) [Citation22]. The created method is consequently recommended for the normal routine analysis in the quality control unit.
2 Experimental
2.1 Materials and chemicals
Salicylhydroxamic acid working standard (>99%) and SHAM (300 mg capsules) were a kind gift from El Nasr Pharmaceutical Chemicals Co. “ADWIC”. Salicylhydroxamic acid raw materials were purchased from Haoyuan Chemexpress Co. Ltd (MOLBASE, Shanghai, China) and Shangrao New Future Environment Protection Technology Co., Ltd. (Shangrao, China). Methanol used in this work was of analytical grade which purchased from Sigma–Aldrich (St Louis, USA). All the other chemicals and reagents used were of analytical grade. Double distilled water was used for the preparation of all solutions.
2.2 Method development
2.2.1 Instrumentation
Spectroscopic analysis was carried out using Double beam Shimadzu recording UV–Visible Spectrophotometer (Kyoto, Japan) model 1800 with 10 mm path length quartz cells. The solutions were made fresh on mass basis using a Mettler Toledo balance (Switzerland) model JB1603-C/FACT with a precision of ±0.01 mg. Double distilled water was produced in our laboratory using GFL-2008 water (Burgwedel, Germany).
2.2.2 Preparation of standard solutions
A stock solution of salicylhydroxamic acid working standard containing 1000 μg mL−1 was prepared in distilled water by transferring the required amount of salicylhydroxamic acid in 500 mL with the aid of 5 mL methanol. It was made up to mark using distilled water. Then, a series of 100 mL volumetric flasks with varying fractions were topped up to mark with distilled water in order to prepare different standard differing in concentration in the range 0.1–50 μg mL−1. All other solutions were stored refrigerated in the dark when not in use.
2.2.3 Preparation of sample (capsules and raw materials)
Twenty capsules of SHAM (300 mg) were weighed. Equivalent to 100 mg salicylhydroxamic acid was quantitatively transferred into 500 mL volumetric flasks. Then it was dissolved using 5 mL of methanol. After that it was shaken for 5 min, 20 mL distilled water was added, shaken again for another 2 min, and finally topped up to the mark with distilled water to attain a final concentration of 200 μg mL−1. The solution was filtered using Whatman filter paper. The filtrate was diluted to obtain the desired concentration within the linearity range studied. The absorbance of sample solutions was measured and the amount of salicylhydroxamic acid was determined using the calibration curve. In the same way, the raw materials were also prepared by transferring 100 mg each and transferred to 500 mL volumetric flask. After that, the same procedure in the preparation of capsules was followed.
2.3 Method optimization
2.3.1 Selection of λmax wavelength
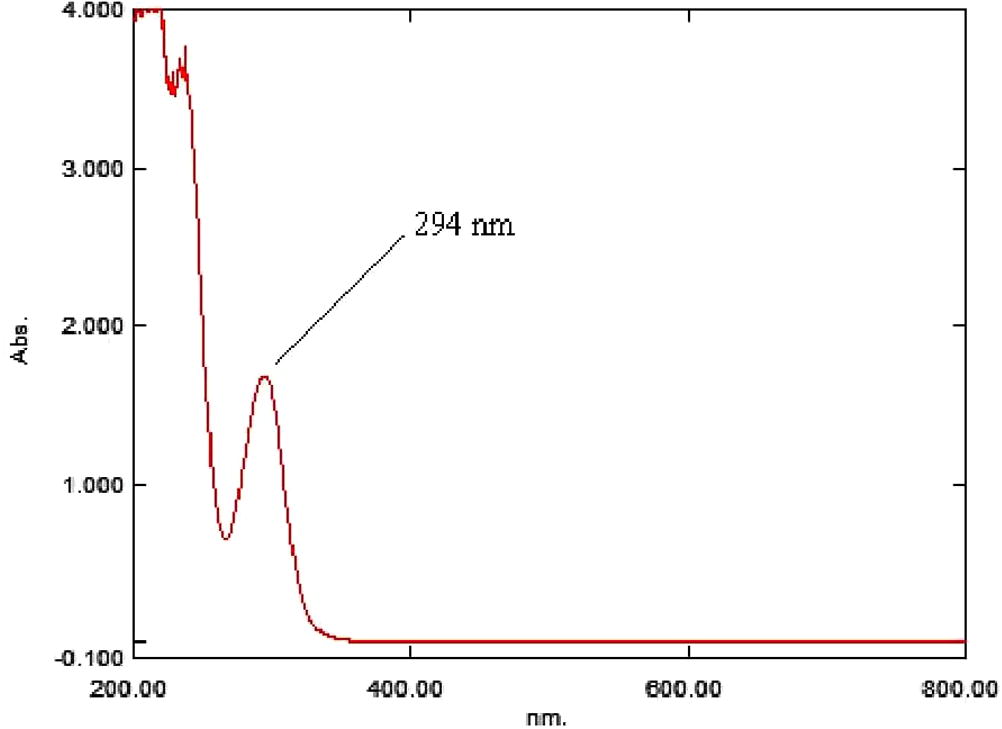
The wavelength at which the maximum absorption (294 nm, ) occurs is selected for further analysis. A definite concentration of salicylhydroxamic acid solution was scanned in UV range of 200–800 nm. Methanol: water (1:99, v/v) was used as a blank. The absorbance of solutions was measured at 294 nm against blank and calibration curve of salicylhydroxamic acid was built up accordingly.
2.4 Method validation
The assay of salicylhydroxamic acid was validated taking into consideration linearity, LOD and LOQ, precision, accuracy, robustness, ruggedness and specificity. Validation of the parameters was carried in the light of the International Conference on Harmonization (ICH) guidelines Q2 (R1) (ICH, 2005) [Citation22]. Below are the parameters that have been investigated.
2.4.1 Sensitivity
The sensitivity of the developed method was investigated by calculating the LOD, and LOQ. This was carried out by preparing a series of concentrations of the drug solutions. LOD and LOQ were carried out by a proper diluting known concentration of salicylhydroxamic acid till the average responses attained were 3 or 10 times the standard deviation of the responses for six measurements [Citation23,Citation24] . LOD and LOQ values were 0.031, and 0.098 μg mL−1, respectively.
2.4.2 Specificity and selectivity
SHAM capsules of label claim 300 mg containing salicylhydroxamic acid of concentration 2 μg mL−1 was prepared in methanol: water (1:99, v/v). On the other hand 2 μg mL−1 of standard salicylhydroxamic acid, in addition, to sample solutions prepared were analyzed using the developed method. The expected amount of capsules was compared with that of the pure salicylhydroxamic acid solution of the same concentration.
2.4.3 Linearity and range
Standard solutions containing salicylhydroxamic acid were prepared in a mixture of methanol: water (1:99, v/v) from a fresh stock solution (1000 μg mL−1) to construct the calibration curve. The least square regression analysis was carried out for the obtained data. Calibration curve consisted of ten different concentrations in the range 0.1–50 μg mL−1 for salicylhydroxamic acid. Each concentration level was performed thrice. The equation of the calibration curve attained was y = 0.02x + 0.004. It was obtained by plotting the absorbance (y) as a function of analyte concentration (x) in μg mL−1.
2.4.4 Accuracy
An appropriate amount of SHAM capsules powder was weighed and then spiked with known amount of the standard compound. After that, each sample was analyzed thrice. In brief, three different concentration levels of SHAM capsules solution using methanol: water (1:99, v/v) as solvent were prepared namely; 1, 5 & 10 μg mL−1 and spiked with three different concentrations of salicylhydroxamic acid standard solution which prepared using methanol: water (1:99, v/v) (2, 15 & 30 μg mL−1). Then the concentrations (x) of the resulting solutions were calculated using the calibration curve. The accuracy was reported as% recovery ± standard deviation. Accuracy values obtained were in the range of 99.00–101.70% as indicated in . The good accuracy results obtained reveal the potential of the developed method for the quantification of the analyte in capsules pharmaceutical formulation.
Table 1 Accuracy results for the determination salicylhydroxamic acid spiked in SHAM capsules.
2.4.5 Precision
Intra- and inter-day precision were used in order to investigate the precision of the developed method. It was done by analyzing three different concentration levels namely; 0.5, 10 and 25 μg mL−1 of standard solutions. The intra-day (repeatability) was estimated by analyzing the nine replicates on the same day. On the other hand, inter-day variation (intermediate precision) was carried out over six consecutive days. Intra-day precision, expressed as the percentage relative standard deviation, RSD, was 0.121–0.322% (), while inter-day precision was 0.114–0.421%, indicating the good precision of the developed method. Reproducibility was also determined by analyzing three different concentrations of salicylhydroxamic acid namely; 0.5, 10 and 25 μg mL−1 on different Shimadzu UV spectrophotometers. The RSD values were less than 0.110%.
Table 2 Intra and inter-day precision for the determination of salicylhydroxamic acid.
2.4.6 Robustness
Robustness of the developed method was also done by slight altering the λmax used in the analysis (λmax 294 nm) i.e., ± 1.0 nm ().
Table 3 Robustness results of salicylhydroxamic acid in capsules and raw materials upon changing λmax 294 nm i.e., ±1.0 nm.
2.4.7 Ruggedness
The ruggedness of the developed method was achieved by analyzing salicylhydroxamic acid by different analysts using similar conditions. The%RSD value was found less than 1.5%.
2.4.8 Analysis of capsules and raw materials using the current method
The content of salicylhydroxamic acid in capsules with label claims 300 mg per capsule and in raw materials were quantified using the developed method. The content of twenty capsules was weighed and the average content per capsule was calculated. Then, equivalent to 100 mg of salicylhydroxamic acid was weighed. The same procedure under Section 2.2.3 was followed for both capsules and raw materials. The prepared solutions were assayed using the proposed method. The% assay results were then reported.
3 Results and discussion
The maximum absorption of salicylhydroxamic acid was detected at 294 nm and overlay spectra of salicylhydroxamic acid working standard, raw materials and capsule were recorded ().
Fig. 3 Overlay spectrum of salicylhydroxamic acid working standard, raw materials, and capsule 300 mg.

The current method was found to be simple, sensitive, accurate, precise, economical and rapid for the routine analysis of salicylhydroxamic acid not only in capsules but also in raw materials.
3.1 Analytical method validation parameters
The developed method was validated in accordance with the ICH guidelines (Q2) (R1) (ICH, 2005) [Citation22].
3.1.1 Linearity and range
The linearity of an analytical method is the figure of merits in method validation step. It is defined as the ability to get results that comply with Beer's law [Citation25]. The characteristic parameters of our newly method are slope 0.02, intercept 0.004, and the correlation coefficient of 0.9999 which show a good linearity of the calibration curve (). Working solutions which contain the standard were prepared as prescribed earlier to draw the calibration curve. Calibration curve contained ten different concentrations (0.1–50 μg mL−1) for salicylhydroxamic acid and each concentration level was performed in a trice. Calibration curve with regression equation was y = 0.02x + 0.004 with good correlation coefficient (0.9999) between the standard concentration (x) and mean absorbance (n = 3) show a good linearity of standard curve ().
Table 4 Results of validation parameters obtained by the newly developed method.
3.1.2 Precision
The precision of an analytical method reflects the degree of scattering occurred between a series of measurements obtained under particular conditions [Citation26]. Intra- and inter-day tests were used to prove the precision of the developed method. The later was conducted by analyzing three concentration levels namely; 0.5, 10 and 25 μg mL−1 of standard solutions. Specifically, intraday precision (repeatability) can be defined as the use of analytical procedure within a laboratory under a short period time through analyzing nine replicates on the same day by the same analyst using the same equipment. On the other hand, inter-day precision (intermediate precision) implies the evaluation of variations in the analysis when a method is used within a laboratory on different days (conducted over six consecutive days), by different analysts [Citation24]. The %RSD for the intra-assay precision and intermediate precision for all the three concentration levels were below 0.322, and 0.421%, respectively () indicating the good precision of the developed method.
3.1.3 Accuracy
The accuracy is defined as the closeness of results to accepted true value. It was determined by conducting recovery tests [Citation27]. An appropriate amount of SHAM capsules powder was weighed and spiked with known amount of the standard compound, and each sample was analyzed in a trice. The results obtained were between 99.00% and 101.70% (). The obtained results support the accuracy of the developed method.
3.1.4 Specificity
The developed method was found selective and specific as there is no interferences occurred as reflected by the accuracy results.
3.1.5 LOD/LOQ
Standard solutions showed good linearity (r2 > 0.9999) over the concentration range tested. The sensitivity of the current method is higher compared with the reported spectrophotometric one (Salem, 2003; [Citation11] the LOQ was 1.53 μg mL−1) or even the potentiometric one reported by Hassan et al., 1997 (the LOD was 0.1 μg mL−1) [Citation15]. The LOD for SHAM was 0.031 μg mL−1, while the LOQ was 0.098 μg mL−1. LOD and LOQ were calculated using the following formulas (LOD = 3.3 σ/S), and (LOQ = 10 σ/S), respectively.
3.1.6 Robustness
The slight variation in the λmax (±1.0 nm) gave% assay results as indicated in , indicating the robustness of the current method.
3.1.7 Application of the newly UV-Spectrophotometer method
The developed method has been successfully applied for the determination of SHAM capsules and raw materials (two different suppliers). In agreement with ICH guidelines the assay values for all formulations studied i.e., capsules and raw materials (1 & 2) were found 90.88, 51.00, and 100.13%, respectively (). Results indicate good agreement between the current method and the manufacturer's claimed values were found ().
Table 5 Assay results of salicylhydroxamic acid in capsules and raw materials.
4 Conclusion
A simple, reliable, accurate and reproducible spectrophotometric method for the determination of salicylhydroxamic acid (SHAM) in capsules and raw materials was successfully developed as per the ICH guidelines. The good analytical performance with regards to validation parameters was achieved. All the validated data attained are in agreement with the ICH guidelines Q2 (R1) (ICH, 2005) [Citation22]. Once compared with the reported spectrophotometric method (Salem, 2003) [Citation11], the developed method exhibits higher sensitivity. The LOD and LOQ were 0.031 μg mL−1 and 0.098 μg mL−1, respectively. Good recoveries of SHAM were obtained in the range of 99.00–101.70% () in different samples confirming the accuracy of developed method. The developed method is thus recommended to be implemented as a quality control protocol in pharmaceutical industries.
Conflict of interest statement
Khaldun Mohammad Al Azzam declares that he has no conflict of interest.
Wafaa El Kassed declares that she has no conflict of interest.
References
- B.GharobiM.G.ZanjanD.E.AsliM.J.JafariEffect of salicylhydroxamic acid (SHAM) on yield and yield components of safflower (Carthamustinctirius L.)Ann Biol Res420137377
- G.-B.LuC.-X.ZhangW.-H.ChenL.-P.ChenY.-S.ZhouThermal hazards and kinetic analysis of salicyl hydroxamic acid under isothermal and adiabatic conditionsThermochim Acta62320164349
- D.M.AlgadiA.SeifA.AlgielaniSynthesis, characterization and biocidal Studies of salicylhydroxamic acid and phathalic salicylhydroxamic acidEur J Acad Essays42017138140
- I.T.IbrahimM.HamedM.Abou ELZahabSynthesis of 125I-salicyl hydroxamic acid for urinary bladder imagingArab J Nucl Sci Appl4820159098
- Hashem El-Sayed N, SALAMA EE. Electrochemical oxidation of salicylhydroxamic acid on Pt electrode. Ovidius Univ Annals Chem. 2016; 27: 53–57.
- Pang S-YM, Tristram S, Brown S. Salicylhydroxamic acid inhibits the growth of candida albicans. Int J Biol Biom Agri Food Biotechnol Eng. 2011; 5: 187–193.
- T.Y.MohamedS.T.AtwaSpectrophotometric microdetermination of Fe(III) and V(V) using schiff base derived from salicylhydroxamic acidInt J Res Stud Biosci12013813
- E.AdiguzelF.YilmazM.EmirikM.OzilSynthesis and characterization of two new hydroxamic acids derivatives and their metal complexes. An investigation on the keto/enol, E/Z and hydroxamate/hydroximate formsJ Mol Struct11272017403412
- E.AlShamailehM.AlawiY.DahdalH.SaadehKinetic stability study of selected hydroxamic acids using HPLC/UVJordan J Pharma Sci120085564
- W.O.FoyeH.S.HongC.M.KimE.L.PrienDegree of sulfation in mucopolysaccharide sulfates in normal and stone-forming urinesInvest Urol1419763337
- A.A.SalemM.M.OmarAtomic absorption and spectrophotometeric determinations of salicylhydroxamic acid in its pure and pharmaceutical dosage formsTurk J Chem272002383393
- A.E.FazaryMetal complexes of salicylhydroxamic acid and 1,10-phenanthroline; equilibrium and antimicrobial activity studiesBull Chem Soc Ethiop282014393402
- Barnicoat AJ, Van T.Hoff WG, Morrison PJ, Bradbrook ID. Determination of salicylhydroxamic acid, a trypanocidal agent, by reversed phase high-performance liquid chromatography. J Chromatogr 1981; 225: 236-239.
- Kanabus-Kaminska J, Urbanski T. Bull Acad Pol Sci Ser Sci Chem. 1979; 27: 891–893 Anal. Abst. 1E48, 41 (1981).
- S.S.M.HassanR.M.El-BahnasawyN.M.RizkPotentiometric determination of salicylhydroxamic acid (urinary struvite stone inhibitor) based on the inhibition of urease activityAnal Chim Acta35119979196
- B.V.ShettyS.MelethilHigh pressure liquid chromatographic assay for salicylic acid and its metabolites in rat urineAnal Lett211988395410
- P.V.KhadikarB.PolS.JoshiS.BhartiPol J Chem611987833838
- L.F.Capitan-VallveyA.Molina-DiazM.L.Fernandez de CordovaM.I.Pascual-RegueraMicrochim Acta11990305311
- A.E.A.A.SalemAtomic absorption and spectrophotometeric determinations of salicylhydroxamic acid in its pure and pharmaceutical dosage formsTurk J Chem272003383393
- H.M.Al-SaidiM.A.Abdel-FadeelA.Z.El-SonbatiA.A.El-BindaryDetermination of bismuth in different samples by dispersive liquid–liquid microextraction coupled with microvolume β-correction spectrophotometryJ Mol Liq2122015635640
- Xiao N, Deng J, Huang K, Ju S, Hu C, Liang J. Application of derivative and derivative ratio spectrophotometry to simultaneous trace determination of rhodamine B and rhodamine 6G after dispersive liquid–liquid microextraction. Spectrochim. Acta A Mol Biomol Spectrosc. 2014; 128 312–318.
- ICH Guideline Q2(R1), Validation of analytical procedures: text and methodology, November 2005.
- G.P.CarrA parallel approach to method validation in pharmaceutical analysisJ Pharm Biomed Anal81990613618
- K.SharmaS.S.AgrawalM.GuptaDevelopment and validation of UV spectrophotometric method for the estimation of Curcumin in bulk drug and pharmaceutical dosage formsInt J Drug Dev Res42012375380
- K.G.ChapmanValidation terminologyI.R.ReddyR.A.NashPharmaceutical process validation2nd ed.1993Maecel DekkerNew York587596
- K.M.Al AzzamB.SaadH.Y.Aboul-EneinSimultaneous determination of atenolol, chlorthalidone and amiloride in pharmaceutical preparations by capillary zone electrophoresis with ultraviolet detectionBiomed Chromatogr242010977981
- L.R.SnyderJ.J.KirklandJ.L.GlajchCompleting the method: validation and transferL.R.SnyderJ.J.KirklandJ.L.GlajchPractical HPLC method development2nd ed.1997John Wiley & Sons IncNew York233312