Abstract
The present study aimed to determine the possible beneficial effects of dimethyl fumarate (DMF) against oxidative stress and inflammation in hypercholesterolemic rabbits. Twenty four New Zealand male rabbits were randomly allocated into 4 groups as the following: Group I (control): rabbits received standard rabbit chow; Group II high cholesterol diet (HCD): rabbits received 1% cholesterol-enriched chow for 4 weeks; Group III (HCD-DMF): rabbits received 1% cholesterol-enriched chow and administered DMF (12.5 mg/kg/day, orally) for 4 weeks; Group IV (DMF): rabbits received standard chow plus DMF (12.5 mg/kg/day, orally) for 4 weeks. At the end of experiment (day 30), blood samples were collected for measuring serum total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C) and C-reactive protein (CRP). In addition, the aorta was removed for measurement of malondialdehyde (MDA), superoxide dismutase (SOD), mRNA expression of cholesteryl ester transfer protein (CETP) and histological assessment of intima/media (I/M) ratio. HCD-fed rabbits showed significant increases in TGs, TC, low-density lipoprotein cholesterol (LDL-C), aortic MDA and aortic I/M ratio levels while they significantly exhibited a reduced SOD level relative to control animals. Moreover, HCD rabbits demonstrated upregulated mRNA expression of CETP. DMF administration significantly decreased HCD-induced elevations in serum TC and LDL-C. Additionally, DMF decreased aortic level of MDA while increased SOD level. Moreover, DMF significantly downregulated mRNA expression of CETP and reduced the elevation in I/M ratio.
Introduction
Hypercholesterolemia is one of the most important risk factors in the development and progression of atherosclerosis, which is a major cause of mortality in world population [Citation1]. Hypercholesterolemia has been shown to increase reactive oxygen species (ROS) production, enhancing oxidative stress. ROS react with lipoproteins specifically low density lipoprotein (LDL), producing oxidized LDL (ox-LDL) [Citation2]. Oxidative modification of LDL plays an important role in inflammation and atherogenesis [Citation3Citation[4]–Citation5] . Thus, antioxidant supplementation could possess a possible protective action against ox-LDL-mediated inflammation, a cornerstone of atherosclerosis.
Dimethyl fumarate (DMF), an ester of fumaric acid (FA), has been recently approved by the FDA for the treatment of relapsing/remitting multiple sclerosis (MS) under the brand name Tecfidera. It is presently employed for the treatment of psoriasis [Citation6].
DMF is rapidly metabolized in the gastrointestinal tract by esterase enzyme into monomethyl fumarate (MMF) which is the main metabolite exerting the therapeutic effects. Half-life of DMF is about 12 min while that of MMF is 36 h. Therefore, orally administered DMF is not detected in the blood stream [Citation7]. DMF has been reported to exhibit an antioxidant effect due to its ability to enhance expression of multiple antioxidant enzymes, including superoxide dismutase (SOD), heme oxygenase-1 (HO-1), glutathione-S-transferase (GST) and catalase (CAT), resulting in reduced oxidative stress possibly via activation of the nuclear factor (erythroid-derived2)-like2-Nrf2 transcriptional pathway, which is the principal regulator of antioxidant enzymes [Citation8] . In addition to possessing antioxidant effect, DMF has anti-inflammatory effect as it reduces inflammatory gene expression and increases anti-inflammatory gene expression [Citation9Citation[10]–Citation11] . Therefore, the present study aimed to investigate the role of anti-inflammatory and antioxidant properties of DMF in preventing oxidative stress and inflammation induced by atherogenic diet in rabbits.
Materials and methods
Materials
Cholesterol, DMF and thiobarbituric acid (TBA) were purchased from Sigma Aldrich chemical Co. (St. Louis, MO, USA). All other chemicals used in this study were of fine analytical grade.
Animals
Adult male New Zealand white (NZW) rabbits with an average body weight of 1.5–2 kg were obtained from Urology and Nephrology Center, Mansoura University, Egypt. The animals were individually housed in cages with food and water available, maintained under standard conditions of temperature about 25 ± 2 °C with a 12 h on/off light schedule. Standard diet pellets were prepared weekly (El Nasr Chemical Co., Abou-Zaabal, Cairo, Egypt). Animals were handled according to rules of Committee of Ethics of Scientific Research, Faculty of Pharmacy, Mansoura University, Egypt. This is in agreement with the Principles of Laboratory Animal Care (NIH publication No. 85–23, revised 1985).
Experimental protocol
Rabbits were randomly distributed into four groups each containing 6 rabbits as the following: Group I (control), rabbits received standard rabbit chow; Group II (HCD), rabbits received 1% cholesterol-enriched chow for 4 weeks [Citation12]; Group III (HCD-DMF), rabbits received 1% cholesterol-enriched chow and administered DMF (12.5 mg/kg/day, orally) for 4 weeks, the dose was converted from rat to rabbit according to Paget and Barnes [Citation13,Citation14] , Group IV (DMF), rabbits received standard chow plus DMF (12.5 mg/kg/day, orally) for 4 weeks. DMF was given to rabbits as a suspension in 0.5% carboxymethyl cellulose (CMC). Both control and HCD groups received 0.5% CMC (1 mL/kg/day, orally) during the treatment period.
Blood samples were collected at the end of experiment (day 30) from marginal ear vein for measurement of serum triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and C-reactive protein (CRP). To obtain serum, blood samples were allowed to clot for 90 min before centrifugation at 3000g. Rabbits were euthanized by an overdose of sodium pentobarbital and exsanguinated for aortic assessment. Aorta was removed for measurement of aortic malondialdehyde (MDA), superoxide dismutase (SOD), histopathological examination, intima/media (I/M) ratio and mRNA expression of cholesteryl ester transfer protein (CETP).
Assessment of serum lipid profile
Allain and co-workers method was used for determination of TC [Citation15] while Fredrickson and colleagues method was used for determination of TGs [Citation16]. Finley et al. method was designed to determine serum HDL-C enzymatically [Citation17]. According to the method described by Friedewald and co-workers, low-density lipoprotein cholesterol (LDL-C) was calculated [Citation18]. Commercial kits (Stanbio Laboratory, Boerne, Texas, USA) were used.
Assessment of serum CRP
For quantitative assessment of CRP in serum, rapid latex agglutination test was used. Commercial kit (Biomed Diagnostics, Badr city, Egypt) was used.
Assessment of aortic antioxidant status
Preparation of aortic homogenate
The aortas were isolated, weighed and homogenized in phosphate buffered saline (PBS, pH 7.4) as 10% (w/v) using a mini handheld homogenizer (Omni international, USA). The supernatants collected after centrifugation of tissue homogenates (1000g, 4 °C, 10 min) were used for assay of MDA and SOD.
Assessment of aortic MDA levels
According to the method of Ohkawa et al., thiobarbituric acid reactive substances (TBARS) were measured as MDA. The concentrations were expressed as nmol/g tissue [Citation19].
Assessment of aortic SOD activity
According to the method of Marklund, the enzymatic activity of SOD was assessed. SOD activity was measured by the degree of inhibition of the auto-oxidation of pyrogallol at an alkaline pH by SOD. The change in absorbance was measured at 420 nm and activity was expressed as U/g tissue [Citation20].
Quantitative real-time polymerase chain reaction (RT-PCR)
The aorta was rapidly isolated, weighed and preserved in RNA Later (Qiagen, Germany) (50–100 mg tissue/1 ml RNA later). Total RNA was extracted from aortic tissues using TRIzol reagent (Invitrogen, USA). One microgram from each sample RNA was reverse transcribed into complementary DNA (cDNA) by using revert aid first strand cDNA synthesis kit (Thermo Scientific Rockford, IL, USA). RT-PCR was performed with Rotor Gene Q thermocycler (Qiagen, Hilden, Germany), using HOT Firepol Evagreen qPCR mix plus kit (Solis BioDyne, Tartu, Estonia).
The mRNA level of CETP was normalized relative to GAPDH ribosomal RNA (Housekeeping gene) in the same sample. The sequences of sense and antisense primers used for amplification are shown as the following: (see ).
Table 1 The sequences of sense and antisense primers used for amplification.
The results were expressed as an n-fold change of the relative expression levels of target genes from control group using ΔΔCt method [Citation21].
Histopathological analysis
At the end of the experiment, the thoracic aorta was carefully removed, fixed in 10% neutral buffered formalin solution (pH 7.4), inserted in paraffin wax, sectioned transversely (5 μm) and stained with hematoxylin and eosin (H&E) stain. Both the intimal cross-sectional area and the medial area were determined then the mean areas were calculated to obtain I/M ratios. The pathologist performing histopathological evaluation was blinded to the study treatment assignment.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM), where n equals the number of rabbits. Statistical analyses were carried out using Graph pad Prism software (GraphPad Software Inc. V4.03, San Diego, CA, USA). Differences were considered significant at P < 0.05.
One-way analysis of variance (ANOVA) followed by Tukey–Kramer’s multiple comparisons post hoc test was used to measure significant differences between groups.
Results
Serum lipid profile
The serum lipid profiles in the four groups of animals are shown in (a-d). The supplementation of HCD for 4 weeks resulted in significant (P < 0.05; n = 6) increases in TC, LDL-C, TGs and HDL-C by 570%, 810%, 220% and 311%, respectively compared to control group. DMF administration significantly (P < 0.05) decreased the elevations of TC, LDL-C and HDL-C by 26%, 34% and 33%, respectively compared to HCD. However, DMF did not significantly affect TGs compared to HCD.
Fig.1 Effect of dimethyl fumarate (DMF, 12.5 mg/kg/day) on serum lipid profile in hypercholestrolemic rabbits. (a) Total cholesterol (TC), (b) Low density lipoprotein-cholesterol (LDL-C), (c) Triglycerides (TGs) and (d) High-density lipoprotein cholesterol (HDL-C). Values represent the mean ± SEM of six rabbits per group. Mean values were compared using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (P < 0.05). * significantly different from the mean value of the control group, # significantly different from the mean value of high cholesterol diet (HCD) group.
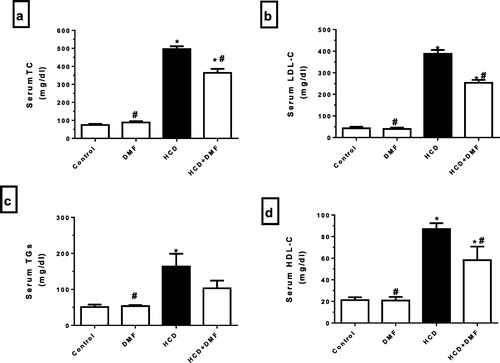
Serum CRP
HCD significantly (P < 0.05) increased serum CRP level (from 1.4 ± 0.24 to 11.6 ± 0.40) compared to control group. In comparison to HCD group, DMF treatment significantly (P < 0.05) decreased CRP level (from 11.6 ± 0.40 to 6.6 ± 0.40) ().
Fig. 2 Effect of dimethyl fumarate (DMF, 12.5 mg/kg/day) on serum C-reactive protein (CRP) in hypercholestrolemic rabbits. Values represent the mean ± SEM of six rabbits per group. Mean values were compared using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (P < 0.05). * significantly different from the mean value of the control group, # significantly different from the mean value of high cholesterol diet (HCD) group.
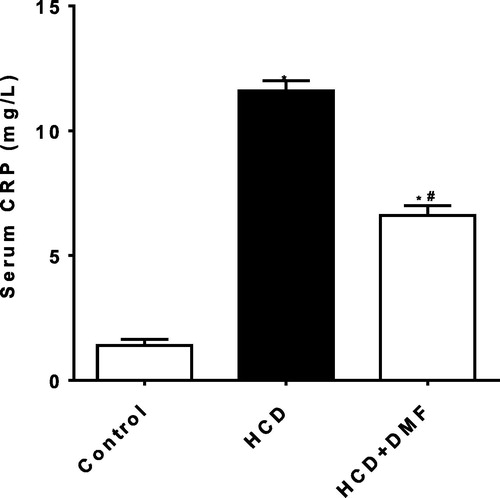
Oxidative stress
Cholesterol feeding resulted in a significant (P < 0.05) increase in aortic MDA level by 321% () while there was a significant reduction in aortic SOD by 52% compared to control group (). DMF treatment significantly (P < 0.05) increased SOD level by 78% compared to the HCD group. However, DMF did not significantly affect MDA compared to HCD.
Fig. 3 Effect of dimethyl fumarate (DMF, 12.5 mg/kg/day) on aortic malondialdehyde (MDA) level in hypercholestrolemic rabbits. Values represent the mean ± SEM of six rabbits per group. Mean values were compared using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (P < 0.05). * significantly different from the mean value of the control group, # significantly different from the mean value of high cholesterol diet (HCD) group.
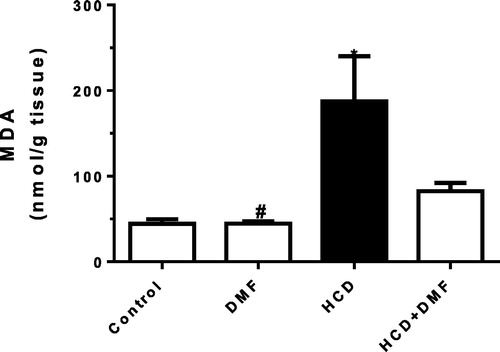
Fig. 4 Effect of dimethyl fumarate (DMF, 12.5 mg/kg/day) on aortic superoxide dismutase (SOD) level in hypercholestrolemic rabbits. Values represent the mean ± SEM of six rabbits per group. Mean values were compared using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (P < 0.05). * significantly different from the mean value of the control group, # significantly different from the mean value of high cholesterol diet (HCD) group.
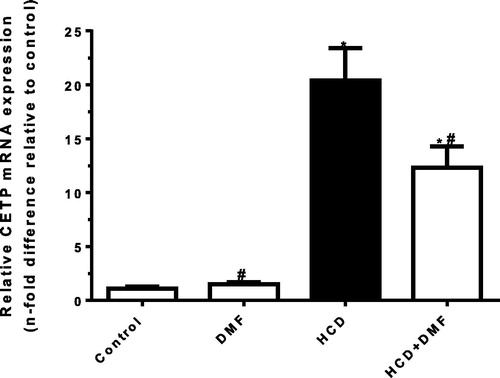
Aortic CETP expression
Chronic cholesterol feeding resulted in a significant (P < 0.05) increase in aortic CETP expression by 17.5 fold in comparison to control group (). DMF treatment significantly (P < 0.05) downregulated CETP expression by 39% compared to HCD group.
Fig. 5 Effect of dimethyl fumarate (DMF, 12.5 mg/kg/day) on aortic cholesteryl ester transfer protein (CETP) expression in high cholesterol diet (HCD)-fed rabbits. Values represent the mean ± SEM of six rabbits per group. Mean values were compared using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (P < 0.05). * significantly different from the mean value of the control group, # significantly different from the mean value of HCD group.
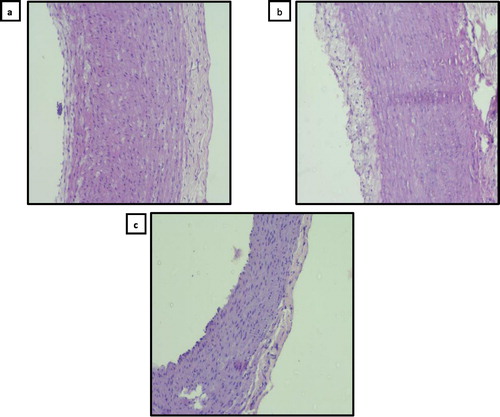
Histopathological analysis of the aorta
In control rabbits, histopathological examination of the aorta using H&E stain showed normal wall of aorta with no fat deposits and intact endothelium (a). There was interrupted endothelium with subendothelial layers of thickened foam cell in HCD group (b). The aorta from DMF-treated rabbits showed normal wall of aorta with intact endothelial lining and marked attenuation of lipid deposition (c).
Fig. 6 Microphotographs of aorta in high cholesterol diet (HCD) fed rabbits. (a) Control: normal aorta with intact endothelium (hematoxylin and eosin, 200×); (b) HCD: interrupted endothelium with subendothelial layers of thickened foam cells (hematoxylin and eosin, 200×); (c) high cholesterol/dimethyl fumarate: intact endothelium with marked reduction in lesion area (hematoxylin and eosin, 200×).
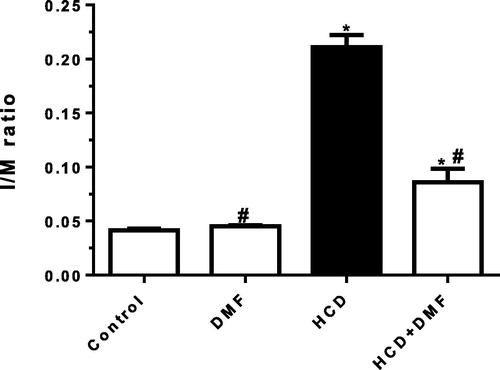
Morphometric analysis of aorta
In control group, the I/M ratios of the thoracic aortas averaged 0.041 ± 0.001. The mean ratio was increased to 0.211 ± 0.011 (P < 0.05) in HCD group. In HCD + DMF group, there was a significant reduction (P < 0.05) in I/M ratio to 0.086 ± 0.012 compared to HCD group ().
Fig. 7 Effect of dimethyl fumarate (DMF, 12.5 mg/kg/day) on intima/media (I/M) ratios of isolated rabbit thoracic aortas in high cholesterol diet (HCD) fed rabbits. Values represent the mean ± SEM of six rabbits per group. Mean values were compared using one-way ANOVA followed by Tukey-Kramer multiple comparisons test (P < 0.05). * significantly different from the mean value of the control group, # significantly different from the mean value of HCD group.
Discussion
The present study aimed to evaluate the beneficial effects of DMF against oxidative stress and inflammation in HCD-fed rabbits. Our findings demonstrate that DMF can decrease oxidative stress and pro-inflammatory proteins as CRP. Possible underlying mechanisms are discussed below.
DMF effects on serum lipid profile of hypercholesterolemic rabbits
The present study revealed that HCD-fed rabbits showed abnormal serum lipid levels with significantly increased TC, LDL-C, TGs and HDL-C levels. These findings are agreed with previous studies [Citation12,Citation22Citation[23]–Citation24] . Our findings indicated that DMF treatment succeeded to significantly decrease TC, TGs and LDL-C when compared to HCD. To our knowledge no previous studies have approached the effect of DMF on hypercholesterolemia. The hypolipidemic effect of DMF may be due to its ability to decrease cholesteryl ester transfer protein (CETP) expression. Circulating CETP lowers the concentration of HDL-C by moving cholesteryl esters from HDLs to VLDLs and LDLs [Citation25,Citation26] . Consequently, CETP levels are positively associated with LDL-C level and negatively associated with HDL-C level [Citation27,Citation28] . In humans and animals, tissue mRNA levels of CETP are increased in response to high-fat and high cholesterol diets [Citation29]. The inhibition of CETP activity reduced atherosclerotic lesions in a rabbit model of atherosclerosis and increased plasma HDL-C concentration [Citation25,Citation30] . DMF has been significally decrease CETP expression in comparison to HCD-fed rabbits. Moreover, the antioxidant activity which mediated via Nrf2 activation may plays a role in hypolipidemic activity of DMF.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is considered as a physiological regulator of ROS generation through expression of numerous genes as hemeoxygenase (HO-1), NADPH dehydrogenase quinone1 (NQO1) and catalase (CAT) through interaction with antioxidant response elements in target gene and protein products of these genes neutralize free radicals.
DMF protects against HCD-induced aortic oxidative stress
HCD-feeding resulted in a significant reduction in aortic SOD level concurrently with increased MDA level. These results support the findings of previous studies, which reported that HCD increased MDA level but decreased the activity of SOD level [Citation31,Citation32] . HCD-feeding causes oxidative stress by increasing the production of reactive oxygen species (ROS), mediated lipoprotein oxidation, particularly LDL particles that cause lipid peroxidation and subsequent influence the development of atherosclerosis [Citation33]. Lipid peroxidation plays an important role in the pathophysiology of atherosclerosis so a decrease in lipid peroxidation leads to a reduction of hypercholesterolemia-induced atherosclerosis [Citation34].
Treatment of rabbits receiving HCD with DMF resulted in a reduction in MDA level. This result was in agreement with previous study, which reported that DMF treated animals showed improvement in the oxidative stress as supported by the lowered levels of MDA in acute pancreatitis in rat model. In addition, there was a significant increase in SOD level [Citation35]. The protective effects of DMF against oxidative stress may be due to its antioxidant effect which could be mediated via activation of Nrf2. Also, DMF has been shown to enhance expression of antioxidant enzyme HO-1 [Citation36]. The upregulation of HO-1 is a likely mechanism, among others, that lead to DMF’s protective effects. Moreover, DMF succeeded to increase HDL-C level which has antioxidant properties [Citation37]. These findings support that DMF treatment could reduce oxidative stress and hence reduce hypercholesterolemia-induced atherosclerosis.
DMF exerts anti-inflammatory effect in hypercholesterolemic rabbits
Hypercholesterolemia enhances the inflammatory response which plays a pivotal role in the development of atherosclerotic changes [Citation38], as it increases circulating monocyte counts and renders these cells more prone for emigration into atherosclerotic lesions [Citation39]. In this study, HCD induced inflammation was manifested by increased serum CRP. This result agreed with previous study, which reported that there is a positive correlation between high cholesterol levels and CRP production [Citation40]. CRP is a pro-inflammatory protein that can be produced by monocytes and smooth muscle cells in atherosclerotic lesions. CRP has been shown to promote atherosclerosis by increasing the expression of adhesion proteins on endothelial cells [Citation41], recruiting monocytes into the arterial wall and increasing the production of inflammatory cytokines by monocytes [Citation42].
In this study, DMF treatment decreased CRP in agreement with previous studies [Citation43,Citation44] . The reduction in CRP level following DMF treatment may be due to antioxidant and anti-inflammatory activity of DMF. Moreover, via activation of Nrf2, inhibition of the nuclear entry of NF-κB in endothelial cells and suppression of T Helper 1 response, DMF has been shown to exert potent anti-inflammatory actions [Citation35].
Effect of DMF on HCD-induced aortic pathologic changes
Chronic cholesterol feeding for 4 weeks resulted in lipid deposition, interrupted endothelium with subendothelial layers of thickened foam cell and increasing in I/M ratios and so promotes the development of atherosclerosis. These results were agreed with previous studies [Citation45,Citation46] .
In comparison to HCD group, DMF significantly attenuated atherosclerotic lesions and reduced the elevations in I/M ratios and. These effects may be due to antioxidant and anti-inflammatory activity of DMF which could be mediated via Nrf2 activation.
Additionally, DMF has been reported to attenuate 7-ketocholesterol (7KC)-induced oxidative stress on 158N murine oligodendrocytes [Citation47]. 7KC, which is formed by cholesterol auto-oxidation, is the main oxidation product of cholesterol found in human atherosclerotic plaque. 7KC could induce atherosclerosis, possibly via reverse sterol transport disruption [Citation48]. It has been reported that 7KC stimulates overproduction of superoxide anions and enhances inflammatory cytokines release [Citation49]. These findings could explain the role of DMF in atherosclerosis.
Conclusion
Our findings suggest that DMF could exert protective effects against inflammation and atherosclerosis in HCD- fed rabbits, possibly by lowering circulating cholesterol levels and reducing vascular oxidative stress and associated inflammation. Further studies are needed to determine the precise mechanism that DMF plays in atherosclerosis.
Conflict of interest
The authors declare that they have no competing interests.
References
- K.RerkasemP.J.GallagherR.F.GrimbleP.C.CalderC.P.ShearmanManaging hypercholesterolemia and its correlation with carotid plaque morphology in patients undergoing carotid endarterectomyVasc Health Risk Manag46200812591264
- A.WarnholtzM.WendtM.AugustT.MunzelClinical aspects of reactive oxygen and nitrogen speciesBiochem Soc Symp712004121133
- I.JialalS.DevarajThe role of oxidized low density lipoprotein in atherogenesisJ Nutr1264 Suppl.19961053S1057S
- T.A.SpringerTraffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigmCell7621994301314
- I.JialalS.DevarajLow-density lipoprotein oxidation, antioxidants, and atherosclerosis: a clinical biochemistry perspectiveClin Chem4241996498506
- R.A.LinkerD.H.LeeS.RyanA.M.van DamR.ConradP.Bistaet al.Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathwayBrain134Pt. 32011678692
- D.WerdenbergR.JoshiS.WolfframH.P.MerkleP.LangguthPresystemic metabolism and intestinal absorption of antipsoriatic fumaric acid estersBiopharm Drug Dispos2462003259273
- J.D.BelcherC.ChenJ.NguyenP.ZhangF.AbdullaP.Nguyenet al.Control of Oxidative Stress and Inflammation in Sickle Cell Disease with the Nrf2 Activator Dimethyl FumarateAntioxid Redox Signal30201610
- R.LoeweW.HolnthonerM.GrogerM.PillingerF.GruberD.Mechtcheriakovaet al.Dimethylfumarate inhibits TNF-induced nuclear entry of NF-kappa B/p65 in human endothelial cellsJ Immunol1689200247814787
- T.J.StoofJ.FlierS.SampatC.NieboerC.P.TensenD.M.BoorsmaThe antipsoriatic drug dimethylfumarate strongly suppresses chemokine production in human keratinocytes and peripheral blood mononuclear cellsBr J Dermatol1446200111141120
- P.SeidelI.MerfortJ.M.HughesB.G.OliverM.TammM.RothDimethylfumarate inhibits NF-{kappa}B function at multiple levels to limit airway smooth muscle cell cytokine secretionAm J Physiol Lung Cell Mol Physiol2972200922
- A.R.El-SheakhH.A.GhoneimG.M.SuddekSM.Ammar elAntioxidant and anti-inflammatory effects of flavocoxid in high-cholesterol-fed rabbitsNaunyn Schmiedebergs Arch Pharmacol38812201513331344
- L.RoblesN.D.VaziriS.LiY.MasudaC.TakasuM.Takasuet al.Dimethyl fumarate protects pancreatic islet cells and non-endocrine tissue in l-arginine-induced chronic pancreatitisPLoS One992014
- G.PagetJ.BarnesToxicity tests. Evaluation of drug activitiesPharmacometrics11964135165
- C.C.AllainL.S.PoonC.S.ChanW.RichmondP.C.FuEnzymatic determination of total serum cholesterolClin Chem2041974470475
- D.S.FredricksonR.I.LevyR.S.LeesFat transport in lipoproteins–an integrated approach to mechanisms and disordersN Engl J Med276119673442
- P.R.FinleyR.B.SchifmanR.J.WilliamsD.A.LichtiCholesterol in high-density lipoprotein: use of Mg2+/dextran sulfate in its enzymic measurementClin Chem2461978931933
- W.T.FriedewaldR.I.LevyD.S.FredricksonEstimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem1861972499502
- H.OhkawaN.OhishiK.YagiAssay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal Biochem9521979351358
- S.MarklundG.MarklundInvolvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutaseEur J Biochem4731974469474
- K.J.LivakT.D.SchmittgenAnalysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) methodMethods2542001402408
- M.S.El-AwadyG.M.SuddekAgmatine ameliorates atherosclerosis progression and endothelial dysfunction in high cholesterol-fed rabbitsJ Pharm Pharmacol6662014835843
- T.HayashiH.Matsui-HiraiA.FukatsuD.SumiH.Kano-HayashiP.J.Raniet al.Selective iNOS inhibitor, ONO1714 successfully retards the development of high-cholesterol diet induced atherosclerosis by novel mechanismAtherosclerosis18722006316324
- S.M.JeonY.B.ParkM.S.ChoiAntihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbitsClin Nutr235200410251034
- A.R.TallPlasma cholesteryl ester transfer proteinJ Lipid Res348199312551274
- P.J.BarterG.J.HopkinsG.D.CalvertTransfers and exchanges of esterified cholesterol between plasma lipoproteinsBiochem J2081198217
- G.J.de GroothJ.A.KuivenhovenA.F.StalenhoefJ.de GraafA.H.ZwindermanJ.L.Posmaet al.Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response studyCirculation10518200221592165
- E.QuinetA.TallR.RamakrishnanL.RudelPlasma lipid transfer protein as a determinant of the atherogenicity of monkey plasma lipoproteinsJ Clin Invest875199115591566
- E.M.QuinetL.B.AgellonP.A.KroonY.L.MarcelY.C.LeeM.E.Whitlocket al.Atherogenic diet increases cholesteryl ester transfer protein messenger RNA levels in rabbit liverJ Clin Invest8521990357363
- M.L.BrownA.InazuC.B.HeslerL.B.AgellonC.MannM.E.Whitlocket al.Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteinsNature34262481989448451
- C.LinJ.RenM.WangX.MaJ.LiuStudy of rongban tongmai granule on anti-oxidant stress in atherosclerosisZhongguo Zhong Yao Za Zhi3622011195199
- G.Del BoccioD.LapennaE.PorrecaA.PennelliF.SaviniP.Felicianiet al.Aortic antioxidant defence mechanisms: time-related changes in cholesterol-fed rabbitsAtherosclerosis8121990127135
- Y.ZhangL.LiJ.YouJ.CaoX.FuEffect of 7-difluoromethyl-5, 4′-dimethoxygenistein on aorta atherosclerosis in hyperlipidemia ApoE(−/−) mice induced by a cholesterol-rich dietDrug Des Devel Ther72013233242
- T.YokozawaA.IshidaE.J.ChoT.NakagawaThe effects of Coptidis Rhizoma extract on a hypercholesterolemic animal modelPhytomedicine10120031722
- L.RoblesN.D.VaziriS.LiC.TakasuY.MasudaK.Voet al.Dimethyl fumarate ameliorates acute pancreatitis in rodentPancreas4432015441447
- J.C.LehmannJ.J.ListopadC.U.RentzschF.H.IgneyA.von BoninH.H.Hennekeset al.Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1J Invest Dermatol12742007835845
- F.E.KuhnE.R.MohlerL.F.SatlerK.ReaganD.Y.LuC.E.RackleyEffects of high-density lipoprotein on acetylcholine-induced coronary vasoreactivityAm J Cardiol6815199114251430
- G.FranciscoC.HernandezR.SimoSerum markers of vascular inflammation in dyslipemiaClin Chim Acta36912006116
- O.SoehnleinM.DrechslerM.HristovC.WeberFunctional alterations of myeloid cell subsets in hyperlipidaemia: relevance for atherosclerosisJ Cell Mol Med1311–12200942934303
- M.M.DuarteJ.B.RochaR.N.MorescoT.DuarteI.B.Da CruzV.L.Loroet al.Association between ischemia-modified albumin, lipids and inflammation biomarkers in patients with hypercholesterolemiaClin Biochem427–82009666671
- V.PasceriJ.S.ChengJ.T.WillersonE.T.YehModulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugsCirculation10321200125312534
- M.J.ThomassenD.P.MeekerS.D.DeodharH.P.WiedemannB.P.BarnaActivation of human monocytes and alveolar macrophages by a synthetic peptide of C-reactive proteinJ Immunother Emphasis Tumor Immunol131199316
- J.SilhavyV.ZidekP.MlejnekV.LandaM.SimakovaH.Strnadet al.Fumaric acid esters can block pro-inflammatory actions of human CRP and ameliorate metabolic disturbances in transgenic spontaneously hypertensive ratsPLoS One972014
- C.TakasuN.D.VaziriS.LiL.RoblesK.VoM.Takasuet al.Treatment with dimethyl fumarate attenuates calcineurin inhibitor-induced nephrotoxicityTransplantation996201511441150
- K.PrasadS.V.ManthaJ.KalraP.LeePrevention of hypercholesterolemic atherosclerosis by garlic, an antixoidantJ Cardiovasc Pharmacol Ther241997309320
- K.PrasadReduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseedCirculation9910199913551362
- A.ZarroukT.NuryE.M.KarymA.VejuxR.SghaierC.Gondcailleet al.Attenuation of 7-ketocholesterol-induced overproduction of reactive oxygen species, apoptosis, and autophagy by dimethyl fumarate on 158 N murine oligodendrocytesJ Steroid Biochem Mol Biol16920172938
- I.C.GelissenA.J.BrownE.L.ManderL.KritharidesR.T.DeanW.JessupSterol efflux is impaired from macrophage foam cells selectively enriched with 7-ketocholesterolJ Biol Chem2713019961785217860
- A.VejuxG.LizardCytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosisMol Aspects Med3032009153170
