?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cancer related morbidity and mortality is a major health care concern. Developing potent anti-cancer therapies which are non-toxic, sustainable and affordable is of alternative medicine. This study was designed to investigate the aqueous natural extracts mixture (NE mix) prepared from common spices turmeric, ginger and garlic for its free radical scavenging potential and anti-cancer property against human breast cancer cell lines (MCF-7, ZR-75 and MDA-MB 231). Qualitative analysis of their bioactive constituents from turmeric, ginger and garlic were done using liquid chromatography-ESI- mass spectrometry (LC-ESI-MS/MS). To the best of our knowledge, NE mix with and without Tamoxifen has not been tested for its anti-cancer potential. We observed that the NE mix induced apoptosis in all the breast cancer cell lines, but it was more prominent in MCF-7 and ZR-75 cell lines in comparison to MDA-MB 231 cell line. The extent of apoptosis due to combined treatment with NE mix-Tamoxifen was higher than Tamoxifen alone, indicating a potential role of the NE mix in sensitizing the ER-positive breast cancer cells towards Tamoxifen. In support to MTT assay, cell cycle analysis, our RT-PCR results also prove that the NE mix 10 μg, Tam 20 μg and combination of NE mix 10 μg-Tam 20 μg altered the expression of apoptotic markers (p53 and Caspase 9) leading to apoptosis in all three cell lines. Our data strongly indicate that our NE mixture is a potential alternative therapeutic approach in certain types of cancer.
1 Introduction
Breast cancer is one of the leading cancers affecting women globally. Incidence of breast cancer is on the rise in countries like India, Japan and other Asian countries. In India, over 50,000 women die of breast cancer every year [Citation1]. Even in western countries, where the rate of incidence is reported to be either stabilized or declining, breast cancer still continues to contribute significantly to cancer-related mortality and morbidity. Adding to existing healthcare concerns of breast cancer, its rising incidence in younger (pre-menopausal) women worldwide [Citation2] and its overall morbidity and mortality underscores a need for developing alternative breast cancer preventive and intervention strategies.
Apoptosis is a conserved and pivotal process of cell death that is required for host defense, suppression of oncogenesis and for normal development and faulty apoptosis is enhances the tumor development and progression. The hallmark of human cancers is evading apoptosis. p53 gene was initially described as an oncogene in 1979, but later it is to known as tumor suppressor gene whose function is to abolish and hinder abnormal cell proliferation, thus preventing neoplastic development [Citation3,Citation4] . It is found in all human cancers that p53 function in controlling the negative growth regulators is lost. Under normal cellular environment, the p53 signalling pathway is always in standby mode in normal cells, but it gets activated in response to cellular stresses, and several autonomous pathways of p53 which are dependent on distinct upstream regulatory kinases. p53’s pivotal role in mammary carcinogenesis has been acquired from enormous information collected from mechanistic, molecular pathological and transgenic animal studies [Citation5].
The in vitro studies on p53 function, and it is found that it continuously suppresses tumorigenesis. Vousden and Lu [Citation6] suggested that p53 might be involved in preventing tumor development especially in humans. The suppression of tumorigenesis is exerted mainly by initiating the apoptosis [Citation7]. Attardi and Jacks [Citation8] demonstrated that loss of p53 activity has accelerated the tumor formation in transgenic mice as a result of apoptosis dysfunction. The integrity and mutation status of p53 decides its qualitative and quantitative activity in different stress-induced signalling pathways. The fact that p53 function is lost due to mutation in breast neoplasia, but its mutation rate is significantly low in other solid tumours [Citation4,Citation9] .
Caspases and numerous upstream regulatory factors execute the cell death process by initiating or directing their proteolytic activity, which could act either as tumor suppressors or oncogenes. These regulatory factors do regulate cell survival or repair signalling pathways often in response to cellular stress, apart from being strong apoptotic inducers [Citation10,Citation11] . Hence, loss of function doesn’t mean that tumor formation is a result of apoptosis dysfunction due to inactivation of caspases. Any injury to outer mitochondrial membranes triggers the release of caspase-9 enzyme found in the mitochondrial intermembrane space into the cytosol together with cytochrome c, hence it interacts with and activates the apoptosis-activating factor (Apaf-1) in a cytochrome c and dATP-regulated manner [Citation12]. Caspase-9 is one of the essential caspases which is required for the initiation of apoptosis signalling on the apoptosome complex through mitochondrial pathway and severe pathophysiological events will arise in case of failure to activate caspase-9 [Citation12]. The activation of caspase 9 and downstream caspase cascade usually occur during mitochondrial disruption [Citation13]. Tumor suppressor proteins and proto-oncogenes with a more direct effect on Caspase activity can be found among p53-transregulated genes harbouring apoptosis-specific functions. Despite an obvious central role of p53 and caspases in the hallmarks of cancer, their status is not yet used for the management of breast cancer [Citation9].
Currently, Tamoxifen is one of the strategies in hormone therapy. Tamoxifen is a selective estrogen receptor modulator (SERM) used for in treatment of breast cancers. However, Tamoxifen-based prevention strategy is limited to a selective group of women who are Estrogen Receptor (ER) positive. Moreover, prolonged use of the drug predisposes women to ovarian [Citation14] uterine and endometrial cancers [Citation15] apart from causing other serious complications such as retinal vein occlusion [Citation16] deep vein thrombosis, pulmonary embolism [Citation17], stroke [Citation18] and cataracts [Citation19], highlighting an urgent need for developing safer scalable approaches. Strategies to lower the required dose of anti-cancer drugs (sensitization) are useful. Identifying the anti-cancer properties of commonly used dietary products and other household herbs [Citation20,Citation21] may offer probable solutions for prevention. Natural anticancer agents exert significantly lower toxicity, safe and easily available; hence a combinational therapy using natural anticancer drug along with commercially available anticancer drugs should be encouraged in order to reduce the limitations in controlling the metastatic cancers [Citation22]. Such a notion is supported by the observed beneficial impact of natural compounds with anti-cancer treatment modalities [Citation23Citation[24]–Citation25] .
A multitude of studies have shown beneficial effects of phyto and marine extracts for intervention and treatment in cancer [Citation26Citation[27]Citation[28]–Citation29] . In view of the above, it may be rationalized that exploiting anti-cancer potential of commonly used food ingredients and easily accessible herbs may be useful in controlling the cancers. Thus in the present study, we have investigated the potential effect of conventional hormone therapy drug i.e. tamoxifen when supplemented with natural extracts mixture made from commonly used dietary spices in inducing cell death and sensitization of immortalized cells i.e. breast cancer cells which are ER-positive (MCF-7 and ZR-75) and ER-negative (MDA-MB 231).
2 Materials and methods
2.1 Materials
Human breast cancer cell lines, MCF-7, ZR-75 and melanoma cell line MDA-MB 231 were obtained from a National cell line repository (National Centre for Cell Science, Pune, India). Hi-Gluta XL™ Dulbecco’s Modified Eagle’s Medium (High Glucose) cell culture medium, Hi-Gluta XL™ RPMI-1640 cell culture medium, L-Glutamine-Penicillin-Streptomycin solution, Dulbecco’s Phosphate buffered saline (DPBS), 0.22 μm sterile syringe driven filters, sterile cell scrapers, 0.25% Trypsin-EDTA solution, Bovine serum albumin (BSA) were obtained from Hi-Media, India. Fetal bovine serum was obtained from Seralab, USA. Sterile cell culture plastic ware was purchased from Thermo Fisher, USA. Flow-cytometer BD FACS caliber Apoptosis Kit – Annexin V Alexa Fluor 488 and propidium iodide (Thermo fishers) and Ultrapure water was generated using Millipore RiOs-DI®3 system.
2.2 Chemicals
Acetonitrile ULC/MS Grade purchased from Biosolve Chimie SARL (Dieuze, France) and formic acid (Optima LC/MS grade) was purchased from Fisher Scientific (Geel, Beljium, Germany). Methanol (LiChrosolv) was purchased from Merck (Darmstadt, Germany). Deionized water was prepared by passing distilled water through a Milli-Q water purification system (Millipore, Milford, MA, USA).
2.3 Methods
2.3.1 Preparation of natural extracts (NE) and tamoxifen solution
Turmeric, ginger and garlic were brought from local markets and the natural extracts mixture was prepared in-house by adding 20 g of each i.e. turmeric, garlic and ginger paste into 500 ml of ultrapure water and heating it at 60 °C for 6 h. Later the mixture was shaken overnight at room temperature followed by centrifugation at 4 °C for 10 min. The supernatant was then separated and filter-sterilized using a 0.22 µm syringe filters and lyophilized.
Tamoxifen stock solution was prepared by dissolving 10 mg of Tamoxifen into 500 µl of absolute ethanol and then adding 4.5 ml of ultrapure water. The solution was then filter-sterilized using a 0.22 µm syringe filter. The required dosages were prepared from diluting this stock solution.
2.4 Antioxidant assays
2.4.1 Superoxide anion scavenging assay
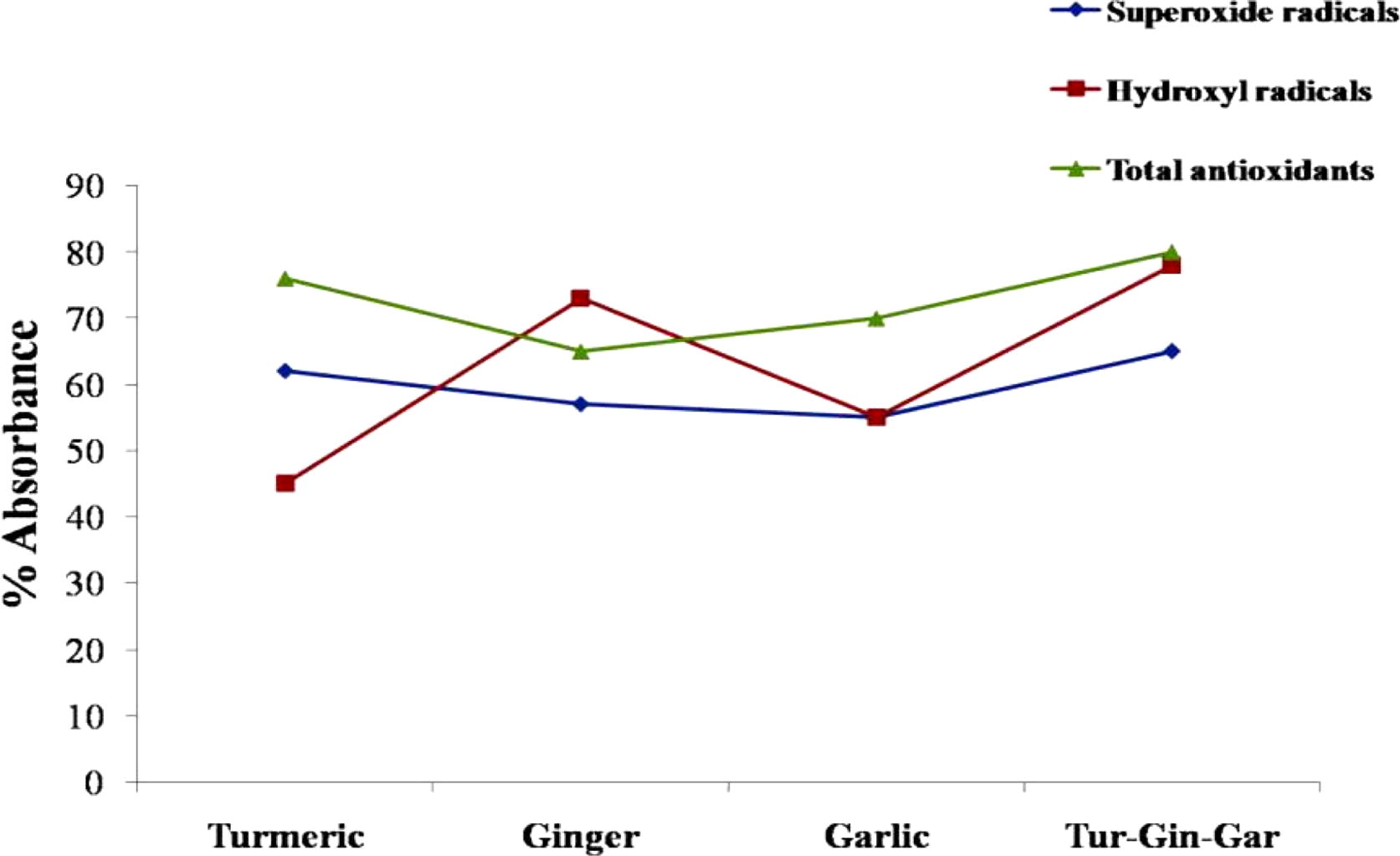
The assay for superoxide anion radical scavenging activity was supported by riboflavin-light-NBT system [Citation30]. Briefly, 1 ml of sample was taken at different concentrations (25–500 μg/ml) and mixed with 0.5 ml of phosphate buffer (50 mM, pH 7.6), 0.3 ml riboflavin (50 mM), 0.25 ml PMS (20 mM), and 0.1 ml NBT (0.5 mM). Reaction was started by illuminating the reaction mixture using a fluorescent lamp. After 20 min of incubation, the absorbance was measured at 560 nm. Ascorbic acid was used as standard. The scavenging ability of the plant extract was determined by the following equation:(1)
(1)
2.4.2 Phosphomolybdate assay (total anti-oxidant capacity)
The total anti-oxidant capacity of the fractions was determined by Phosphomolybdate method using ascorbic acid as a standard [Citation31]. An aliquot of 0.1 ml of sample solution was mixed with 1 ml of reagent solution (0.6 M Sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and incubated in a water bath at 95 °C for 90 min. After the samples had cooled to room temperature; the absorbance of the mixture was measured at 765 nm against a blank. A typical blank contained 1 ml of the reagent solution and the appropriate volume of the solvent and incubated under the same conditions. Ascorbic acid was used as standard. The anti-oxidant capacity was estimated using following formula:(2)
(2)
2.4.3 Hydroxyl radical scavenging assay
Hydroxyl radical scavenging activity was measured by the ability of the different fractions of plant extracts to scavenge the hydroxyl radicals generated by the Fe3+-ascorbate-EDTA-H2O2 system (Fenton reaction) [Citation32]. The reaction mixture contained; 500 μl of 2-deoxyribose (2.8 mM) in phosphate buffer (50 mM, pH 7.4), 200 μl of premixed ferric chloride (100 mM) and EDTA (100 mM) solution (1:1; v/v), 100 μl of H2O2 (200 mM) with or without the extract solution (100 μl). The reaction was triggered by adding 100 μl of 300 mM ascorbate and incubated for 1 h at 37 °C. 0.5 ml of the reaction mixture was added to 1 ml of TCA (2.8%; w/v; aqueous solution), then 1 ml of 1% aqueous TBA were added to the reaction mixture. The mixture was heated for 15 min on a boiling water bath. After the mixture being cooled the absorbance at 532 nm was noted against a blank (the same solution but without reagent). The scavenging activity on hydroxyl radical was calculated as follows:(3)
(3)
2.4.4 Cell culture
Human breast adenocarcinoma MCF-7 cells and MDA-MB 231 melanoma cells were aseptically cultured in T-25 cell culture flasks or multi-well cell culture plates in Hi-Gluta XL™ Dulbecco’s Modified Eagle’s Medium, (High Glucose) supplemented with 10% Fetal bovine serum (FBS) (Hi-Media or Seralab), 20 mM L-Glutamine, Streptomycin (100 μg/ml) and Penicillin (100 Units/ml). Whereas, breast ductal carcinoma ZR-75 cells were aseptically cultured in HiGluta XL™ RPMI-1640 medium supplemented with 10% FBS Streptomycin (100 μg/ml) and Penicillin (100 Units/ml). The cells were grown in a cell culture incubator (HEPA Class 100-Steri cycle CO2 incubator), supplied with 5% CO2 and maintained at 37 °C.
2.4.5 Treatment of the cells with natural extracts and Tamoxifen
Cells at 60% confluency were serum starved for 24 h and the serum-free media was replaced with appropriate media containing 10% FBS. Various amounts of natural extracts mix (5, 10 and 20 µg/mL) and Tamoxifen (Tam 20 µg/mL) were added to the cells and total volume of each well was made up to 2 ml, using corresponding media. Control cells were only treated with respective vehicle solutions without the agents. Experiments were carried out in triplicate and the experiments were repeated twice. After 42 h of treatment, cells along with the medium were collected into 15 ml polypropylene tubes. The tubes were spin down at 1500 rpm for 5 min at room temperature.
2.4.6 MTT assay/cytotoxic assay
The MTT assay was used for measuring toxicity as previously described [Citation30,Citation31] . Briefly, cells were seeded in 96-well plates overnight. After 42 h of incubation with crude extracts, the cells were rinsed with 1X PBS and incubated with 100 μL of 0.5 mg/mL MTT at 37 °C. After 30 min of incubation, the dark blue crystals of formazan (MTT metabolites) were dissolved with 100 μL of DMSO and incubated at 37 °C for 30 min. The level of reduced MTT was determined by measuring the difference in absorbance at 570 and 650 nm using a microplate reader (Spectra Max M5, Molecular Devices). According to the U.S. NCI plant screening program, a crude extract is generally considered to have in vitro cytotoxic activity with an IC50 value ≤20 μg/mL [Citation33,Citation34] .
2.4.7 Cell cycle analysis
Cells were serum starved for 24 h as above, treated with different extracts and/or Tamoxifen for 42 h, trypsinized and fixed in 70% ethanol overnight. Fixed cells were then treated with a mixture of Propidium Iodide, RNAseA and Triton-X-100 and analyzed for DNA content in different phases of the cell cycle in a TALI-Image based cytometer (Thermo Fisher).
2.4.8 Quantification of apoptosis and cell death
Apoptosis and cell death were determined as described below, using Annexin V Alexa Fluor® 488 conjugate (Apoptosis kit) and propidium iodide (Cell death assay kit), respectively. The treated cells were centrifuged at 1500 rpm for 5 min and the formed pellet was re-suspended in 200 μl of Annexin binding buffer and 10 μl of Annexin-V Alexa Fluor® 488, and 5 μl of propidium iodide (PI) was added to approximately 5 × 105–5 × 106 cells/ml and mixed well, later the mixture was incubated at room temperature in dark for 20 min. After the incubation cell suspension was analysed by TALI-Image based cytometer (Thermo Fisher). The number and percentage of apoptotic and dead cells were assessed by Annexin-V and PI staining, respectively. For the fluorescent imaging of dead and apoptotic cells was done using EVOS Digital inverted fluorescence microscope (Invitrogen) with a 10X LPlanFL PH fluorescence objective was used to image the labelled cells in the plate.
2.4.9 Reverse transcriptase-PCR (RT-PCR) quantitative analysis
Expression of p53 and Caspase-9 genes was performed by a semiquantitative Reverse transcriptase-PCR technique. Total RNA was extracted from immortal cell lines i.e. MCF-7, ZR-75 and MDA MB 231 using the RNASure mini isolation kit (cat no: #NP-84105, Nucleo-pore, Genetix Biotech Asia Pvt Ltd, New Delhi), as per the instructions given by the manufactures. RNA (2 µg) was then converted to cDNA using Verso cDNA synthesis kit (#AB-1453/A, Thermo scientific) at 42 °C for 30 min, and 95 °C for 2 min. PCR amplification was performed on the cDNA solution with EmeraldAmp® GT PCR Master Mix (Cat# RR310A, Takara) at a final volume of 10 µL. The PCR conditions and primers sequence were as follows: for p53 mRNA amplification: Forward primer- 5_AGTCTAGAGCCACCGTCCA, Reverse: AGGTCTGAAAATGTTTCCTGACTCA-3 _, generating a 270-bp PCR product. For Caspase-9 mRNA amplification: Forward: 5_-CTCAGACCAGAGATTCGCAAAC, Reverse: GCATTTCCCCTCAAACTCTCAA-3_, generating a 118-bp PCR product. GAPDH primers sequence: Forward primer: 5_-GGCTCTCCAGAACATCATCCCTGC, Reverse: GGGTGTCGCTGTTGAAGTCAGAGG -3_, is generating a 250-bp PCR product. PCR program for p53: 95 °C for 5 min, 95 for 30 s, 62 °C for 30 s, 72 °C for 30 s for 40 cycles and final extension step at 72 °C for 7 min. PCR program for Cas9: 95 °C for 5 min, 95 for 30 s, 61 °C for 30 s, 72 °C for 30 s for 40 cycles and final extension step at 72 °C for 7 min. PCR program for GAPDH: 95 °C for 5 min, 95 for 30 s, 66 °C for 30 s, 72 °C for 30 s for 40 cycles and final extension step at 72 °C for 7 min. RT-PCR products were separated on 1.5% agarose (Lonza).
2.5 Mass spectrometry
2.5.1 Sample preparation
Lyophilized extracts (10mg/1 ml) of turmeric, ginger and garlic were dissolved in methanol (HPLC grade) and filtered using 0.45 µm PTSC filter.
2.5.2 LC conditions
The chromatographic analysis was performed on a Shimadzu Nexera X2 UPLC system (Shimadzu Corporation, Nakagyo, Kyoto, Japan) equipped with a Quaternary pump, an online degasser (DGU-20A 5R), a thermostatic auto sampler (SIL-30AC), a thermostatically controlled column compartment (CTO-20AC), and a diode array detector (DAD) (SPD-M20A) which were communicated through Bus module (CBM-20A). Chromatographic separation was performed on a reverse-phase column Eclipse XDB C18 column (4.6 × 150 mm, 4.6 μm, Agilent Technologies) maintained at ambient temperature. The mobile phases consisted of water containing 0.1% formic acid (A) and acetonitrile (B), and the elution was set at Isocratic mode as follows: (40%A: 60%B. The flow rate was set at 1.0 mL/min, and the injection volume was 10 μL [Citation35].
2.5.3 Data processing
For the LC-ESI-MS experiments, Shimadzu Triple Quadruple mass spectrometer (LCMS 8040) (Shimadzu Corporation, Kyoto, Japan) equipped with an electrospray ionization (ESI) source was connected to the UPLC instrument. The ESI source parameters were set as follows: ion spray voltage, 4.5 kV; DL temperature, 250 °C; Heat Block Temperature, 400 °C; and Nebulising (N2) and Dry gas (N2) flow rates, 3.0 and 10 L/min, respectively. The mass analyzer was operated in positive ion mode, with a mass range of 50–900. Similar to the technique described for ESI-MS. Accurate masses were calibrated according to the manufacturer’s guidelines using TQ- Standard Sample (PEG, PPG and Raffinose) (Shimadzu corporation, Kyoto, Japan). The data were recorded and processed using the Lab Solutions 5.60 SP2 software (Shimadzu Corporation, Kyoto, Japan).
2.5.4 Statistical analysis
Statistical analysis of the data was performed using two way ANOVA and followed by student T-test. Difference between the values was considered significant, if ∗p < 0.05.
3 Results
The anti-cancer potential of the aqueous natural extracts was assessed by treating different ER- positive and negative breast cancer cell lines (MCF-7, ZR-75 and MDA-MB-231) with the extracts alone and in combination with Tamoxifen, for 42 h (–). We noted detachment of cells from the substratum, denoting events associated with cell death. The impact was more prominent in MCF-7 cells, followed by ZR-75 and MDA-MB 231 cells.
3.1 Free radical scavenging capacity of natural extracts
On comparison of the free radical scavenging and total antioxidant capacity of different aqueous extracts (), it is evident that the aqueous extract of the combination seems to be more potent than the individual extracts, from its ability to neutralize oxygen radicals. The total antioxidant and free superoxide neutralizing capacity is similar between turmeric and the extract mixture, but those of ginger and garlic extracts are relatively less compared to the mixture. The combination of extracts clearly shows better capacity to neutralize hydroxyl radicals compared to turmeric and garlic extracts. The ginger extract showed high free hydroxyl radical scavenging capability in comparison to turmeric and garlic individually, whereas the turmeric extract has slightly better superoxide neutralizing capacity than other extracts individually.
3.2 Cell Proliferation/Viability Assay- MTT assay
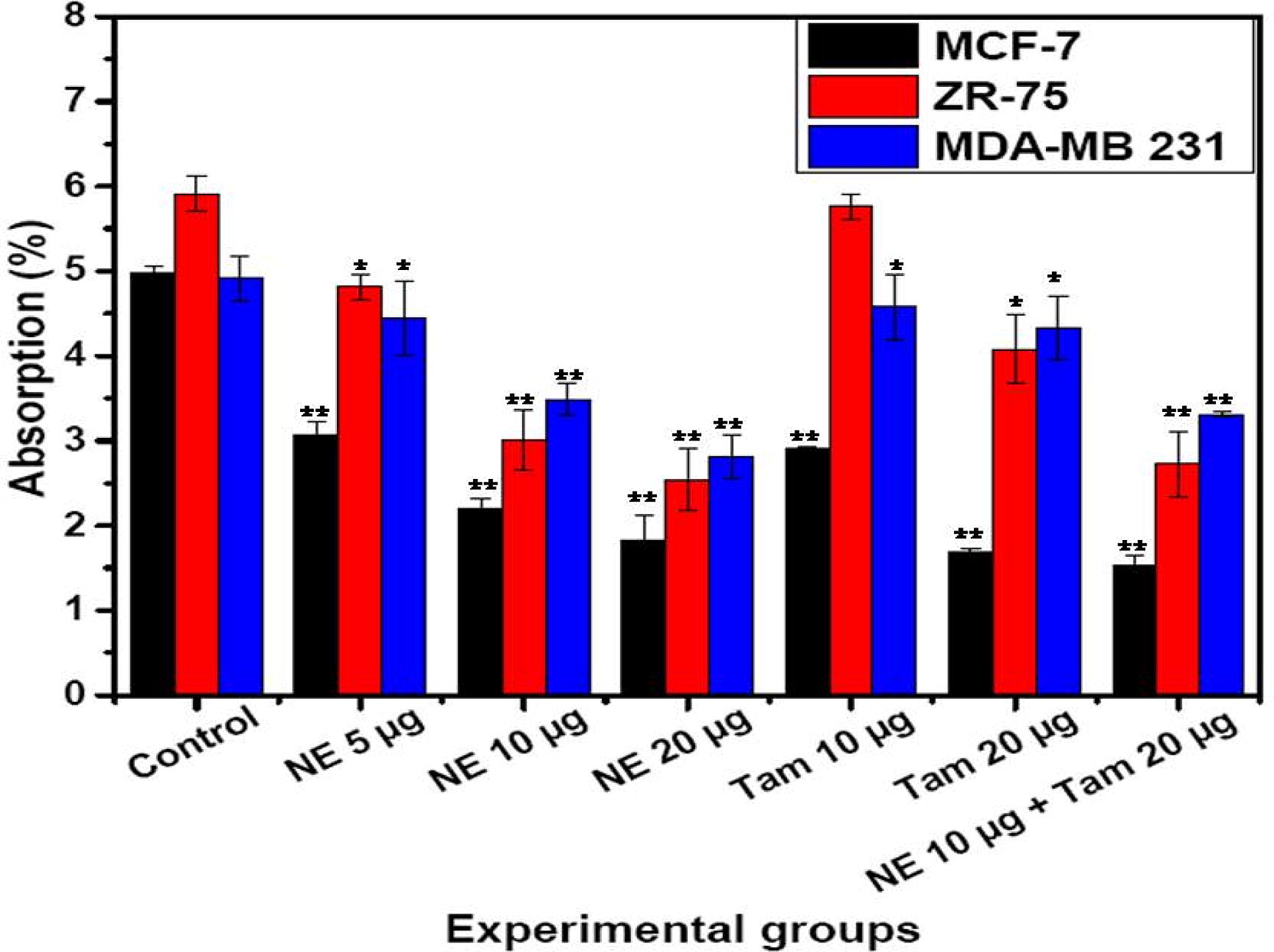
Cells were plated at a density of 1 × 104 cells/well in 96-well plate, incubated at 37 °C overnight and treated in triplicate with increasing concentrations of NE mix or a combination of NE and Tam. After 42 h of treatment, a microtiter plate reader was used to record absorbance at 570 nm, based on the ability of viable cells to convert the yellow tetrazolium MTT (3-(4, dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide).
The NE mix (10 µg/mL) with or without Tamoxifen (20 µg/mL) has the ability to control the cell proliferation in comparison to the individual treatments in ER +ve and ER −ve cell lines. The control and NE mix (5, 10 and 20 µg/mL) treated samples did not show any signs of cytotoxicity. MDA MB 231 which is a triple negative cells as shown susceptibility to towards Tamoxifen upon combination treatment with NE mix in dose dependent manner. The results were expressed as percentage of absorbance ().
Fig. 2 Cell proliferation in breast cancer cells with NE mix and Tamoxifen treatment. Number of proliferating cells (Average ± SE) is shown as assessed by MTT (3-(4, dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) colour formation following the treatment with different concentrations of NE extracts mix and Tamoxifen (Tam). Two way ANOVA followed by student T-test was used for statistical analysis and significance between the groups and the difference was considered significant for P values <0.05 (*P < 0.05, **P < 0.005).
3.3 Cell cycle analysis
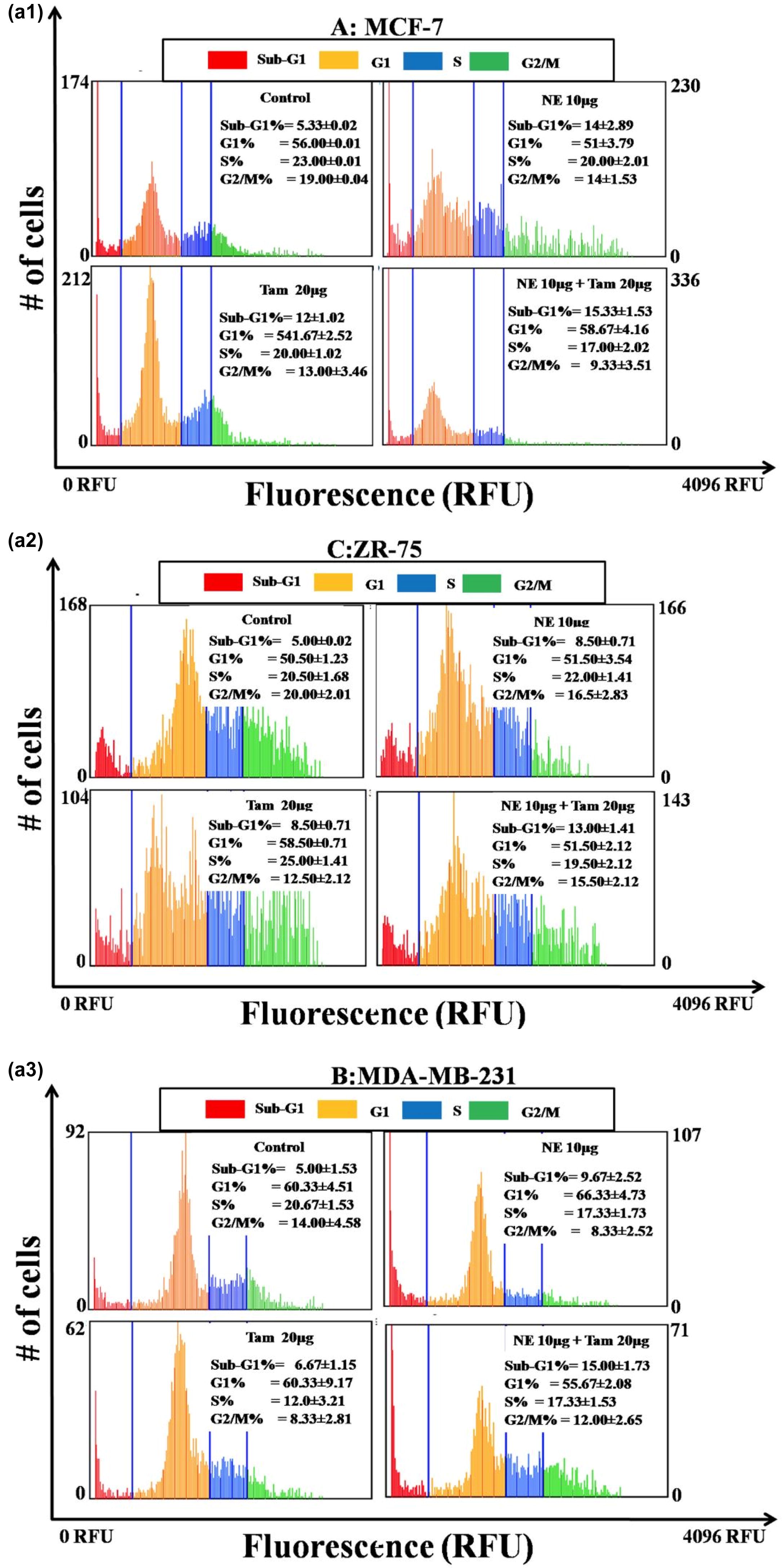
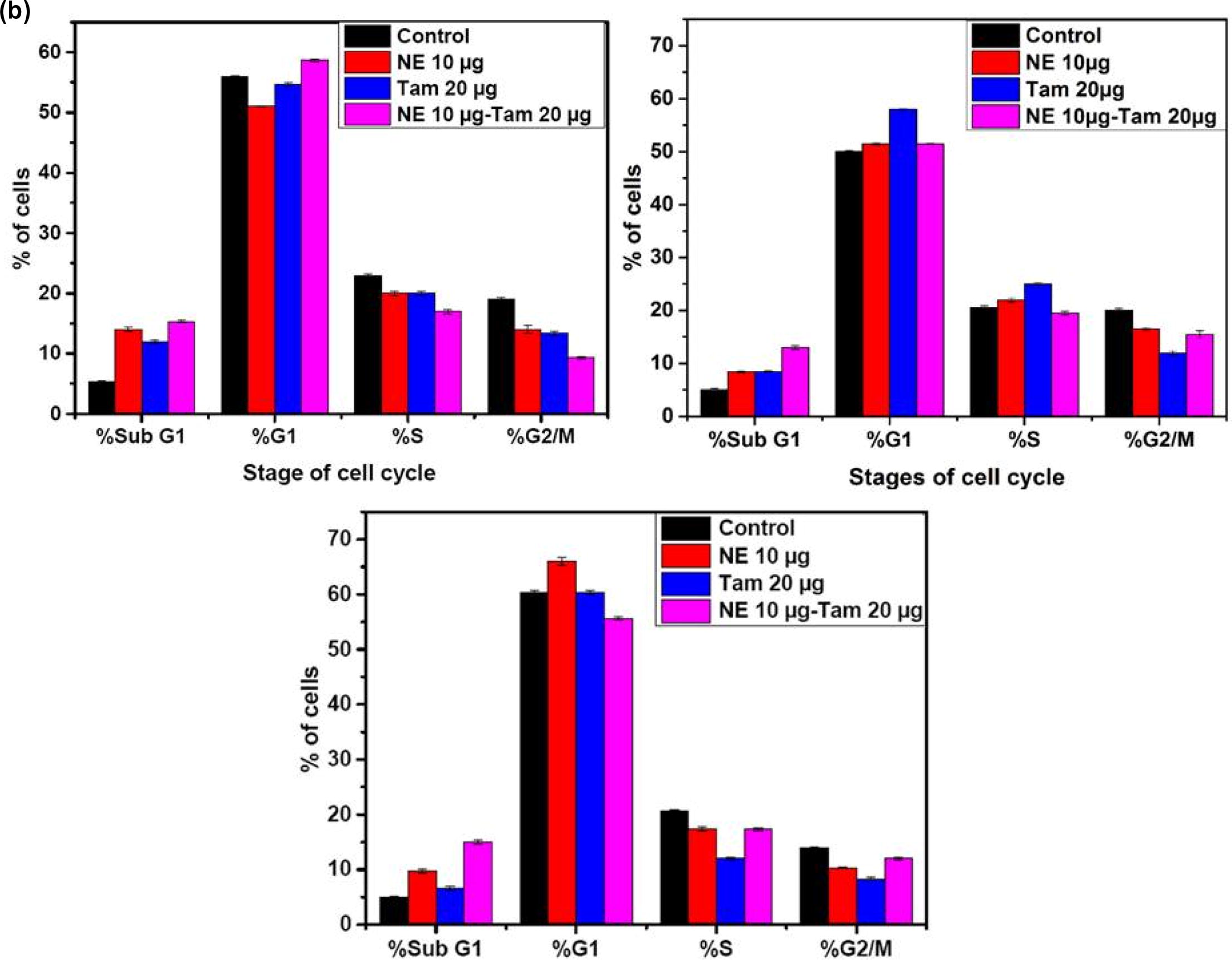
The cell cycle analysis reveals the efficiency of natural extract mixture with and without Tamoxifen. The a and b, represents the percentage of cells being arrested in different stages of cell cycle when treated with 10 µg/mL concentration of natural extract mixture (NE mix 10 µg), Tamoxifen 20 µg/mL and combination of NE mix 10 µg – Tamoxifen 20 µg/mL. The percentage of cells being arrested in G1 phase is significantly high in all the immortal cell lines when treated with NE mix with and without Tamoxifen. The efficiency of NE mix has increased the potency of Tamoxifen when the cells were treated simultaneously. The MDA MB 231 cell lines showed less susceptibility towards NE mix, Tamoxifen alone but it’s more susceptible to combinational treatment. The MCF-7 and ZR-75 cell lines showed more susceptibility towards NE mix, Tamoxifen alone and combinational treatment in comparison to MDA MB 231 cell lines.
Fig. 3 a (1, 2, 3) and b: Panels A, B, C represent the cell cycle histograms of MCF-7, ZR-75 and MDA-MB-231 cells respectively after treatment with different extracts. Medium control is the vehicle for NE mix and Ethanol control is the vehicle for Tamoxifen. The blue coloured gatings in the histogram segregate the Sub-G1, G1, S and G2/M phases of the cell cycle. Percentages of cells in each phase of the cell cycle are indicated inside the individual histogram representing different treatments. The X-axis of each histogram represents the propidium iodide (PI) fluorescence based on the DNA content in each phase of the cell cycle. The Y-axis of each histogram represents the number of cells in each phase.
3.4 Apoptosis (Annexin V Staining)
As determined by the Annexin-V binding, Natural extract (NE) mix with or without Tamoxifen has the ability to induce apoptosis in all the three cell lines, though the degree of the impact varied with the treatment and the cell line (). NE mix induced apoptosis in MCF-7 cells and ZR-75 cells in a dose-dependent manner (NE mix 5–20 µg) but the effect was not similar with MDA-MB 231 cells as apoptosis was minimal and was not found to be dose-dependent. The effect of NE mix was more prominent in MCF-7 and followed by ZR-75 cells at a dose of 20 µg. The cells underwent apoptosis on Tamoxifen treatment alone, but when cells were treated with NE mix and Tamoxifen together, the extent of apoptosis was clearly higher except in the case of ZR-75 cells. The total number of cells undergoing apoptosis in MCF-7 and ZR-75 cells was more compared to MDA-MB 231 cells.
Fig. 4 Apoptotic cell death in breast cancer cells with NE mix and Tamoxifen treatment. Percentage of apoptotic cells (Average ± SE) as assessed by Annexin-V-Alexa Fluor 488 binding, following the treatment with different concentrations of extracts NE mix and Tamoxifen (Tam). Two way ANOVA and student’s T-test were used to test for statistical significance between the groups and the difference was considered significant for p values <0.05*.
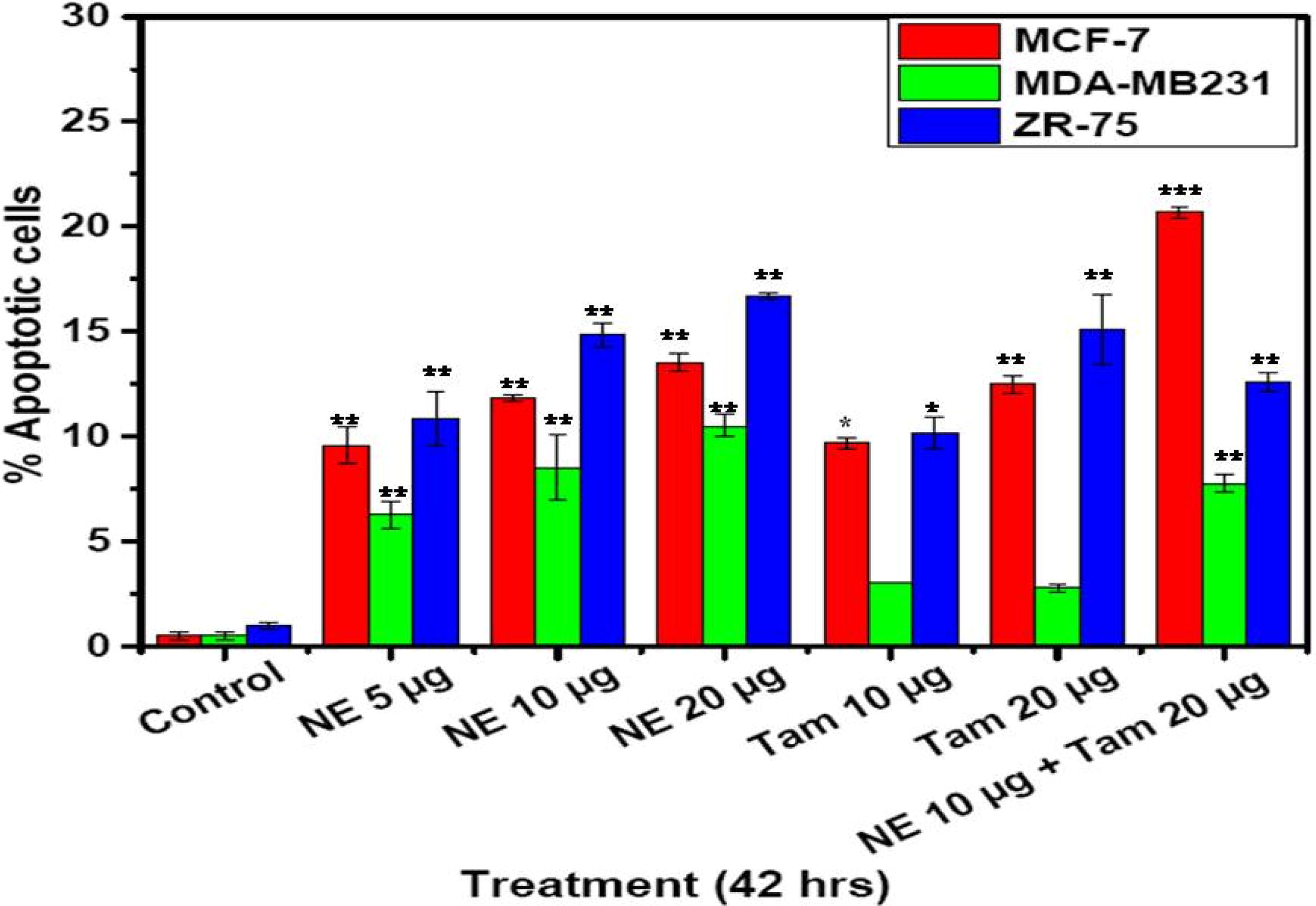
Fig. 5 Apoptosis in breast cancer and melanoma cells treated with NE mix and Tamoxifen. Percentage of PI-positive (Average ± SE) in cells after treatment with different concentrations of extracts NE mix, and Tam. One way ANOVA was used to test for statistical significance between the groups and the difference was considered significant for P values <0.05 (*P < 0.05, **P < 0.005, ***P < 0.001).
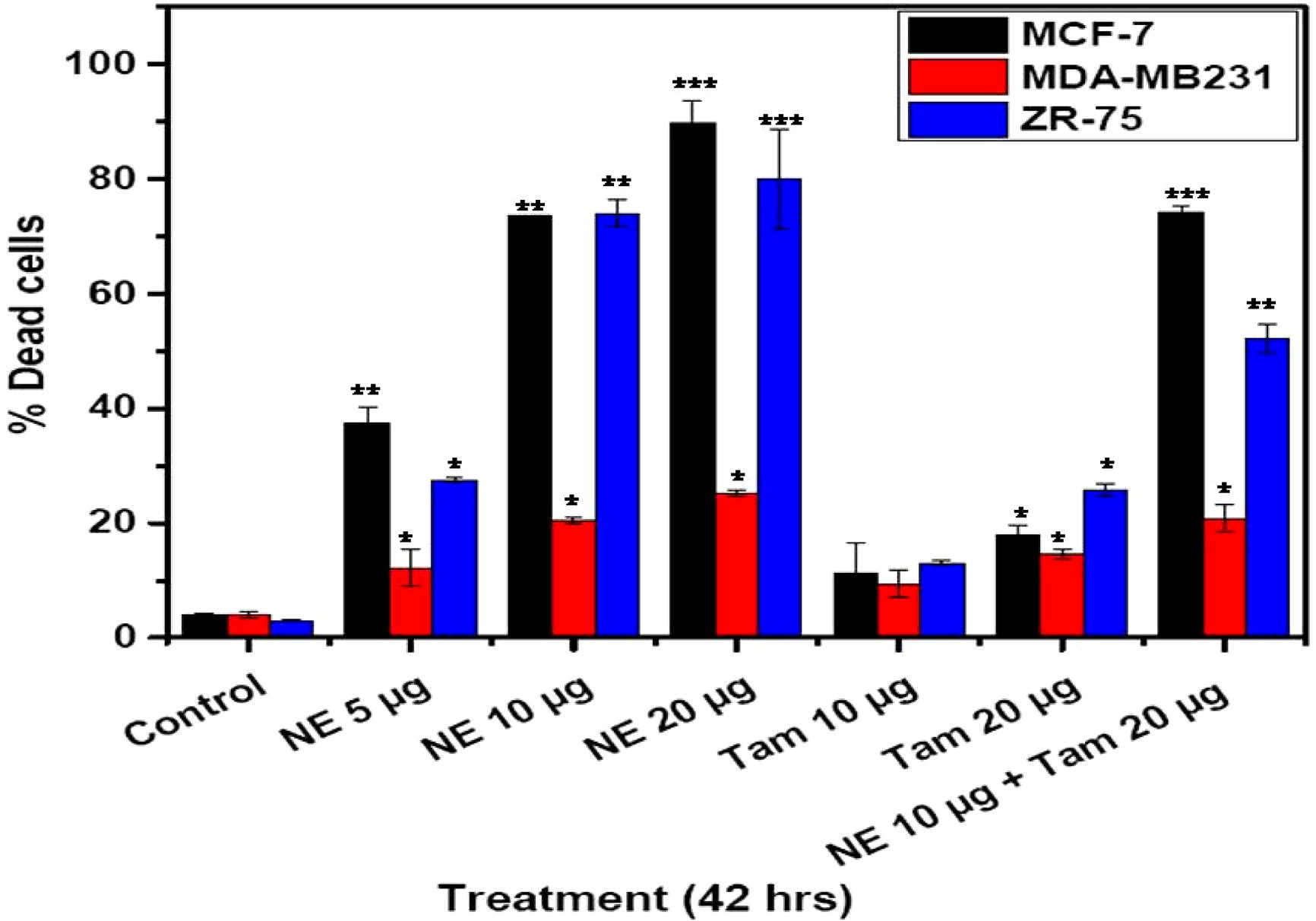
3.5 Cell death (propidium iodide staining)
The data presented in shows an additional measure of apoptosis induced by NE mix, Tamoxifen and NE-Tam in MCF-7, ZR-75 and MDA-MB 231 cell lines at varying concentrations, determined by propidium iodide staining. As shown in , natural extract-induced apoptosis in MCF-7 and ZR-75 cells was dose-dependent. The NE mix concentrations were increased from 5 to 20 µg. Cell death was more intense in MCF-7 cells and ZR-75 cells when treated with NE mix and this effect was dose dependent (5–20 µg). However, such a dose dependent increase in cell death was not found in MDA-MB 231 cells. Cell death induced by Tam was lesser across the cell lines when compared to the effect of NE-Tam combination. However, combined treatment of the cells with NE and Tam showed a higher effect in MCF-7 cells and ZR 75.
3.6 Fluorescence microscopy
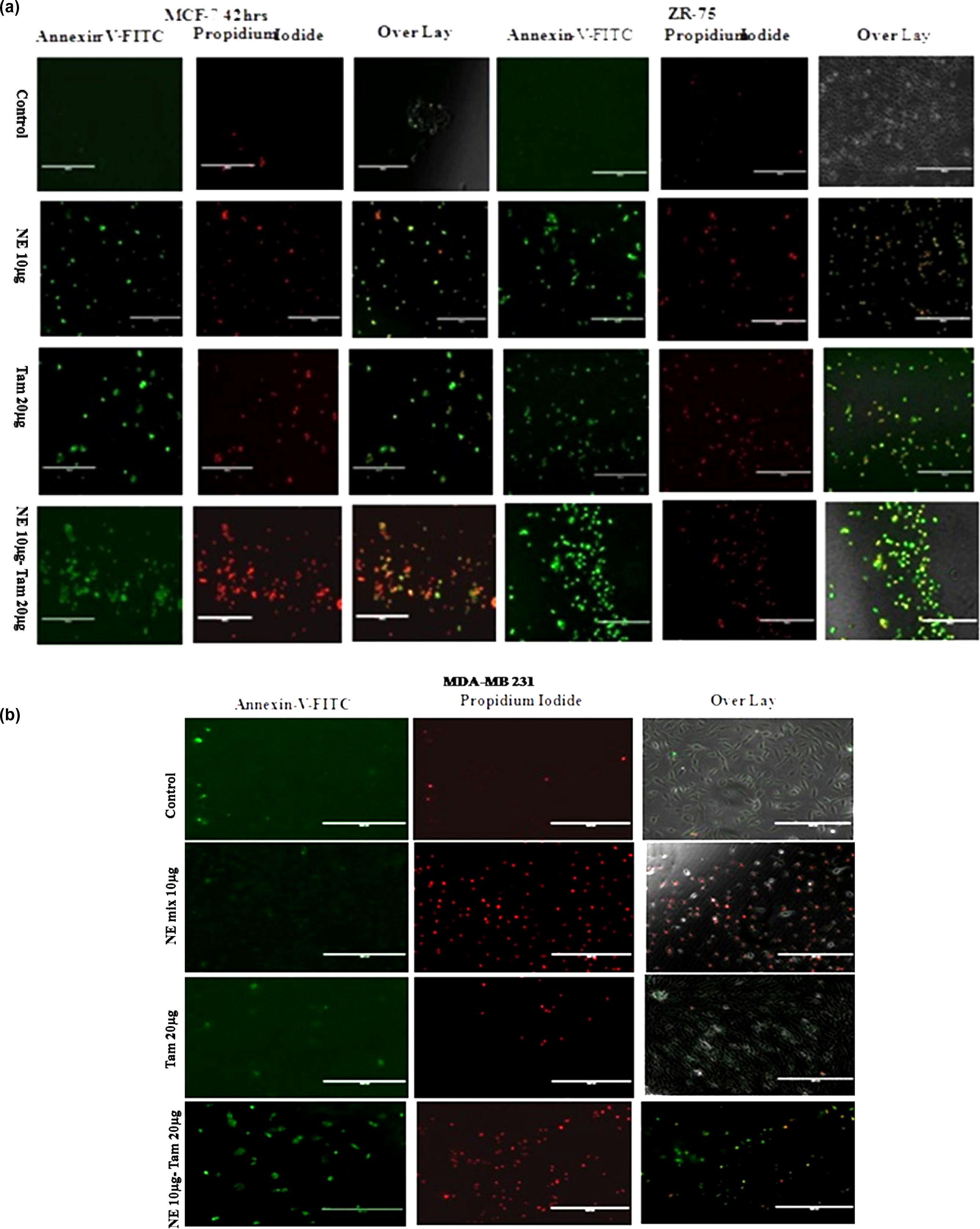
To investigate the mechanism by which NE mix and tamoxifen induces apoptosis in MCF-7, ZR-75 and MDA-MB 231 cells, the cells were stained using Annexin V Alexa Fluor® 488 conjugate (Apoptosis kit) and propidium iodide (Cell death assay kit) (). Alexa Fluor staining of NE mix-treated MCF-7, ZR-75 and MDA-MB 231 cells (NE 10 µg for 42 h) and Tamoxifen (20 µg for 42 h) revealed nuclear fragmentation and chromatin condensation, typical characteristics of apoptosis. The cells undergoing apoptosis was confirmed by staining with propidium iodide (a membrane impermeable dye) and Annexin V Alexa Fluor® 488 which has high affinity towards the phospholipid phosphatidylserine. Phosphatidylserine is normally present in the inner side of the cellular membrane, and is translocated to the outer membrane leaflet in apoptotic cells. The cell lines exposed to NE mix and Tam, stained positive for Annexin V and propidium iodide (a and b), a pattern reflecting membrane disruption that is characteristic of later stages of apoptosis. Taken together, these data indicate that NE mix and Tam-induced cell death is at least in part the result of apoptosis.
Fig. 6 a and b: For fluorescent imaging of apoptotic cells, cells were labelled with 5 µl of Annexin-V-Alexa Fluor 488 and 1 µl of propidium iodide and incubated for 20 min at 37 °C. EVOS Digital inverted fluorescence microscope (Invitrogen) with a 10X LPlanFL PH fluorescence objective was used to image the labelled cells in the plate.
3.7 RT-PCR quantification studies – gene expression of apoptotic markers
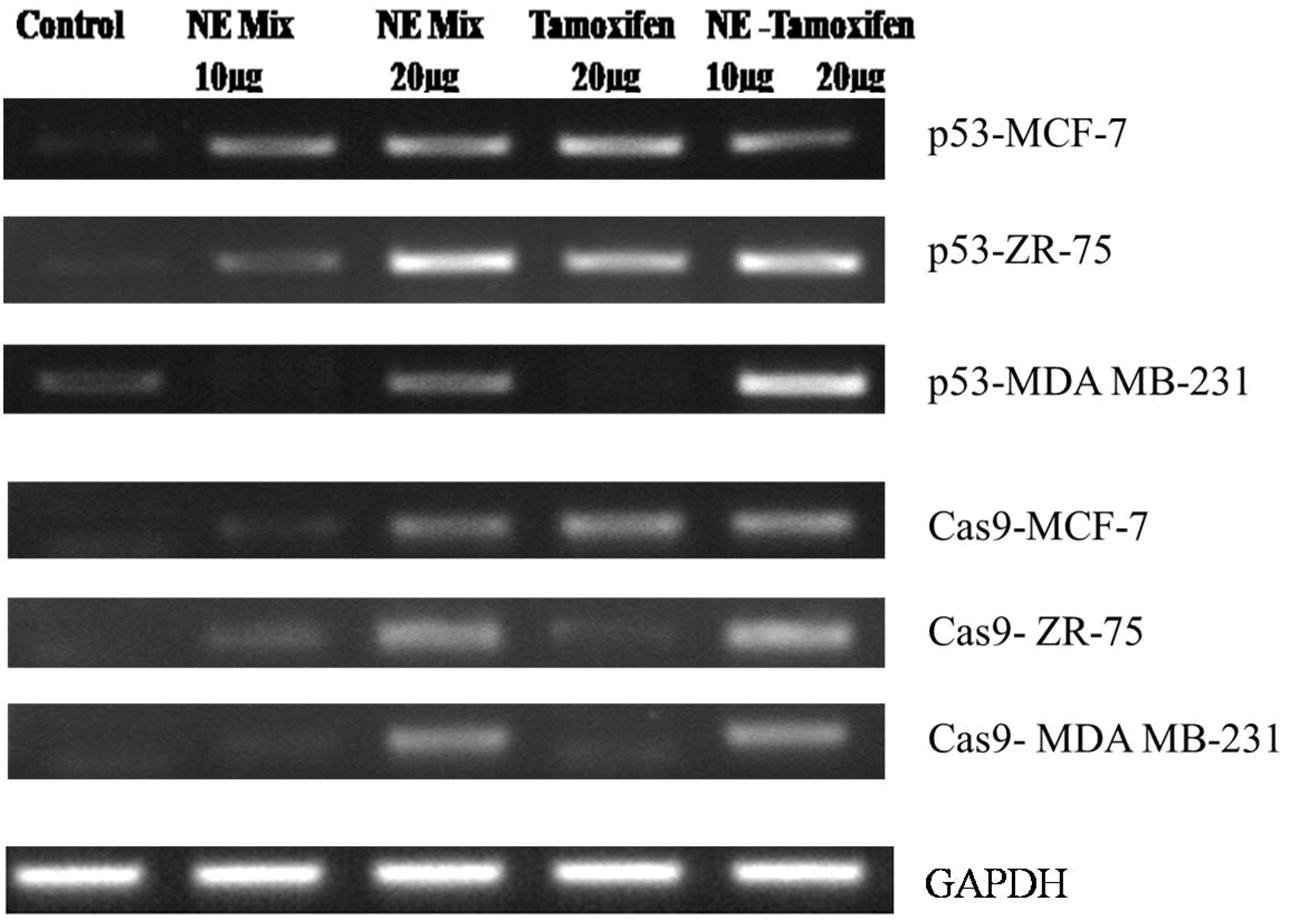
The gene expression studies of p53 and Cas9 genes in immortal cell lines i.e. MCF-7 and MDAMB-231 and ZR-75 cells when treated with NE mix (10 and 20 µg), Tamoxifen 20 µg and NE mix 10 µg – Tamoxifen 20 µg. Results from RT-PCR studies are represented in , wherein the expression of p53 was less significant in all controls samples, but on treatment with NE mix (10 and 20 µg), Tamoxifen 20 µg and NE mix 10 µg – Tamoxifen 20 µg there was increased expression indicating the induced apoptosis in all cell lines and the effect appeared to be dose dependent and combinational (). Similarly the Caspase-9 gene expression was not observed in all the control samples, but the expression increased in treated groups i.e. NE mix (10 and 20 µg), Tamoxifen 20 µg and NE mix 10 µg – Tamoxifen 20 µg and the enhanced efficiency appeared to be dose dependent and combinational (). Expression of GAPDH in all the three immortal cell lines
3.8 Mass spectrometry analysis
LC-ESI-MS/MS analyses: The analytes were detected in positive ionization mode by monitoring the product scan spectra for the protonated molecules [M + H]+ in, turmeric, ginger and garlic the IS are shown in (a–c). The chromatograms show qualitative analysis of the metabolites and active compounds present in the natural extract (turmeric, ginger, garlic).
4 Discussion
Concern over cancer-related morbidity and mortality exists, notwithstanding significant advancements in developing chemotherapeutic drugs and radiotherapy. Further, preventive medicine modalities are few and are of limited use. Our present study was envisaged to examine the anti-cancer potential of food components consumed routinely. Such food components could have potential in preventive medicine, can be widely acceptable and put into use with little effort.
In India, different natural extracts including turmeric, ginger and garlic are used in cooking on a daily basis. These spices contain bio-active components such as; curcumin from Curcuma longa L. (turmeric), allicin from Allium sativum L. (garlic), 6-gingerol and oleoresin from Zingiber officinale R. (ginger). These natural extracts have been a part of Ayurveda, an ancient system of Indian medicine. Curcumin, a bio-active component of turmeric which is an important constituent of natural extracts powder is used in Indian culinary dishes [Citation36,Citation37] and extracts of ginger [Citation38] or garlic [Citation39] have been shown to induce apoptosis.
In the present communication, we report that aqueous extracts of commonly used food ingredients have excellent anti-oxidant and free radical scavenging properties () and they can induce cell death in cancer cells, particularly those of breast cancer cells. The treatment also decreased number of actively dividing cells, both in MCF-7 and in ZR-75 cells. Additionally, we found a higher effect on the cancer cells of Tamoxifen (Tam) when administered in combination with natural extracts.
The MTT assay suggests that the NE mix (5, 10 and 20 µg) has dose dependent effect on the different immortal cell lines (MCF-7, ZR-75, MDA MB231), similarly the Tamoxifen treatment also showed dose dependent activity (10 and 20 µg). Whereas the combination of NE mix 10 µg – Tamoxifen 20 µg showed enhanced efficiency in controlling the cell proliferation in comparison to the individual treatments i.e. NE mix and Tamoxifen. Based on the results it is safe to mention that our NE mix is sensitizing the cells towards Tamoxifen treatment, as a result the cells are getting arrested at G1 phase of cell cycle and ultimately death ().
Our novel aqueous extracts made from a blend of natural extracts exhibited pro-apoptotic and anti-proliferative properties in breast cancer cell lines. Cell cycle analysis shown in indicates perturbed cell cycle events in treated cells. MCF-7 cells treated with NE and Tam or a combination of NE and Tam had over 50% cells in G1 phase when compared to the untreated control cells The percentage of cells in the Sub-G1 phase was also found to be low in all the treatments. NE extract concomitantly reduced the number of cells in the G2/M phase and substantially increased the number of cells in G1 phase (a and b). MDA-MB-231 cells treated with NE had more cells in the Sub-G1 phase (19.67%) compared to the control or Tam or the combination of NE and Tam. More number of cells tended to accumulate in G1-phase after the treatments (b). ZR-75 cells treated with NE 20 µg or Tam 20 µg had more than 50% of the cells under G1 phase while the percentage of Sub-G1 phase cells were equivalent to the respective control. When treated with a combination of NE and Tam, the percentage of cells in sub-G phase was slightly higher than the individual treatments.
The NE-induced varied amounts of cell death in the breast cancer cell lines. The NE induced significantly higher apoptosis in MCF-7 and ZR-75 cells when compared to MDA-MB 231cells. ZR-75 cells appeared to be more sensitive to natural extracts and Tam-induced apoptotic cell death as compared to MCF-7, though both the breast cancer cell lines are positive for ER. The underlying mechanisms need to be investigated. The apoptosis induced by the natural extracts was more prominent in MCF-7 cells than in ZR-75 or MDA-MB 231 cells.
Tamoxifen induced relatively higher amounts of apoptotic cells in MCF-7 and ZR-75 cell lines than in MDA-MB 231. This may be due to the presence of the Estrogen Receptor (ER) in MCF-7 and ZR-75 cells [Citation40] whereas lower apoptosis was observed in MDA-MB 231 cells duet to absence of ER [Citation41]. Tam also induced minimal levels of cell death in the ER-negative MDA-MB 231 cells which may be due to ER-independent actions of Tamoxifen. Tamoxifen was shown to induce apoptosis through cancerous inhibitor of protein phosphatase 2A and phospho-AKT [Citation42], independent of its actions mediated through ER. Additionally, absence of PR in MDA-MB 231cells [Citation43] is linked with over expression of Her-2 [Citation44], and Her-2 has been reported to reduce Tam’s ability to induce cell death [Citation45]. Signalling molecules such as, TGF-β, MAPK, c-Myc and JNK were also shown to be sensitive to Tam [Citation46]. 4-hydroxy- tamoxifen was also reported to induce autophagic degradation of K-Ras in breast cancer cells [Citation47].
Additionally, the natural extracts were found to have an enhanced effect when combined with Tamoxifen, as judged by the increased effect on the cell lines ( and ). MCF-7 and ZR-75 cell lines clearly showed greater Tam-mediated cell death when treated together with NE mix. On the other hand, MDA-MB 231 cells did not exhibit such an increased effect when treated with both natural extracts and Tamoxifen together.
The gene expression studies of p53 and CASP-9 genes in immortal cell lines i.e. MCF-7, ZR-75 and MDAMB-231 cells when treated with NE mix (10 and 20 µg), Tamoxifen 20 µg and NE mix 10 µg – Tamoxifen 20 µg. Results from RT-PCR studies are represented in , wherein the expression of p53 was less significant in all controls samples, but on treatment with NE mix (10 and 20 µg), Tamoxifen 20 µg and NE mix 10 µg – Tamoxifen 20 µg there was increased expression indicating the induced apoptosis in all cell lines, but in comparison to MCF-7 was more significant than in ZR-75 and MDA MB 231. MDA MB231 cells undergoing apoptosis was least among all three cell lines. The NE mix 10 µg and Tamoxifen 20 µg treatment did induce apoptosis but it wasn’t as significant as NE mix 20 µg and NE mix 10 – Tamoxifen 20 µg, hence it evident that the NE mix is sensitizing the immortal cell lines (ER +ve and −ve cell lines) to Tamoxifen treatment.
Similarly the Cas9 gene expression was not observed in all the control samples, but with the treatment with NE mix (10 and 20 µg), Tamoxifen 20 µg and NE mix 10 µg – Tamoxifen 20 µg showed enhanced expression of Cas9 gene in all the drug concentrations and combinational treatment. The Cas9 gene was significant high in MCF-7 cell line in comparison to the ZR-75 and MDA MB 231 cell lines, where as the MDA MB 231 cells showed least expression among all three cell lines. The NE mix 10 µg concentration has enhanced the Cas9 expression when compared to NE mix 20 µg, Tamoxifen 20 µg, but the combinational treatment showed more Cas9 expression in all the cell lines suggesting the cells becoming sensitive towards Tamoxifen and undergoing apoptosis.
Anti-cancer effects of the natural extracts observed in the present study when taken together with other reported benefits of plant products [Citation48Citation[49]Citation[50]Citation[51]–Citation52] indicate potential anti-cancer and preventive potential of the extracts and call for further investigations to realize the benefits of the extracts. If the natural extracts prove to possess cancer prevention potential, preventive strategies based on the commonly used dietary sources will be ideal as they are likely to be free of side effects and could be implemented with ease. Moreover, the human system is adapted to these dietary ingredients. Additionally, these extracts may be taken along-side with the conventional chemotherapy and radiotherapy treatment regimens to augment their therapeutic potency. Such a notion is in line with the present study and the reported enhancement of Tamoxifen-induced cell death of MCF-7 cells with equol, a soya phytochemical [Citation52]. Several other reports also suggest that bio-active compounds in natural ingredients reduce proliferation [Citation12] and transformation of cells, tumor angiogenesis and metastasis, though the underlying mechanisms of action of these compounds are yet to be understood [Citation53Citation[54]–Citation55] .
A sensitive and reliable LC-MS/MS method was developed for the determination of bioactive compounds in turmeric, ginger and garlic (). The specificity of the extraction indicates that the chromatographic method tested possessed the capacity to differentiate and quantitate the analyte in the presence of other endogenous constituents in samples, as well as to detect potential interferences. The analytical procedure used was based on liquid–liquid extraction to obtain sufficiently high recoveries. Chromatographic peak areas from Curcumin standards and turmeric extract spiked with neat standards at concentrations of the test samples of the three compounds were monitored. The results obtained showed that the matrix effect of the turmeric extract was significant. Similar results were observed in case of ginger and garlic extracts and the bioactive composition was in concord with the literature [Citation56].
5 Conclusions
The hormone therapy drugs raloxifene and tamoxifen are used to treat breast cancer. Reported studies support an explicit role of selected phyto and dietary products in the prevention and treatment of cancers by targeting different signal transduction pathways. Additive or synergistic effect of natural compounds combined with chemopreventive agents has been reported and it is also helping in mitigating drug-associated toxicities [Citation49]. Genistein which is used for its chemopreventive effects in human breast cancers has also shown anti-proliferatory effect in both ER-positive and ER-negative breast cancer cells [Citation56]. According to literature, it is found that genistein was sensitizing the prostate cancer to radiation, and similarly iso-flavones from soya were able to reduce the adverse effects of radiation in men. Since the bioactive compounds of turmeric, ginger and garlic have been reported to possess anti-cancer properties such as cell atrophy, anti-proliferative effect, cytotoxicity and free scavenging properties, they have the potential to act as chemopreventive agents.
We have examined the anti-proliferative, apoptotic properties of the natural extract mix against both ER-positive and ER-negative breast cancer cell lines (MCF 7, ZR-75 and MDA-MB 231) with or without Tamoxifen. Our results show increased effects of tamoxifen when combined with the natural extracts. In conclusion, our findings indicate that commonly used aqueous natural extracts can induce cell death and lower the number of dividing cells. In addition, our data also suggest the possibility that the extracts may be sensitizing the cancer cells to Tamoxifen, which needs further study. Our results open up the possibility to develop natural extracts that can target breast cancers and also give understanding of the exact mechanism of action i.e. which signalling molecules are getting activated or repressed.
Conflict of interest
We declare that we have no conflict of interest with anyone.
Acknowledgments
We extend our sincere thanks to Sunshine Hospitals for their support and facilities and we want to extend our sincere thanks to Dr. Suresh Babu (senior scientist) and Mr. Kumar Katragunta, Indian Institute of Chemical Technology for helping with us LC-ESI-MS-MS analysis. We acknowledge Dr. Kiranam Chatti, Dr. Reddy’s Institute of Life Sciences for providing us with cancer cells and Ms. Prasanthi Sampara, SMART-Sunshine Hospitals for her assistance in manuscript editing.
References
- P.KumarN.B.BolshetteV.S.JamdadeN.A.MundheK.K.ThakurK.K.Saikiaet al.Breast cancer status in India: an overviewBiomed Prevent Nutr32013177183
- D.R.YouldenS.M.CrambC.H.YipP.D.BaadeIncidence and mortality of female breast cancer in the Asia-Pacific regionCancer Biol Med112014101115
- E.AziziM.H.AbdolmohammadiS.H.FouladdelA.ShafieeG.H.AminS.M.GhaffariEvaluation of p53 and Bcl-2 genes and proteins expression in human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss.) Drude in comparison to TamoxifenDARU1732009181186
- P.BertheauJ.Lehmann-CheJ.VarnaA.DumayB.PoirotR.Porcheret al.P53 in breast cancer subtypes and new insights into response to chemotherapyBreast2222002S279
- W.LuoF.WuR.ElmaouedB.B.BeckE.FischerX.Menget al.Amifostine enhancement of the anti-cancer effects of paclitaxel in endometrial cancer is TP53-dependentInt J Oncol37201011871194
- K.H.VousdenX.LuLive or let die: the cell’s response to p53Nat Rev Cancer282002594604
- S.HauptM.BergerZ.GoldbergY.HauptApoptosis-the p53 networkJ Cell Sci116Pt 20200340774085
- L.D.AttardiT.JacksThe role of p53 in tumor suppression: lessons from mouse modelsCell Mol Life Sci55119994863
- G.MilenaS.ShukriTimC. The p53 pathway in breast cancerBreast Cancer Res420027010.1186/bcr426
- H.C.MichaelR.NatalieR.S.HenningS.S.GuyF.F.ThomasS.Ericet al.Regulation of cell death protease caspase-9 by phosphorylationScience2825392199813181321
- C.U.I.QiaoY.U.Jing-huaW.U.Jin-nanT.Shin-ichiO.SatoshiM.Mutsuhikoet al.P53-mediated cell cycle arrest and apoptosis through a caspase-3-independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cellsActa Pharmacol Sin287200710571066
- M.L.WürstleM.A.LaussmannM.RehmThe central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosomeExp Cell Res31811201112131220
- A.D.GuerreroI.SchmitzM.ChenJ.WangPromotion of caspase activation by caspase-9-mediated feedback amplification of mitochondrial damageJ Clin Cell Immunol33201210.4172/2155-9899.1000126
- C.MadedduG.GramignanoP.KotsonisF.ParibelloA.MacciòOvarian hyperstimulation in premenopausal women during adjuvant tamoxifen treatment for endocrine-dependent breast cancer: a report of two casesOncol Lett8201412791282
- L.ShenL.HongG.ZhangR.MaiSynchronous uterine carcinosarcoma and contralateral breast cancer after tamoxifen therapy: a case reportInt J Clin Exp Pathol7201452955301
- H.I.OnderA.C.KilicS.A.KoseA.KaratasE.KayaM.Kayaet al.Branch retinal vein occlusion associated with tamoxifen useSemin Ophthalmol28220138890
- R.K.HernandezH.T.SørensenL.PedersenJ.JacobsenT.L.LashTamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism, a Danish population-based cohort studyCancer11519200944424449
- B.FisherJ.P.CostantinoD.L.WickerhamR.S.CecchiniW.M.CroninA.Robidouxet al.Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 StudyJ Natl Cancer Inst9722200516521662
- M.B.GorinR.DayR.J.CostantinoB.FisherC.K.RedmondL.Wickerhamet al.Long-term tamoxifen citrate use and potential ocular toxicityAm J Ophthalmol12541998493501
- O.L.DmitriM.D.ValeryAnti-breast cancer agents derived from plantsNat Prod Bioprospect512015116
- C.C.LeeP.HoughtonCytotoxic of plants from Malaysia and Thailand used traditionally to treat cancerJ Ethnopharmacol10032005237243
- M.KasturiB.A.IbrahimG.B.RenuL.Hwei-SanM.SivakumarExceedingly higher co-loading of curcumin and paclitaxel onto polymer-functionalized reduced graphene oxide for highly potent synergistic anticancer treatmentSci Rep620163280810.1038/srep32808
- C.CharalambousC.A.PittaA.I.ConstantinouEquol enhances tamoxifen’s anti-tumor activity by induction of caspase-mediated apoptosis in MCF-7 breast cancer cellsBMC Cancer13201323810.1186/1471-2407-13-238
- A.GoelB.B.AggarwalCurcumin, the golden spice from indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organsNutr Cancer627201091993010.1080/01635581.2010.509835
- M.R.SartippourR.PietrasD.C.Marquez-GarbanH.W.ChenD.HeberS.M.Henninget al.The combination of green tea and tamoxifen is effective against breast cancerCarcinogenesis2712200624242433
- Ho YS, Pan MH. Tea extracts confer its antiproliferating effects through inhibition of nicotine- and estrogen-induced 9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. In: Nutrition, Functional and Sensory Properties of Foods. 2013, pp. 256-268. doi:10.1039/9781849737685-00256.
- BNooluARajannaAChauhanBNagallaRManchalaAIsmailMurraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cellsBMC Complement Altern Med13720131472688210.1186/1472-6882-13-7
- A.OuhtitR.L.GaurM.AbdrabohS.K.IrelandP.N.RaoS.G.Rajet al.Simultaneous inhibition of cell-cycle, proliferation, survival, metastatic pathways and induction of apoptosis in breast cancer cells by a phytochemical super-cocktail: Genes that underpin its mode of actionJ Cancer49201370371510.7150/jca.7235 eCollection 2013
- M.SrivastavaM.HegdeK.K.ChiruvellaH.J.KorotS.BhattacharyaB.Choudharyet al.Sapodilla plum (Achrassapota) induces apoptosis in cancer cell lines and inhibits tumor progression in miceSci Rep42014614710.1038/srep06147
- M.N.RavishankaraN.ShrivastavaH.PadhM.RajaniEvaluation of antioxidant properties of root bark of Hemidesmus indicus R. Br. (Anantmul)Phytomedicine9222002153160
- M.UmamaheswariT.K.ChatterjeeIn vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extractAfr J Tradit Complement Altern Med5120076173
- D.Y.KwonS.J.KimJ.W.LeeY.C.KimComparison of hydroxyl radical, peroxyl radical, and peroxynitrite scavenging capacity of extracts and active components from selected medicinal plantsToxicol Res264201032132710.5487/TR.2010.26.4.321
- M.NiharikaS.K.SamantaAmeliorative action of aqueous extract of Acalypha indica against puffer fish Lagocephalus lunaris induced toxicityInt J Drug Dev Res522013257271
- R.R.BanalaS.K.VemuriA.V.G.ReddyG.P.V.SubbaiahAqueous extract of Acalypha indica leaves for the treatment of Psoriasis: In-vitro studiesInt J Bioassays654201753605364 https://doi.org/10.21746/ijbio.2017.04.007
- W.Xiu-MeiZ.Qi-ZhiY.JianZ.Rong-HuaZ.JunC.Li-Jinget al.Validated HPLC–MS/MS method for simultaneous determination of curcumin and piperine in human plasmaTrop J Pharm Res1142011621629
- T.ChoudhuriS.PalT.DasS.GaurisankarCurcumin selectively induces apoptosis in deregulated cyclin d1-expressed cells at G2 phase of cell cycle in a p53-dependent mannerJ Biol Chem2802020052005920068
- A.I.ElkadyO.A.AbuzinadahN.A.BaeshenT.R.RahmyDifferential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinaleJ Biomed Biotech2012201261435610.1155/2012/614356
- A.TsuburaY.C.LaiM.KuwataN.UeharaK.YoshizawaAnticancer effects of garlic and garlic-derived compounds for breast cancer controlAnticancer Agents Med Chem1132011249253
- A.FreundA.ChauveauJ.P.BrouilletA.LucasM.LacroixA.Licznaret al.IL-8 expression and its possible relationship with estrogen-receptor- negative status of breast cancer cellsOncogene2222003252265
- E.A.VladusicA.E.HornbyF.K.Guerra-VladusicJ.LakinsR.LupuExpression and regulation of estrogen receptor beta in human breast tumors and cell linesOncol Rep712000157167
- C.Y.LiuM.H.HungD.S.WangP.Y.ChuJ.C.SuT.H.Tenget al.Tamoxifen induces apoptosis through cancerous inhibitor of protein phosphatase 2 A-dependent phospho-Akt inactivation in estrogen receptor-negative human breast cancer cellsBreast Cancer Res165201443110.1186/s13058-014-0431-9
- K.SubikJ.F.LeeL.BaxterT.StrzepekD.CostelloP.Crowleyet al.The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell linesBreast Cancer420103541
- H.J.KimX.CuiS.G.HilsenbeckA.V.LeeProgesterone receptor loss correlates with human epidermal growth factor receptor 2 overexpression in estrogen receptor-positive breast cancerClin Cancer Res123 Pt 220131013 s1018 s
- C.K.OsborneR.SchiffG.ArpinoA.S.LeeV.G.HilsenbeckEndocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapyBreast1462014458465
- S.MandlekarA.N.T.KongMechanisms of tamoxifen-induced apoptosisApoptosis662001469477
- L.KohliN.KazaT.CoricS.J.ByerN.M.BrossierB.J.Klockeet al.4-hydroxytamoxifen induces autophagic death through K-Ras degradationCancer Res731420134395440510.1158/0008-5472.CAN-12-3765
- B.B.AggarwalC.SundaramN.MalaniH.IchikawaCurcumin: the Indian solid goldAdv Exp Med Biol5952007175
- N.S.YaacobN.N.KamalM.N.NorazmiSynergistic anticancer effects of a bioactive subfraction of Strobilanthes crispus and tamoxifen on MCF-7 and MDA-MB-231 human breast cancer cell linesBMC Complementary Altern Med14201425210.1186/1472-6882-14-252
- J.ChenL.LiJ.SuB.LiT.ChenY.S.WongSynergistic apoptosis-inducing effects on A375 human melanoma cells of natural borneol and curcuminPLoS One962014e101277
- K.BrusselmansE.De SchrijverW.HeynsG.VerhoevenJ.V.SwinnenEpigallocatechin-3- gallate is a potent natural inhibitor of Fatty acid synthase in intact cells and selectively induces Apoptosis in prostate cancer cellsInt J Cancer10662003856862
- S.ModemS.E.DiCarloT.R.ReddyFresh garlic extract induces growth arrest and morphological differentiation of MCF-7 breast cancer cellsGenes Cancer32201217718610.1177/1947601912458581
- F.S.GiudittaD.S.MauroB.GiorgioF.MircoB.AnahiG.Lauraet al.Inhibition of breast cancer cell proliferation and in vitro tumorigenesis by a New Red Apple CultivaPLoS ONE1082015e013584010.1371/journal.pone.0135840
- A.BhanotR.SharmaM.NoolviNatural sources as potential anti-cancer agents: A reviewIntl J Phytomedicine32011926
- F.H.SarkarY.LiZ.WangD.KongCellular signaling perturbation by natural productsCell Signal211120091541154710.1016/j.cellsig.2009.03.009
- M.K.ShanmugamR.KannaiyanG.SethiTargeting cell signalling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancerNutr Cancer632201116117310.1080/01635581.2011.523502
- S.Zhi-MingS.Zhen-ZhouL.Can-HuiR.S.MaryamL.G.VayH.Davidet al.Curcumin exerts multiple suppressive effects on human breast carcinoma cellsInt J Cancer9822002234240