?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Preeclampsia, a hypertensive disorder of pregnancy, was related to hypertension, diabetes, oxidative stress, obesity, in addition to polycystic ovarian diseases. Visfatin, potentially a new adipokine has emerged having high contribution in pathogenesis of preeclampsia. Oxidative stress was increased lipid peroxidation and caused vascular endothelial damage. This study was conducted with the aim of evaluating the level of visfatin gene expression in placenta and measuring some oxidative stress parameters. Eighty Egyptian patients of newly diagnosed pregnant hypertensive disorder were selected for the study which was recruited from Mansoura University Hospital, Department of Obstetrics and Gynecology and also normotensive pregnant women were collected. The pregnant women groups were classified into four groups: gestational hypertension (n = 20), mild preeclampsia (n = 16), severe preeclampsia (n = 25), chronic hypertension with superimposed preeclampsia (n = 19) and compared with normotensive pregnant women (n = 10) as control group. Visfatin gene expression level was decreased in placenta of pregnant hypertensive disorder women groups with mean 1.28 ± 0.42, 1.01 ± 0.24, 0.40 ± 0.14 and 0.32 ± 0.11 respectively when compared to normotensive pregnant women with mean 1.56 ± 0.69. Additionally, catalase activity, total antioxidant capacity and reduced glutathione levels were decreased in hypertensive pregnant women groups compared with normotensive ones. On the other hand malondialdhyde level was increased in preeclampsia groups when compared with normalized pregnant women. Decreased visfatin level has an important pathophysiology of preeclampsia and suggests the complication in pregnancy. Also the imbalance between oxidant and antioxidant has an important causative factor in the pathogenesis of preeclampsia.
Introduction
A condition in pregnancy characterized by high blood pressure contributes greatly to maternal morbidity and mortality and the fetus as well around the world [Citation1]. The mere solution for preeclampsia and pregnancy induced hypertension is delivery of the fetus and placenta [Citation2]. Blood pressure’s interruptions that happen in pregnancy have harmful influence on organ systems of both the pregnant woman and the fetus [Citation3]. Complications in pregnant women suffer from preeclampsia are seizure activity, placental abruption, stroke, hemolysis, hemolysis elevated liver enzymes and low platelets (HELLP) syndrome, liver hemorrhage, pulmonary edema, acute renal failure, and disseminated intravascular coagulation [Citation4].
The exact reasons of preeclampsia are not well known so risk of it may be elevated by some factors such as the first pregnancy [Citation5]. Also women with a history of preeclampsia [Citation4] and multiple gestations elevate the risk [Citation2]. Furthermore, some disease present before pregnancy such as obesity, diabetes mellitus, insulin resistance, chronic hypertension, gestational diabetes, lupus, vascular or connective tissue disorders and also chronic kidney disease may be increase the risk of preeclampsia [Citation6]. High blood pressure during pregnancy is divided into four groups as recommended by the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. The first group is chronic hypertension, the second group is gestational hypertension, the third group is mild and severe preeclampsia and the fourth group is preeclampsia superimposed on chronic hypertension [Citation7]. Gestational hypertension and preeclampsia generally can be diagnosed by measurements of high blood pressure and proteinuria [Citation8]. Also some symptoms may be associated with increasing in blood pressure such as vision interruption, continuous acute headaches, unexpected expand in face, hands and feet, vomiting, epigastric pain, lowering in platelets and increasing in liver enzymes and serum creatinine [Citation9].
Adipokines play an important role in some process including inflammation, organization of food intake, and arrangement of body weight homeostasis, proliferation, insulin sensitivity, immunity and vascular homeostasis [Citation10]. In obesity and type 2 diabetes mellitus have an alteration in adipokine production. Also, there is imbalance in adipokine production at the onset of insulin resistance, adipose tissue inflammation, chronic systemic inflammation, cardiovascular disease and endothelial dysfunction [Citation11]. Fukuhara et al. [Citation12] are the first one that characterize visfatin as an adipokine and show that insulin mimetic properties in mice with binding to and promoting the insulin receptor. Visfatin was found identical to pre-B cell colony enhancing factor (PBEF). It is a highly conserved 52 kDa cytokine-like protein. It increases the maturation of B cell precursors in relation to interleukin-7 and stem cell factor [Citation11] and also suppresses the apoptosis of neutrophils [Citation13]. Rongvaux et al. [Citation14] reported that visfatin displays intrinsic enzymatic activity as a nicotinamide phosphoribosyl transferase (Nampt). However, the physiological connection of NAMPT stay argumentative [Citation15], Revollo et al. [Citation16] showed that the critical role of NAMPT in the monitoring of glucose metabolism through the NAD biosynthetic activity.
Pathogenesis of preeclampsia is influenced by oxidative stress. It could lead to tissue damage by the end [Citation17]. An adaptive mechanism promoting the antioxidant defense system in pregnant women to oppose the effect of oxygen active species through enzymatic antioxidants like superoxide dismutase, glutathione peroxidase, catalase and also non-enzymatic antioxidants like reduced glutathione can hinder the occurrence of oxidative stress in preeclampsia [Citation18]. Elevation of lipid peroxidation products causes impaired antioxidant enzyme defense mechanism and this imbalance may result in preeclampsia pathogenesis [Citation19].
The purpose of this study was to evaluate the visfatin gene expression level in placental hypertensive and normotensive pregnant women groups as well as some antioxidants were determined in all groups.
Subjects and methods
Patients
The study was conducted from May 2014 until December 2016; patients chosen from the Obstetrics and Gynecology Department, Mansoura University. The study group was comprised 80 Egyptian pregnant women with hypertensive disorder and was divided into four groups. Group I (gestational hypertensive): twenty of pregnant women were gestational hypertensive with the age range 27 to 41 years with the mean age 34.40 ± 4.27 years which defined as blood pressure ≥ 140/90 mmHg after gestational age 20 weeks without proteinuria. Group II (mild preeclampsia): Sixteen of pregnant women were mild preeclampsia with the age range 33 to 46 years with the mean age 39.63 ± 4.40 years which defined as BP ≥ 140/90 mmHg after gestational age 20 weeks with significant proteinuria (+ protein in urine on dipstick). Group III (severe preeclampsia): Twenty-five of pregnant women were severe preeclampsia with the age range 21 to 40 years with the mean age 30.28 ± 6.39 years. Severe preeclampsia was determined with blood pressure ≥ 160/110 mmHg with excessive proteinuria (≥ ++++ protein in urine on dipstick). Group IV (chronic hypertension with superimposed preeclampsia): Nineteen of pregnant women were chronic hypertension with superimposed preeclampsia with the age range 28 to 45 years with the mean age 36.84 ± 5.40 years. The preeclampsia with hypertension present before pregnancy was defined as certified BP ≥ 140/90 prior to pregnancy with new onset proteinuria (+ protein in urine on dipstick). Group V (Normotensive group): The normotensive pregnant women comprised 10 with the age range 21 to 42 years with the mean age 31.20 ± 7.79 years. Normal participants were at the same maternal and gestational age. Also, their pre-pregnancy parity and maternal body mass index were equal.
An informed written approval was provided by all study participants. Additionally, the Ethical Board of Mansoura University approved conducting the study. Also, the study was delimited to patients who did not provide a written approval, those who smoke, those with gestational diabetes, infectious disease, and premature rupture of membrane or suffer from other medical diseases. Moreover, participants with abnormal glucose tolerance who participated in the gestation test administration at weeks 24–28 or suffer from other medical diseases were also eliminated.
Tissue preparation
Placental biopsy samples were obtained during Caesarean sections from both normotensive patients and those with hypertensive pregnant women. Only some placental samples taken from a woman by a caesarean operation were included in the study to prevent any probable effects of labor on visfatin gene expressions. An anatomization of a central area of chronic tissue and extraction of maternal deciduas and amnionic membranes were taken for analysis. Large tissue samples cut to ≤ 0.5 cm in any single dimension then placed the fresh tissue in in epindorff containing 500 µl of RNA later tissue collection (RNA later Inc., Austin, Texas, U.S.). Tissue samples in RNA later solution were stored at 4 °C for 2 week without compromising RNA quality.
Blood collection
Morning fasting blood samples were collected from the study participants to be estimated after applying all aseptic precautions. One ml blood was collected in EDTA tubes for estimation of reduced glutathione level and the rest blood was collected into clean and dry test tubes then allowed to clot, serum was separated by centrifugation at 3000 rpm for 10 min and then stored in deep frozen at −20 °C until used. Serum was used for estimation of catalase activity, total antioxidant capacity level and malondialdhyde level.
RNA isolation and quantitative PCR
Total RNA was collected from placental tissue samples utilizing vivantis kit (Vivantis Technologies Sdn. Bhd., Malaysia), based on the manufacturer’s instructions. The extracted RNA was transcript into cDNA by using seni cDNA synthesis kit (Bioline USA Inc., USA), based on the manufacturer’s instructions. The housekeeping gene (GAPDH) was used to normalize mRNA concentrations. Quantitative PCR was performed for comparing expression levels of visfatin transcripts. The relative expression levels of visfatin in the tissue samples and reference sample were assessed by quantitative PCR using real-time RT–PCR analyses (Piko Real 96 type 5100, thermo scientific). RT–PCRs were performed using a seni
SYBR NO-ROX kit (Bioline USA Inc., USA) in a final volume of 20 ml. An initial PCR activation step was 2 min at 95 °C followed by 40 cycles of 5 s at 95 °C, and 20 s at 60 °C and 10 s at 72 °C. The sequence of primers, used in real-time PCR, was designed according to Vivantis Technologies Sdn. Bhd., Malaysia to span introns to prevent detection of genomic DNA as shown in (). It was used to reverse-transcribe and expand the RNA template for 40 cycles so finally Ct was estimated.
Table 1 The sequence of primers used in real-time PCR.
Assessment of the biochemical parameters
Serum catalase activity was determined by the method of Fossati et al. [Citation20] and Aebi [Citation21] by using a commercially available kit (Biodiagnostic, Dokki, Giza, Egypt). 0.5 ml of reagent 1 (chromogen- buffer) was added to 50 µl of serum sample then 100 µl of reagent 2 (). 0.2 ml of reagent 3 (catalase – inhibitor) and 0.5 ml of reagent 4 (peroxidase enzyme) were added. The pink color appears measured at 510 nm. Serum total antioxidant capacity level was determined by the method of Karacevic et al. [Citation22] by using a commercially available kit (Biodiagnostic, Dokki, Giza, Egypt). 0.5 ml of reagent 1 (H2O2) was added 20 µl of sample serum then 0.5 ml of working reagent (chromogen- enzyme- buffer) was added. The absorbance was recorded at 505 nm.
Blood reduced glutathione content was determined by the method of Beutler et al. [Citation23] by using a commercially available kit (Biodiagnostic, Dokki, Giza, Egypt). 0.5 ml of reagent 1 (trichloroacetic acid) was added to 0.1 ml of blood sample, centrifuged. 1.0 ml of reagent 2 (buffer) was added to 0.5 ml of the supernatant then add 0.1 ml of reagent 3 (5,5′-dithiobis(2-nitrobenzoic acid)). The color was measured at 405 nm. Thiobarbituric acid (TBA) test is widely used assay for the measurement of lipid peroxidation (malondialdehyde) according the method of Draper and Hadly, [Citation24]. 0.5 ml serum was mixed with 2.5 ml of TCA to precipitate proteins. After centrifugation 1.0 ml of TBA was added to 2.0 ml of the supernatant and a pink chromogen was measured at 532 nm.
Statistical analysis
Statistical Package for the Social Sciences Version 16.0 (SPSS) was used in the study analysis. Exploration of data revealed preserved normality. We used mean and standard deviation for description of the central tendency and dispersion. Analysis of differences between two groups as regards quantitative parameters was done using independent t-test with the probability of <.05 considering significant. Correlation between two quantitative parameters was assessed using Pearson correlation with (r) representing the correlation coefficient and its significance was starred if <.05.
Results
There was no statistically significant difference (P = .196) in age between hypertensive pregnant women patients with mean age 34.74 ± 6.27 years and normalized group with mean age 31.20 ± 7.79 years.
The level of placental visfatin gene expression in hypertensive pregnant women groups compared to normotensive group
There was no statistically significant decreased in placental visfatin gene expression level with mean 1.28 ± 0.42 in gestational hypertensive pregnant women when compared with normotensive group with the mean 1.56 ± 0.69 (). Also there was statistically significant decrease of placental visfatin gene expression level with mean 1.01 ± 0.24 in mild preeclampsia pregnant women as compared with normotensive pregnant women group with mean 1.56 ± 0.69 (). Severe preeclampsia and chronic with superimposed preeclampsia pregnant women have highly statistically significant decrease of placental visfatin gene expression level with mean 0.40 ± 0.14 and 0.32 ± 0.11 respectively as compared with normotensive pregnant women group with mean 1.56 ± 0.69 ().
Table 2 The placental visfatin gene expression in hypertensive pregnant women groups compared to normotensive pregnant women group.
Oxidative stress parameters in hypertensive pregnant women groups compared to normotensive pregnant women group
Serum catalase activity, serum total antioxidant capacity and blood reduced glutathione levels were decreased in gestational hypertension pregnant women, mild preeclampsia pregnant women group, severe preeclampsia and chronic with superimposed pregnant women group when compared to normotensive group (). In contrast, serum malondialdhyde level was elevated in gestational hypertension pregnant women, mild preeclampsia pregnant women group and severe preeclampsia and also chronic with superimposed pregnant women group when compared to normotensive group ().
Table 3 The oxidative stress measured parameters in hypertensive compared to normotensive pregnant women groups.
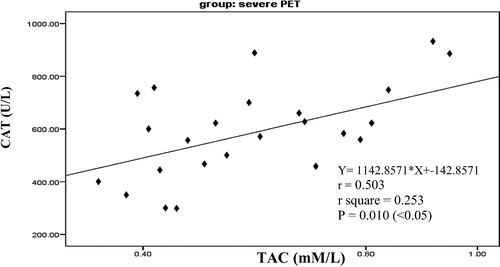
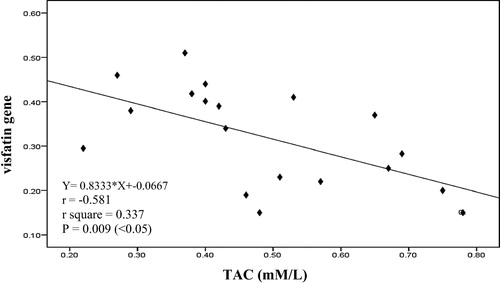
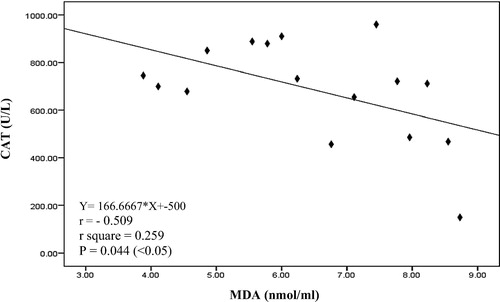
It is clearly that there was a significant negative correlation between serum CAT and serum MDA (p = .044) as in in mild preeclampsia pregnant women group. Also there was a significant positive correlation between serum CAT and serum TAC (p = .010) as in in severe preeclampsia pregnant women group. There was no correlation between visfatin gene and oxidative stress measured parameter except a significant negative correlation between tissue visfatin gene and serum TAC (p = .009) as shown in in chronic hypertension with superimposed preeclampsia pregnant women group.
Fig. 1 Linear Pearson correlation between each serum CAT and serum MDA among mild preeclampsia pregnant women group (n = 16).
Discussion
The etiology of preeclampsia is unknown but adipokines have an important role in pathogenesis of preeclampsia [Citation25]. Moreover, adipokines regulation problems may be present in the pathophysiology of insulin resistance [Citation26], obesity [Citation27], dyslipidemia and the metabolic syndrome [Citation28]. These findings were consistent with adipokine have been comprised in metabolic adaptations to health pregnancy [Citation29], as well as in preeclampsia [Citation30]. The expression of visfatin is not only in adipose tissue but also in placenta, fetal membranes, neutrophils [Citation31], myometrium [Citation32], bone marrow, liver, kidney, heart, muscle, lung [Citation11] and macrophages [Citation33]. In this study placental visfatin gene expression level was decreased in all hypertensive pregnant women groups when compared with normotensive pregnant women group.
Preeclampsia is related with elevated cardiovascular disease risk later in life [Citation7]. Preeclampsia and cardiovascular risk factors have the same work in inflammation, insulin resistance, and obesity. Since visfatin levels are increased in all above metabolic syndrome, authors were purposed that Nampt may contribute to preeclampsia. Ferreira et al. [Citation34] showed that serum visfatin levels in preeclampsia pregnant women were increased in comparison to pregnant controls. In contrast, other authors were proposed that expression of visfatin was decreased due to act as a proangiogenic factor so may be related to the pathogenesis of preeclampsia. Thus, Hu et al. [Citation35] showed that plasma Nampt levels were decreased in mild preeclampsia and excessive decreased in severe preeclampsia. In the same idea, Kim et al. [Citation36] reported that the expression of visfatin was decreased in placental biopsies in comparison to the Nampt levels in placentas from normal pregnant.
The first state in preeclampsia is decreased placental perfusion and begins set of statements that change vascular function and hypertension. Consequently, impaired placental perfusion induced endothelial dysfunction [Citation37]. Visfatin induction in preeclampsia was not affected by placental perfusion damaging mechanism [Citation34]. Ognjanovic et al. [Citation38] have elucidated that Nampt secretion from amniotic epithelium cells in the human placenta was increased due to pro-inflammatory stimuli such as lipopolysaccharide and interleukin-1[Citation38]. Also visfatin has a protective role in preventing apoptosis induced by infection in the placenta [Citation39]. Thus, it is still an open question [Citation40].
Oxidative stress is the abnormal levels of reactive oxygen species and the natural antioxidants present in the body [Citation41]. Elevation of oxidative stress markers lead to endothelial cell dysfunction and so elevated the blood pressure in preeclamptic patients [Citation42]. In the present study, there was decreased in catalase activity, total antioxidant capacity and reduced glutathione levels in hypertensive pregnant women groups when compared with normotensive ones. A significantly reduced of antioxidant in preeclampsia may be due to increased attack of free radicals and thus resulted in low production of them [Citation43]. In contrast to this study, few others reported showed an increased in antioxidants [Citation44].
Lipid peroxidation has the main originator factor for oxidative stress and the etiopathogeneiss of preeclampsia [Citation45]. In this result, there was increased in malondialdhyde level in hypertensive pregnant women groups when compared with normotensive pregnant women group. Products of lipid peroxidation are the candidate factors that mediate disturbance of the maternal vascular endothelium and may inhibit prostacyclin synthesis and also stimulate smooth muscle contraction that lead to widespread vasospasm, a prominent feature of preeclampsia [Citation1].
In conclusion, pregnancy induced hypertension women showed decreased in visfatin gene expression level when compared to normal pregnant women. Our observation revealed that an imbalance between lipid peroxidation and antioxidants in preeclampsia lead to free radical mediated endothelial dysfunction so we strongly recommend that all pregnant women have to be supplemented with antioxidants to avoid overwhelming effect of oxidative stress.
References
- L.GhulmiyyahB.SibaiMaternal mortality from preeclampsia / eclampsiaSemin Perinatol36120125659
- Dattel BJ, Chescheir N, Lockwood C. Your pregnancy & birth. 4th ed. Washington, D.C.: The American College of Obstetricians and Gynecologists; 2005.
- L.M.BorgeltM.B.O’ConnellJ.A.SmithK.A.CalisWomen’s health across the lifespan2010American Society of Health-System PharmacistsMaryland
- A.R.VestL.S.ChoHypertension in pregnancyCardiol Clin302012407423
- Barss V, Repke J. Preeclampsia (beyond the basics). South Holland: Up-todate; 2012.
- S.McCoyK.BaldwinPharmacotherapeutic options for the treatment of preeclampsiaAm J Health Syst Pharm662009337344
- E.A.SteegersP.von DadelszenJ.J.DuvekotR.PijnenborgPre-eclampsia Lancet3762010631644
- ACOG Committee on Practice Bulletins-ObstetricsACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002Obstet Gynecol.992002159167
- DiPiro J, Talbert RL, Yee G, Matzke G, Wells B, Posey LM. Pharmacotherapy A Pathophysiologic Approach. 8th ed. McGraw Hill; 2011.
- A.FukuharaM.MatsudaM.NishizawaK.SegawaM.TanakaK.KishimotoRetractionScience31858502007565
- B1SamalY.SunG.StearnsC.XieS.SuggsI.McNieceCloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factorMol Cell Biol142199414311437
- A1FukuharaM.MatsudaM.NishizawaK.SegawaM.TanakaK.KishimotoVisfatin: a protein secreted by visceral fat that Mimics the effects of insulinScience30757082005426430
- S.H.JiaY.LiJ.ParodoA.KapusL.FanO.D.Rotsteinet al.Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsisJ Clin Invest113200413181327
- A.RongvauxR.J.SheaM.H.MulksD.GigotJ.UrbainO.Leoet al.Pre-B-cell colony enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyl-transferase, a cytosolic enzyme involved in NAD biosynthesisEur J Immunol3211200232253234
- D.de LuisM.G.SagradoR.AllerR.CondeO.Izaolaet al.Circulating visfatin in obese non-diabetic patients in relation to cardiovascular risk factors, insulin resistance, and adipocytokines: a contradictory piece of the puzzleNutrition2620081112
- J.R.RevolloA.KörnerK.F.MillsA.SatohT.WangA.Gartenet al.Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzymeCell Metab652007363375
- P.PhalakJ.KulkarniM.TilakA.P.ThoratRole of lipid peroxidation and antioxidant status in pathogenesis of preeclampsiaIndian J Basic Appl Med Res262013536539
- S.B.PatilM.V.KodliwadmathS.M.KodlwadmathM.B.PatilLipid peroxidation and non-enzymatic antioxidants status in preeclampsia and postpartum preeclmaptic womenNatl J Basic Med Sci3120123943
- K.T.SharmilaR.D.RajaR.E.VenkataR.R.AparnaJ.N.NaiduCorrelation between lipid peroxidation product – malondialdehyde (MDA) and reduced glutathione (GSH) in preeclampsiaInt J Appl Biol Pharmaceut Technol622015196199
- P.FossatiL.PrencipeG.BertUse of 3,5- dichloro-2-hydroxybenzene sllforic acid /4-amino phenazone chromogenic system in direct enzymic assay of uric acid in serum and urineClin. Chem.261980227231
- H.AebiCatalase in vitroMethod Enzymol1051984121126
- D.KoracevicG.KoracevicV.DjordjevicS.AndrejevicV.CosicMethod for the measurement of antioxidant activity in human fluidsJ Clin Pathol542001356361
- E.BeutlerO.DuronM.B.KellyImproved method for the determination of blood glutathioneJ Lab Clin Med611963882888
- W.DraperM.HadleyIndirect determination of oxygen free radicalMethod Enzymol1861990421431
- K.WatanabeK.NaruseK.TanakaH.MetokiY.SuzukiOutline of definition and classification of “pregnancy induced hypertension (PIH)”Hyperten Res Preg11201334
- P.M.CatalanoM.HoeghJ.MiniumL.Huston-PresleyS.BernardS.Kalhanet al.Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolismDiabetologia49200616771685
- K.N.FraynObesity and metabolic disease: is adipose tissue the culprit?Proc Nutr Soc642005713
- Y.MatsuzawaT.FunahashiS.KiharaL.ShimomuraAdiponectin and metabolic syndromeArterioscler Thromb Vasc Biol2420042933
- S.Mazaki-ToviR.RomeroJ.P.KusanovicO.ErezE.VaisbuchF.Gotschet al.Adiponectin multimers in maternal plasmaJ Matern Fetal Neonatal Med21112008796815
- S.Mazaki-ToviR.RomeroE.VaisbuchJ.P.KusanovicO.ErezF.Gotschet al.Maternal serum adiponectin multimers in preeclampsiaJ Perinat Med3742009349363
- C.E.Kendal-WrightD.HubbardG.D.Bryant-GreenwoodChronic stretching of amniotic epithelial cells increases pre B cell colony-enhancing factor (PBEF/visfatin) expression and protects them from apoptosisPlacenta292008255265
- M.S.EsplinM.B.FausettM.R.PeltierS.HamblinR.M.SilverD.W.Branchet al.The use of cDNA microarray to identify differentially expressed labor associated genes within the human myometrium during laborAm J Obstet Gynecol19322005404413
- T.B.DahlA.YndestadM.SkjellandE.ØieA.DahlA.Michelsenet al.Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilizationCirculation11582007972980
- A.F.FerreiraJ.C.RezendeC.O.R.de CassiaR.AkolekarK.H.NicolaidesMaternal serum visfatin at 11–13 weeks’ gestation in preeclampsiaJ Hum Hypertens2742013261264
- W.HuZ.WangH.WangH.HuangM.DongSerum visfatin levels in late pregnancy and preeclampsiaActa Obstet Gynecol Scand8742008413418
- S.C.KimM.J.ParkB.S.JooJ.K.JooD.S.SuhK.S.LeeDecreased expressions of vascular endothelial growth factor and visfatin in the placental bed of pregnancies complicated by preeclampsiaJ Obst Gynaecol Res3842012665673
- J.M.RobertsD.W.CooperPathogenesis and genetics of preeclampsiaLancet357924920015356
- S.OgnjanovicT.L.KuG.D.Bryant-GreenwoodPre-B cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epitheliumAm J Obstet Gynecol19312005273282
- S.OgnjanovicG.D.Bryant-GreenwoodPre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranesAm J Obstet Gynecol1874200210511058
- T.D.FilippatosH.S.RandevaC.S.DerdemezisM.S.ElisafD.P.MikhailidisVisfatin/PBEF and atherosclerosis-related diseasesCurr Vasc Pharmacol8120101228
- G.BuonocoreS.PerroneM.L.TatarannoOxygen toxicity: chemistry and biology of reactive oxygen speciesSemin Fetal Neonatal Med152010186190
- J.B.SharmaS.MittalOxidative stress and preeclampsiaObstet Gynacol Today92004551554
- Sarkar P, Jayaram S. Estimation of primary enzymatic antioxidants in pregnancy induced hypertension. Webmed Central: 2013; <http://www.webmedcentral.com/ article_view/3980> cited on 31.01.13.
- P.S.SheenaComparative study of oxidative stress in pregnancy induced hypertension preeclampsia and eclampsiaInt J Biomed Adv Res3112012810814
- K.T.SharmilaD.R.RajaR.E.VenkataR.R.AparnaJ.N.NaiduCorrelation between lipid peroxidation product – malondialdehyde (MDA) and reduced glutathione (GSH) in preeclampsiaInt J Appl Biol Pharmaceut Technol622015196199