Abstract
Salvinia molesta, commonly known as giant Salvinia, is a floating fern belonging to the family of Salviniaceae. In this study the active fractions of the fern extract were separated using column chromatography and phenolic compounds present in the active fractions were determined by RP-HPLC. Ethyl acetate extract was found to possess significant pharmacological activity when compared to other extracts under study and therefore an attempt was made to fractionate ethyl acetate extract. The analysis was performed through two different mobile phases involving solvent A (acetonitrile) and solvent B (0.1% phosphoric acid in water) and solvent A (methanol) and Solvent B (4% acetic acid). HPLC analysis indicated the presence of phenolic compounds namely ascorbic acid, quercetin, gallic acid, resorcinol, catechol, vanillin and benzoic acid with specific retention times. The detected compounds possess antioxidant and antitumour activities. The results of the present study suggests the possibility to use S. molesta as a source for a plausible antioxidant agent which could be isolated and used as a lead candidate for the development of antioxidant drugs that help stop or limit damage caused by free radicals and to counteract oxidative stress leading to the prevention of a variety of chronic and degenerative diseases.
1 Introduction
The phenolic compounds are ubiquitous in plant kingdom. They synthesize several thousand different chemical structures and are characterized by hydroxylated aromatic rings. These compounds are secondary metabolites which are derived from the pentose phosphate, shikimate and phenylpropanoid pathways in plants [Citation1]. These are one of the most widely occurring groups of pytochemicals which are of appreciable physiological and morphological importance in plants [Citation2]. A number of studies have been aimed to characterize the health promoting activities of phenolic compounds due to their antioxidant properties. They are useful in treatment and management of cancer, cardiovascular and neurodegenerative diseases or as components in anti-aging or cosmetic products [Citation3].
The antioxidant activity of phenolic compounds are mainly due to their redox potential which empower them to function as reducing agents, donors of hydrogen atoms or electrons, singlet oxygen quenchers or metal chelators [Citation4Citation[5]–Citation6] . Phenolic compounds exhibit a wide range of physiological properties such as anti-allergic, anti-microbial, anti-thrombotic, anti-inflammatory, anti-arthritic, antipyretic, analgesic, antioxidant, cardio-protective, immunomodulatory and vasodilatory effects [Citation7Citation[8]Citation[9]Citation[10]–Citation11] . These activities of phenolic-flavonoidic compounds may be due to the presence of gallic acid, ellagic acid, ascorbic acid, quercetin, tannic acid, vanillin, resorcinol, catechin etc. [Citation12Citation[13]–Citation14] .
Modern studies have shown that ferns possess biological properties such as anti-microbial, antioxidant, anti-proliferative, anti-inflammatory, antitussive, antitumor, anti-HIV, enzyme modulation and stimulation, hormonal action, interference of DNA replication and physiological action [Citation15,Citation16] . Iqbal Choudhary et al. [Citation17] have isolated phenolic compounds together with few other phytoconstituents for the first time from the aquatic fern S. molesta. The isolated compounds were two glycosides, 6′-O-(3,4-dihydroxy benzoyl)-β-d-glucopyranosyl ester and 4-O-β-d-glucopyranoside-3-hydroxy methyl benzoate, along with five already known compounds viz., methyl benzoate, hypogallic acid, caffeic acid, paeoniflorin and pikuroside. They exhibited potent free radical scavenging activity in a non-physiological assay. These compounds possess interesting characteristics, noteworthy of further study.
Basing on these data the aim of the present study was to fractionate ethyl acetate extract of S. molesta using column chromatography and to quantify the phenolic compounds present in the fractions by RP-HPLC with photo diode array detection (PDA). This study was the first to quantify seven antioxidant phenolic compounds in the fern extract applying two different mobile phases.
2 Materials and methods
2.1 Chemicals and phenolic standards
Hexane, ethyl acetate, ethanol, methanol, acetone, vanillin-H2SO4 spray, acetonitrile, phosphoric acid, acetic acid, chromanorm water, gallic acid, catechol, benzoic acid, resorcinol, ascorbic acid, vanillin, quercetin, silica gel and sea sand. All the above chemicals were of analytical grade and were purchased from Hi media, Pvt. Ltd., Mumbai, India.
2.2 Plant materials
Plants of S. molesta were collected from the paddy fields, rivers and ponds of Kalliyad and Kaiyamkulam, Kaithachira, Thrissur, Kerala, India. The specimen was identified and authenticated by Dr. G. Jeya Jothi, Taxonomist, Loyola College, Chennai, Tamil Nadu, India. The voucher specimen (No: LCH-130) of the plant has been preserved in Loyola College Herbarium for further reference. The plant materials were cleansed under running tap water three to four times, after which it was shade dried at room temperature for three weeks. The dried plant materials were pulverized into fine powder, passed through a sieve (mesh No. 40) and were stored in airtight containers [Citation18].
2.3 Preparation of plant extracts
The extraction from the plant materials was performed by maceration. Four different solvents namely hexane, ethyl acetate, ethanol and methanol were used for the sequential extraction starting from low polarity to high polarity. 50 g of the powdered plant materials were soaked in 200 ml of hexane in a stoppered container and was placed on the orbital shaker at 120 rpm for 72 h at room temp. The mixture was then pressed and filtered through Whatman No.1 filter paper and was concentrated under reduced pressure using a rotary evaporator. The same procedure was followed for the other three solvents. The extraction process was carried out in triplicates with each solvent. The dried crude extracts were stored in amber vials and were placed in a refrigerator at 4 °C [Citation18,Citation19] .
2.4 Column chromatographic fractionation of ethyl acetate extract
The ethyl acetate extract (EAE) was subjected to Silica gel column chromatography for the isolation of phytoconstituents. A vertical glass column (40 mm width × 60 mm length) made of borosilicate material was used for the fractionation. The column was rinsed well with acetone and was completely dried before packing. A piece of glass wool was placed at the bottom of the column with the help of a glass rod. Sea sand (50–70 particle size) was added to the top of the glass wool to 1 cm height. The sand particles were rinsed down using the solvent. Hexane was poured into the column up to 3/4th level by closing the stopcock. 200 g of silica gel (60–120 mesh size) was used as the packing material. Silica slurry was prepared with hexane and was poured from the top of the column approximately 2/3rd of the column with simultaneous draining of the solvent to aid proper packing of the column. Sea sand was added to the top of silica slurry to 1 cm height and the sand particles were rinsed down with the solvent. 20 g of EAE was mixed with minimum quantity of hexane and was poured down from the top of the column along the sides and was rinsed down with the solvent. Sea sand was added to the top of the extract to 1 cm height. Solvent level 6 cm from above the extract was maintained to prevent drying of the column. Gradient elution method was followed to separate fractions from EAE by using solvents from low polarity to high polarity (i.e. hexane to methanol) in varying ratios. The flow rate was adjusted to 5 ml/min and 40 ml solvent was collected for each fraction.
2.4.1 TLC of fractions
The fractions were collected separately and subjected to TLC (20 × 20 cm aluminium sheets coated with silica gel 60 F254) to detect the presence of phytocompounds. The TLC plates were sprayed with vanillin-con. H2SO4 spray (15 g of vanillin in 250 ml of ethanol + 2.5 ml of con. H2SO4) and dried at 100 °C in hot air oven for 20–30 min. The Rf value of each spot was calculated. Fractions with the same Rf values were pooled and concentrated to dryness using rotary evaporator. The dry weight of the fractions was measured. The condensed fractions and EAE were further analyzed by HPLC for the presence of antioxidant phenolic compounds.
2.5 HPLC analyses of fractions and EAE
HPLC profiles of EAE and isolated fractions of S. molesta were determined by two methods using two different mobile phases selected on the basis of varying gradations of solvent systems in specific retention times and elute detections [Citation20]. Analysis of all samples was performed using Shimadzu LC-10 AT VP, Luna 5u C18 reverse-phase analytical column (250 × 4.6 mm) with binary gradient mode, SPD-M10A VP photo diode array detector (PDA), injection volume 20 µl, total flow 1 ml/min, column oven temperature 25 °C and detection wavelength 280 nm. 55 mg of EAE and each fraction were dissolved in 3 ml of methanol for the analysis. The solvents used for the mobile phases were previously filtered through millipore and degassed prior to use. Quercetin, ascorbic acid, benzoic acid, gallic acid, vanillin, resorcinol and catechol were used as standard solutions for the quantification of phenolic compounds.
2.5.1 Method A
HPLC analyses of ascorbic acid, benzoic acid, gallic acid, vanillin, resorcinol and catechol were performed by Method A. Gradient elution of two solvents was used for the quantification of ascorbic acid, benzoic acid, gallic acid, vanillin, resorcinol and catechol: Solvent A (acetonitrile) and solvent B (0.1% phosphoric acid in water) [Citation21]. Gradient elution program was begun with 92% of solvent B and was held at this concentration for 0–35 min. This was followed by 78% of solvent B for the next 35–45 min. Total run time was 45 min.
2.5.2 Method B
HPLC analysis of quercetin was performed by Method B. Gradient elution of two solvents was used for the quantification of quercetin: Solvent A (methanol) and Solvent B (4% acetic acid) [Citation22]. Gradient elution program was begun with 100% of solvent B and was held at this concentration for 0–4 min. This was followed by 50% of solvent B for 4–10 min and then reduced to 20% of solvent B for the next 10–20 min and then increased to 50% of solvent B for the next 20–22 min. Total run time was 22 min.
3 Results
The fractions obtained from silica gel column chromatography of S. molesta EAE were tested for the detection of various phytocompounds using TLC and sprayed with vanillin-con. H2SO4 spray and dried at 100 °C in hot air oven for 20–30 min. The phytocompounds showing the same Rf values were pooled into a single fraction. The total number of active fractions obtained after pooling were as follows: The elutes 1–164 aliquots of 40 ml each in solvent systems H:EA (100:0 and 90:10) formed Fraction A; the elutes 165–375 aliquots of 40 ml each in solvent systems H:EA (80:20, 70:30 and 60:40) formed Fraction B; the elutes 376–531 aliquots of 40 ml each in solvent systems H:EA (50:50, 40:60 and 30:70) formed Fraction C; the elutes 532–583 aliquots of 40 ml each in solvent systems H:EA (20:80, 10:90 and 0:100) formed Fraction D and the elutes 584–650 aliquots of 40 ml each in solvent systems EA:MEOH (100:0, 90:10 and 80:20) formed Fraction E. The yields of the fractions obtained are shown in .
Table 1 Experimental yield of S. molesta fractions.
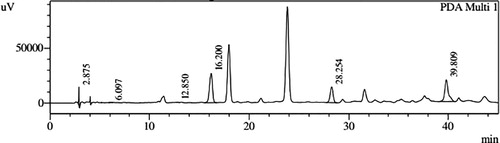
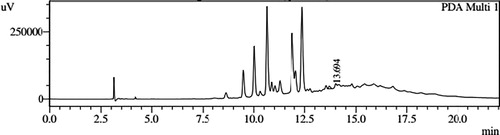
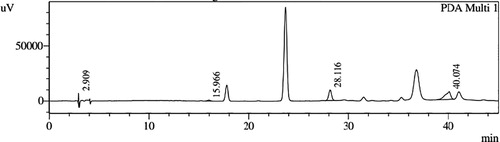
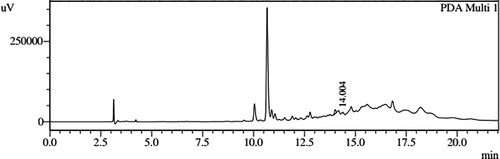
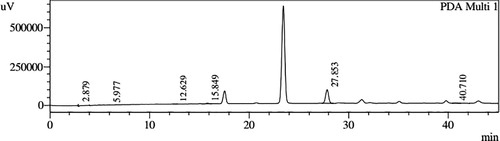
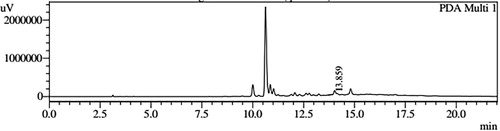
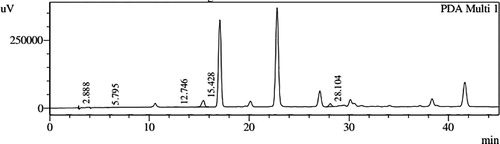
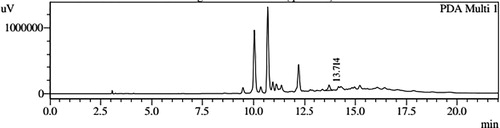
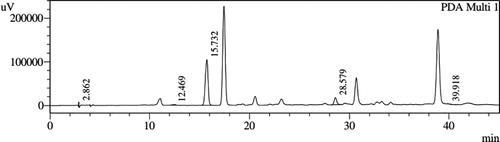
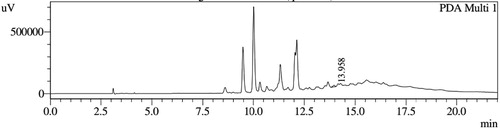
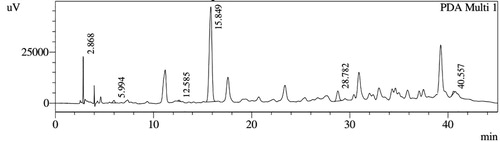
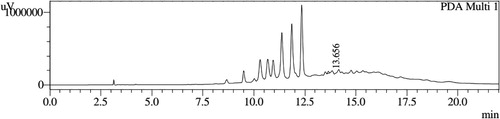
HPLC profiles of S. molesta fractions and EAE were analyzed for seven phenolic compounds viz., ascorbic acid, quercetin, gallic acid, resorcinol, catechol, vanillin and benzoic acid. Phenolic compounds present in each fraction and EAE are shown in , and with peaks showing different retention times (RT). Phenolic compounds present in EAE (Figs. 1 and 2) were vanillin (28.254 min), benzoic acid (39.809 min), quercetin (13.694 min), ascorbic acid (2.875 min), gallic acid (6.097 min), resorcinol (12.850 min) and catechol (16.200 min). Quercetin (14.0 min), ascorbic acid (2.909 min), catechol (15.966 min), vanillin (28.116 min) and benzoic acid (40.074 min) were present in Fraction A (Figs. 3 and 4). Ascorbic acid (2.879 min), quercetin (13.859 min), gallic acid (5.977 min), resorcinol (12.629 min), catechol (15.849 min), vanillin (27.853 min) and benzoic acid (40.710 min) were present in Fraction B (Figs. 5 and 6). Gallic acid (5.795 min), ascorbic acid (2.888 min), quercetin (13.714 min), resorcinol (12.746 min), catechol (15.428 min) and vanillin (28.104 min) were present in Fraction C (Figs. 7 and 8). Catechol (15.732 min), ascorbic acid (2.862 min), resorcinol (12.469 min), quercetin (13.958 min), vanillin (28.579 min) and benzoic acid (39.918 min) were present in Fraction D (Figs. 9 and 10). Resorcinol (12.585 min), catechol (15.849 min), vanillin (28.782 min), benzoic acid (40.557 min), quercetin (13.656 min), ascorbic acid (2.868 min) and gallic acid (5.994 min) were present in Fraction E (Figs. 11 and 12).
Table 2 Retention times of phenolic compounds present in EAE and Fraction A of S. molesta.
Table 3 Retention times of phenolic compounds present in Fractions B and C of S. molesta.
Table 4 Retention times of phenolic compounds present in Fractions D and E of S. molesta.
4 Discussion
A major study conducted in S. molesta by Li et al. [Citation23] using bioactivity guided fractionation of ethanol extract yielded 50 compounds, including 17 abietane diterpenes (1, 17–22), nine phenolics (2–4, 29–32, 49 and 50), five fatty acids (24–28), five triterpenes (35–39), four apocarotenoids (42–45), two acyclic sesquiterpenoids (6 and 23), two monoterpenes (5 and 46), two jasmonates (33 and 34), two steroids (40 and 41) and two coumarins (47 and 48). All the abietane diterpenes were isolated from S. molesta for the first time, and out of the 6 compounds, (1–6), salviniol (1) was a rare abietane diterpene with new ferruginol-menthol coupled skeleton and both salviniside I (2) and salviniside II (3) were novel benzofuran glucose conjugates with unique 10-membered macrodiolide structures. Another study has shown that naringinin was the major phenolic compound present in acetone: methanol (1:1) extract of S. molesta which was identified and quantified by HPLC followed by myricetin along with rutin, epicatechin, catechin, quercetin, kaempferol and vanillin. These compounds were also found to have free radical scavenging potential [Citation24].
A study by Cary and Weerts [Citation25] showed that S. molesta grew most rapidly in high concentration of phosphorous and nitrogen (2–20 mg N 1−1 and 2 mg PO4-P 1−1). Since this plant can uptake nitrogen and other minerals from the aquatic environment, it is presumed that this plant contains nutritious biomass which could serve as an alternative unconventional plant protein source. It also possesses high crude fiber, tannin, lignin, and ash content which could limit its usage in the non-ruminant animal feeding operations [Citation26]. According to Moozhiyil and Pallauf, [Citation27] the crude protein content of S. molesta is relatively high in all stages of growth (young: 32.2%; medium: 37.5% and mature: 36.8%) compared to terrestrial forages. It was also found out that lignin content was as high as 13.7% while the average crude ash was 17.3% and the crude fiber was 35.2%. According to the result of the above study the level of tannin increased as the plant matured. The present study has identified seven penolic compounds such as ascorbic acid, quercetin, gallic acid, resorcinol, catechol, vanillin and benzoic acid in S. molesta and, therefore it can be concluded that this plant is one of the plausible natural antioxidants that could be used as a lead candidate for synthesizing antioxidant drugs which can be used for the treatment of many oxidative stress related diseases.
5 Conclusion
The present study has reported the presence of phenolic compounds such as ascorbic acid, quercetin, gallic acid, resorcinol, catechol, vanillin and benzoic acid in the fractions of ethyl acetate extract of S. molesta. Ethyl acetate extract was found to possess significant pharmacological activities; hence it was fractionated using silica gel column chromatography using different solvents in varying polarity. The study has found that S. molesta, an aquatic fern has promising medicinal properties and is a potent natural antioxidant owing to the presence of a number of phenolic compounds. Therefore, further investigation is needed to purify these phenolic components to be used as lead compounds for the development of novel antioxidant drugs.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgements
This study was financially supported by University Grants Commission Maulana Azad National Fellowship Scheme (F1-17.1/2012-13/MANF-2012-13-CHR-KER-7693), Ministry of Minority Affairs, New Delhi, India. We thank Dr. Jayaraj, CIU, KFRI, Peechi, Thrissur, India, for helping us with HPLC analyses. We are immensely grateful to Prof. Cinzia Forni (Italy), Dr. Sr. Ignatius Mary (France) and Dr. T.V. Poonguzhali (Chennai) for their valuable suggestions and comments.
References
- R.RandhirY.T.LinK.ShettyPhenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitorsAsia Pac J Clin Nutr132004295307
- L.BravoPolyphenols: chemistry, dietary sources, metabolism and nutritional significanceNutr Rev561998317333
- A.M.BoudetEvolution and current status of research in phenolic compoundsPhytochem6822–24200727222735
- R.A.JacobThe integrated antioxidant systemNutr Res151995755766
- I.B.AfanasevA.I.DorozhkoA.V.BrodskiiV.A.KostyukA.I.PotapovitchChelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidationBiochem Pharmacol38198917631769
- R.AmarowiczR.B.PeggP.Rahimi-MoghaddamB.BarlJ.A.WeilFree radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairiesFood Chem842004551562
- O.Benavente-GarciaJ.CastilloF.R.MarinA.OrtunoJ.A.Del RioUses and properties of Citrus flavonoidsJ Agric Food Chem45199745054515
- C.ManachA.MazurA.ScalbertPolyphenols and prevention of cardiovascular diseasesCurr Opin Lipidol1620057784
- E.MiddletonC.KandaswamiT.C.TheoharidesThe effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancerPharmacol Rev522000673751
- R.Puupponen-PimiaL.NohynekC.MeierM.KahkonenM.HeinonenA.HopiaK.M.Oksman-CaldenteyAntimicrobial properties of phenolic compounds from berriesJ Appl Microbiol902001494507
- S.SammanP.M.Lyons WallN.C.CookFlavonoids and coronary heart disease: Dietary perspectivesC.A.Rice EvansL.PackerFlavonoids in health and disease1998Marcel DekkerNew York469482
- M.WangK.LiY.NieY.WeiX.LiAntirheumatoid arthritis activities and chemical compositions of phenolic compounds-rich fraction from Urtica atrichocaulis, an endemic plant to ChinaEvid Based Complement Altern Med20122012110
- N.BalasundramK.SundramS.SammanAnalytical, nutritional and clinical phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence and potential usesFood Chem9912006191203
- N.S.GillR.AroraS.R.KumarEvaluation of antioxidant, anti-inflammatory and analgesic potential of Luffa acutangula Roxb. var. amara. ResJ Phytochem52011201208
- H.C.ChangK.G.SushimH.S.TsayStudies on folk medicinal fern: an example of “Gu-Sui-Bu”H.FernandezA.KumarM.A.RevillaWorking with ferns: issues and applications2010SpringerNew York285293
- N.N.SospeterM.JosphatG.M.CharlesM.M.CharlesK.K.GeorgeA review of some phytochemicals commonly found in medicinal plantsPhoton Int J Med Plants1052013135140
- M.Iqbal ChoudharyNadraNaheedAhmedAbbaskhanSyed GhulamMusharrafHinaSiddiquiAtta-ur-RahmanPhenolic and other constituents of fresh water fern Salvinia molestaPhytochem69200810181023
- T.G.GiniG.Jeya JothiPreliminary phytochemical screening of whole plant extracts of Peperomia pellucida (Linn.) HBK (Piperaceae) and Marsilea quadrifolia Linn. (Marsileaceae)Int J Pharmacog Phytochem Res532013200214
- Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction technologies for medicinal plants and aromatic plants. United Nations Industrial Development Organization and the International Centre for Science and High Technology. 1st ed. Italy, No. 66, 2008.
- A.A.BoligonT.F.De BrumJ.K.FrolhichA.L.F.FroederM.L.AthaydeHPLC/DAD Profile and determination of total phenolics, flavonoids, tannins and alkaloids contents of Scutia buxifolia Reissek stem barkRes J Phytochem6320128491
- W.SameeS.VoraratSimultaneous determination of gallic acid, catechin, rutin, ellagic acid and quercetin in flower extracts of Michelia alba, Caesalpinia pulcherrima and Nelumbo nucifera by HPLCThai Pharm Health Sci J22007131137
- Y.L.LinY.L.ChenY.C.LiangJ.K.LinComposition of polyphenols in fresh tea leaves and associations of their oxygen radical absorbing capacity with anti-proliferative actions in fibroblast cellsJ Agric Food Chem44199613871394
- ShiyouLiPingWangGuangruiDengWei.YuanSuZushangCytotoxic compounds from invasive Giant Salvinia (Salvinia molesta) against human tumor cellsBioorg Med Chem Lett23201366826687
- P.ChantiratikulP.MeechaiW.NakbanpotecAntioxidant activities and phenolic contents of extracts from Salvinia molesta and Eichornia crassipesRes J Biol Sci410200911131117
- P.R.CaryP.G.J.WeertsGrowth of Salvinia molesta as affected by water temperature and nutrition. II. Effects of phosphorus levelAquat Bot1719836170
- NRC. Nutrient requirements for poultry. 9th ed., Natl. Acad. Press, Washington, DC; 1994.
- M.MoozhiyilJ.PallaufChemical composition of the water fern, Salvinia molesta, and its potential as feed source for ruminantsEcon Bot401986375383