Abstract
Medicinal plants have been used as an alternative medicine to promote human health and longevity in many regions of the world since ancient times. In recent years, many novel secondary metabolites from higher fungi have been isolated and reported to provide lead compounds for new drug discovery like antimicrobial, antioxidant, and anticancer agents, etc. A total of 42 endophytic fungi were isolated from 11 different medicinal plants collected from Tamil Nadu, India. The capability of medicinal plant endophytic fungi for the production of bioactive secondary metabolites was evaluated under in vitro conditions. The most frequently isolated fungi were Alternaria sp. Fusarium sp. and mycelia sterilia. The bioactive secondary metabolites of these endophytes were obtained by liquid-liquid extraction with ethyl acetate. Among which 15 showed antimicrobial activity towards potential human pathogens such as Escherichia coli, Salmonella typhi, Proteus mirabilis, Staphylococcus aureus, and Corynebacterium diphtheriae with variable zone of inhibition. Maximum secondary metabolite production with good mycelial growth and antimicrobial activity was observed in Alternaria sp. MGTMMP031. Moreover, in-vitro antioxidant and anticancer activity were also evaluated. The crude secondary metabolite was characterized by ultraviolet spectrum (UV), infrared spectrum (FT-IR) and thin layer chromatography (TLC) with Rf value of 0.35 and the bioactive secondary metabolite was identified as alternariol methyl ether. The results concluded that secondary metabolite from Alternaria sp. may represent a potential ingredient for pharmaceutical application and are worthy of future study.
1 Introduction
Medicinal plants have extensively being used for human welfare since ancient period and also act as a major stockpile for pharmaceuticals [Citation1]. There was a positive linear correlation between endophytes and medicinal plants, in metabolite production due to genetic recombination with the host over evolutionary time [Citation2]. Endophytic fungi which indwell the healthy living tissues without causing side effects also yield certain biologically active secondary metabolites [Citation3]. Endophytes must synthesize metabolites for the competence with co-occurring endophytes, host and pathogens in order to colonize the host, and as well as for the nutrition [Citation4]. They act as major resources for structurally unique, bioactive natural metabolites such as alkaloids, benzopyranones, benzoquinones, flavonoids, phenols, steroids, terpenoids, tetralones and xanthones, with wide potential for the exploration of novel therapy [Citation5]. They act as highly potent producers of antifungal, antiviral, antibacterial, cytotoxic and immunosuppressive metabolites. Several novel endophytic fungal derived natural products were isolated and described earlier [Citation6]. These bioactive metabolites may play a crucial role in infectious diseases therapy. It has been found that the endophytes produce significant amount of antioxidants, which prevent oxidative damage to cellular components. In the present study, a total of 42 endophytic fungi were isolated from healthy medicinal plant leaves and their secondary metabolites were obtained by solvent extraction. Chosen fungal strains were analyzed for antimicrobial activity against five different bacterial human pathogens. Based on the preliminary antimicrobial studies, the active metabolites from endophytic fungi MGTMMP031 was found to be potential towards all the tested pathogens with maximum zone of inhibition and further evaluated for antioxidant and anticancer potential. The culture conditions and nutritional factors were optimized. In addition, the potential secondary metabolite producing endophytic fungi was identified further using ITS 18S rDNA sequence and named as Alternaria sp. (MGTMMP031). The partially purified secondary metabolite was characterized by TLC, UV–VIS and FT-IR spectrum.
2 Material and methods
2.1 Isolation and identification of endophytic fungi
Healthy medicinal plant leaves were collected from botanical garden at Madurai Kamaraj University, Madurai (9°56′38.6″N, 78°00′27.1″E). The leaves were washed with running tap water for 5 min to remove the dust and debris. The leaves were cut into small pieces with the dimensions of 1 cm × 1 cm. Surface disinfection was done by sequentially dipping the leaves in 0.3% sodium hypochlorite for 30 s and in 70% ethanol for 60 s followed by rinsing them in sterile distilled water and further dried with paper towel. The leaf segments were uniformly placed on PDA (potato dextrose agar) medium supplemented with streptomycin (100 μg/ml). The plates were incubated at 25 °C for 6 to 7 days under 12 h's light-dark cycle [Citation7]. The pure fungal strains were obtained and morphologically differentiated and the spores were identified by using lacto phenol cotton blue staining [Citation8].
2.2 Selection of optimal culture medium
Selected fungal strain (MGTMMP031) was grown for 14 days in five different fungal growth medium to determine the optimal media for the growth and production of secondary metabolites. The media namely, potato dextrose broth (PDB), malt extract glucose yeast extract peptone broth (MGYP), yeast extract sucrose broth (YES) [Citation9], malt extract broth (MEB) and mineral salts broth (M1D) [Citation10]. Maximum growth and production of secondary metabolites was observed in PDB medium incubated at 26 °C for 14 days. The extracts were subjected to analyze its antibacterial, antioxidant and anticancer properties.
2.3 Fermentation
The pure fungal strains were inoculated into 500 ml of potato dextrose broth (PDB) medium in 1000 ml Erlenmeyer flask and incubated for 14 days at 26 °C for secondary metabolite production. After the incubation period, the culture was filtered using 4 layers of cheese cloth and the culture filtrate was extracted three times with the equal volume of ethyl acetate using separating funnel. Solvent phase was collected and condensed using rotary vacuum evaporator. The dry crude secondary metabolite was obtained and it was used to study antimicrobial, antioxidant and anticancer potential of the fungal extract.
2.4 Characterization of alternariol like compound
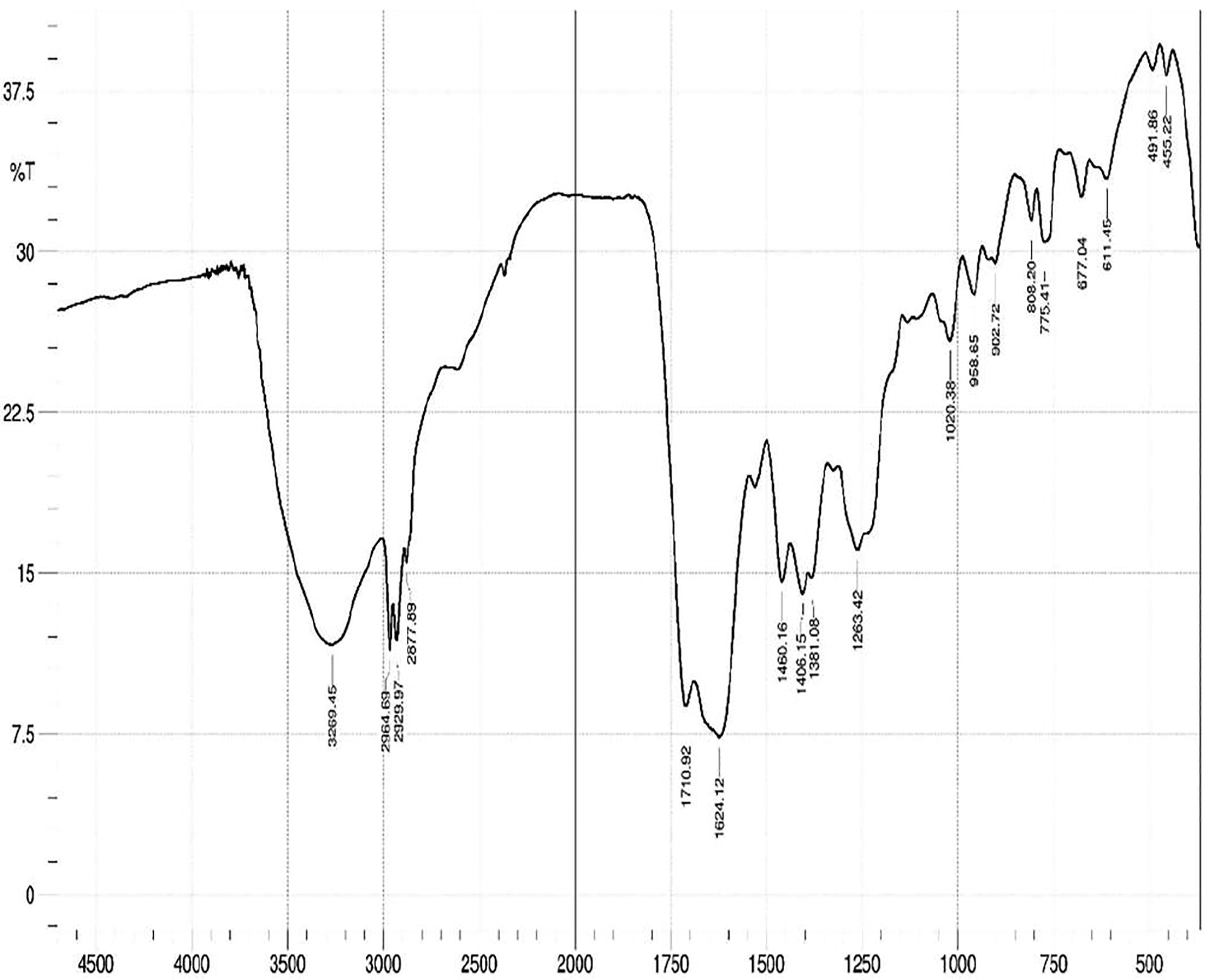
Thin layer chromatography (TLC) was carried out with hexane: ethyl acetate (2:3) solvent system in a pre-coated silica gel TLC plates of grade F274 (E-Merck, Germany) [Citation11]. The eluted plates were dried completely and the chromatograms were visualized under UV at 365 nm and the movement of the metabolites along with solvent was measured (Rf value). The metabolites were dissolved in ethyl acetate and subjected to UV–VIS (200 nm to 700 nm) spectral analysis using Shimadzu UV-1800 series. Fourier transform-infrared spectrum was recorded in the range of 400–4500 cm−1 in dry ethyl acetate using Shimadzu FTIR 8400 series.
2.5 Assessment of antibacterial activity of fungal extracts
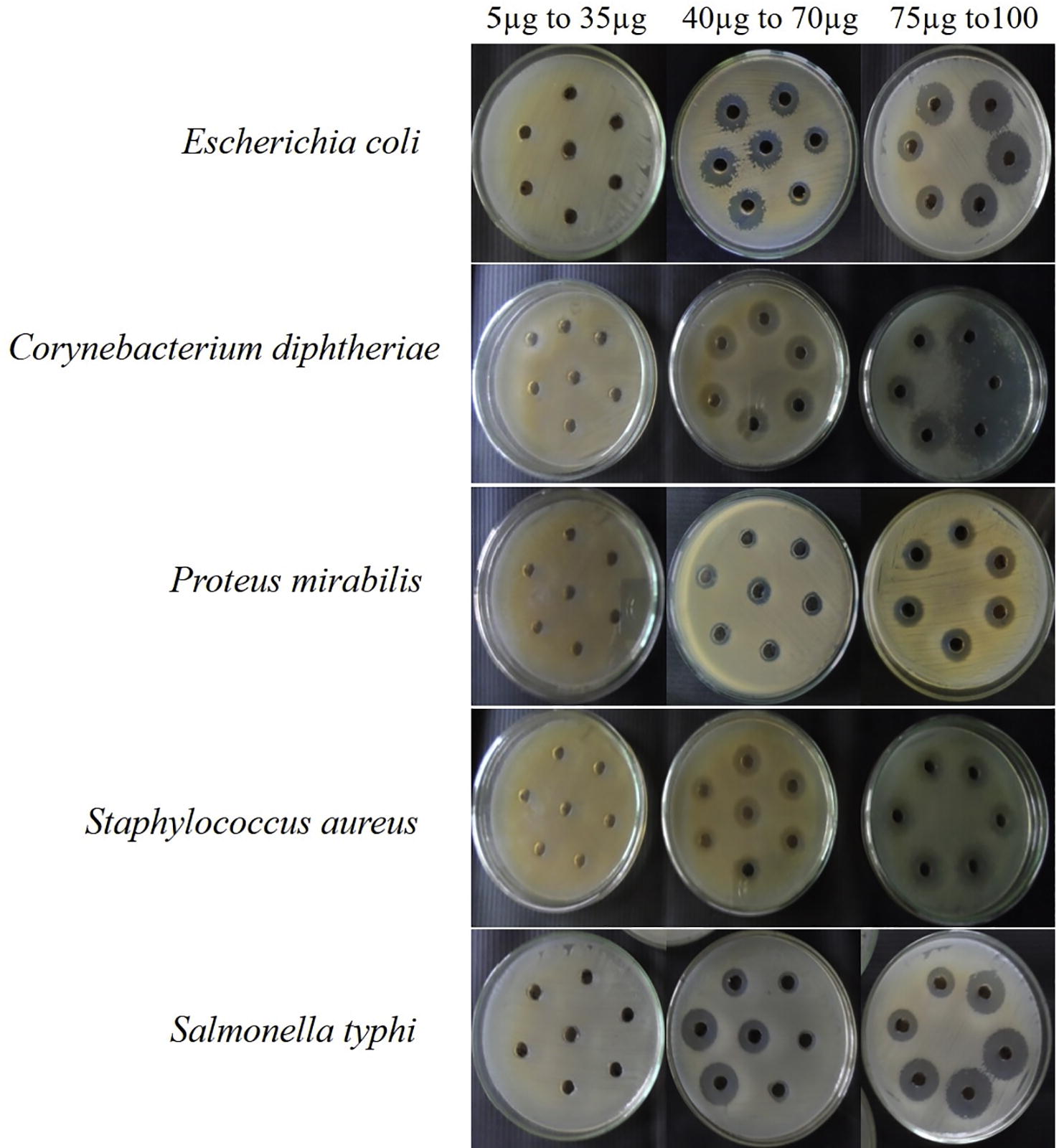
The antibacterial activity of the crude fungal extracts was evaluated by agar well diffusion method [Citation12] against the following human pathogens such as Escherichia coli, Salmonella typhi, Proteus mirabilis, Staphylococcus aureus and Corynebacterium diphtheriae. All the human pathogens were obtained from Apollo hospital Madurai. Streptomycin was used as a positive control and ethyl acetate was used as negative control. Plates were incubated at 37 °C for 24 h. Minimum inhibitory concentrations of the crude fungal extract against individual pathogens were determined and tabulated.
2.6 Estimation of total phenolic and flavonoid content
TPC of the extracts were determined by modified Folin-Ciocalteau method [Citation13]. 0.1 ml of the extract was made up to 3.5 ml with distilled water. 125 μl of Folin’s phenol reagent was added to every tube. After 3 min interval, 1 ml of 7% sodium carbonate (Na2CO3) was added. The mixture was kept for 90 min at room temperature in the dark. The absorbance was measured at 760 nm against the prepared blank. The total phenolic content is expressed as µg of rutin equivalents (RE) per 100 µg of dry extract. TFC of the crude extracts were analyzed by modified aluminium chloride (AlCl3·H2O) method [Citation14]. 0.1 ml of extract was diluted to 2.5 ml with distilled water. 75 μl of 5% sodium nitrite solution were added to each tube. After 5 min incubation, 150 μl of 10% AlCl3·H2O was added and mixed well. After 6 min of interval, 0.5 ml of 1 M sodium hydroxide was added. The absorbance was measured against the blank at 510 nm. Rutin was used as a standard and the results were expressed as µg of rutin equivalents (RE) per 100 µg of dry extract.
2.7 Estimation of in vitro antioxidant property
2.7.1 DPPH radical scavenging activity
The scavenging effect of extracts on DPPH free radicals was determined by the method reported by Shimada et al. [Citation15]. 0.1 ml of extracts was diluted with 1.5 ml of methanol. Tubes were added with 1.5 ml of methanolic solution containing 0.2 mM DPPH radicals. The solution was mixed completely and kept for 20 min at dark. The absorbance was measured at 517 nm. Rutin was used as positive control.
2.7.2 Superoxide radical scavenging activity
The ability of fungal extracts to scavenge superoxide anion was determined through the spectrophotometric measurement of nitro blue tetrazolium reduction [Citation16]. Superoxide anion was generated by a non-enzymatic system. 0.1 ml of the extracts was mixed with equal volume of 60 µM phenazine methosulphate, 468 µM nicotinamide adenine dinucleotide, and 150 µM nitro blue tetrazolium in 0.1 M sodium phosphate buffer pH 7.4. The reaction mixture was incubated at room temperature for 5 min, and the absorbance was read at 560 nm against blank. Rutin was used as positive control.
2.7.3 Hydroxyl radical scavenging activity
Hydroxyl radical scavenging property of the crude extracts was analyzed based on the modified method by Klein et al. [Citation17]. 0.1 ml of crude extracts were taken and made up to 1 ml with methanol. To this 1 ml of iron-EDTA solution (0.13% ferrous ammonium sulphate and 0.26% EDTA), 0.5 ml of 0.018% EDTA, 1 ml of 0.85% (V/V) dimethyl sulfoxide in 0.1 M sodium phosphate buffer pH 7.4. The reaction was initiated by adding 0.5 ml of 0.22% ascorbic acid. The tubes were closed and heated in a water bath at 90 °C for 1 min. The reaction was terminated by adding 1 ml of 17.5% ice-cold trichloroacetic acid. Three ml of NASH reagent (75 g ammonium acetate, 3 ml glacial acetic acid and 2 ml acetyl acetone were mixed and water was added to a total volume of 1L) was added to each tube. The tubes were incubated at room temperature for 15 min. The intensity of the yellow color formed was measured at 412 nm against blank. Rutin was used as positive control.
2.7.4 Nitric oxide radical scavenging activity
The radical scavenging efficiency of crude extracts on nitric oxide radicals was evaluated by Griess reaction [Citation18]. 0.1 ml of crude extracts were made up to 0.5 ml with methanol and mixed with 1.5 ml of 10 mM sodium nitroprusside in phosphate buffer pH 7.4. The reaction mixture was incubated at 25 °C for 30 min. After incubation, 0.5 ml of aliquot was removed and mixed with 0.5 ml of Griess reagent (1% sulphanilamide (w/v), 2% orthophosphoric acid (v/v) and 0.1% naphthyl ethylenediamine dihydrochloride). The absorbance was measured at 546 nm. Rutin was used as reference standard and was treated the same way as that of crude extracts. Sodium nitroprusside in phosphate buffer (2 ml) was used as control.
2.7.5 Reducing power assay
The reducing power of the crude extracts was determined according to the method of Oyaizu, [Citation19]. 0.1 ml of extracts were mixed with 0.25 ml of 0.2 M sodium phosphate buffer (pH 6.6). To this, 0.25 ml of 1% potassium ferricyanide was added and the mixture was incubated at 50 °C for 20 min. The reaction was terminated by adding 0.25 ml of 10% trichloroacetic acid (w/v) and the mixture was centrifuged at 5000 rpm for 10 min. The upper layer (0.5 ml) was mixed with 0.5 ml of deionized water and 1 ml of 0.1% of ferric chloride. The absorbance was measured at 700 nm.
2.8 Evaluation of anti-proliferative property
The Vero cell line, MCF-7, HUH-7 and A549 cell line were grown in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 µg/ml of streptomycin. All cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. After attaining confluency, the cells were trypsinized and the number of viable cells was counted with a hemocytometer to prepare a cell suspension [Citation20].
1 ml of MEM was added to the dissolved fungal extract. The dissolved extracts were filtered through Whatman syringe filter (0.2 µm). The fungal extracts were made up into 1 ml stock. 100 µl of fugal extract was added in each well of 96-well microtiter plate and incubated at 37 °C and 5% CO2. The cells were observed after 24 h, 48 h and 72 h. After 72 h incubation, each well was treated with 20 µl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in PBS and the plate was incubated for 4 h at 37 °C and 5% CO2. Formazan crystals were formed and dissolved in 100 µl of DMSO. The cell viability was measured using an ELISA reader at 620 nm [Citation21].
2.9 Genomic DNA Isolation, ITS region amplification and phylogenetic analysis
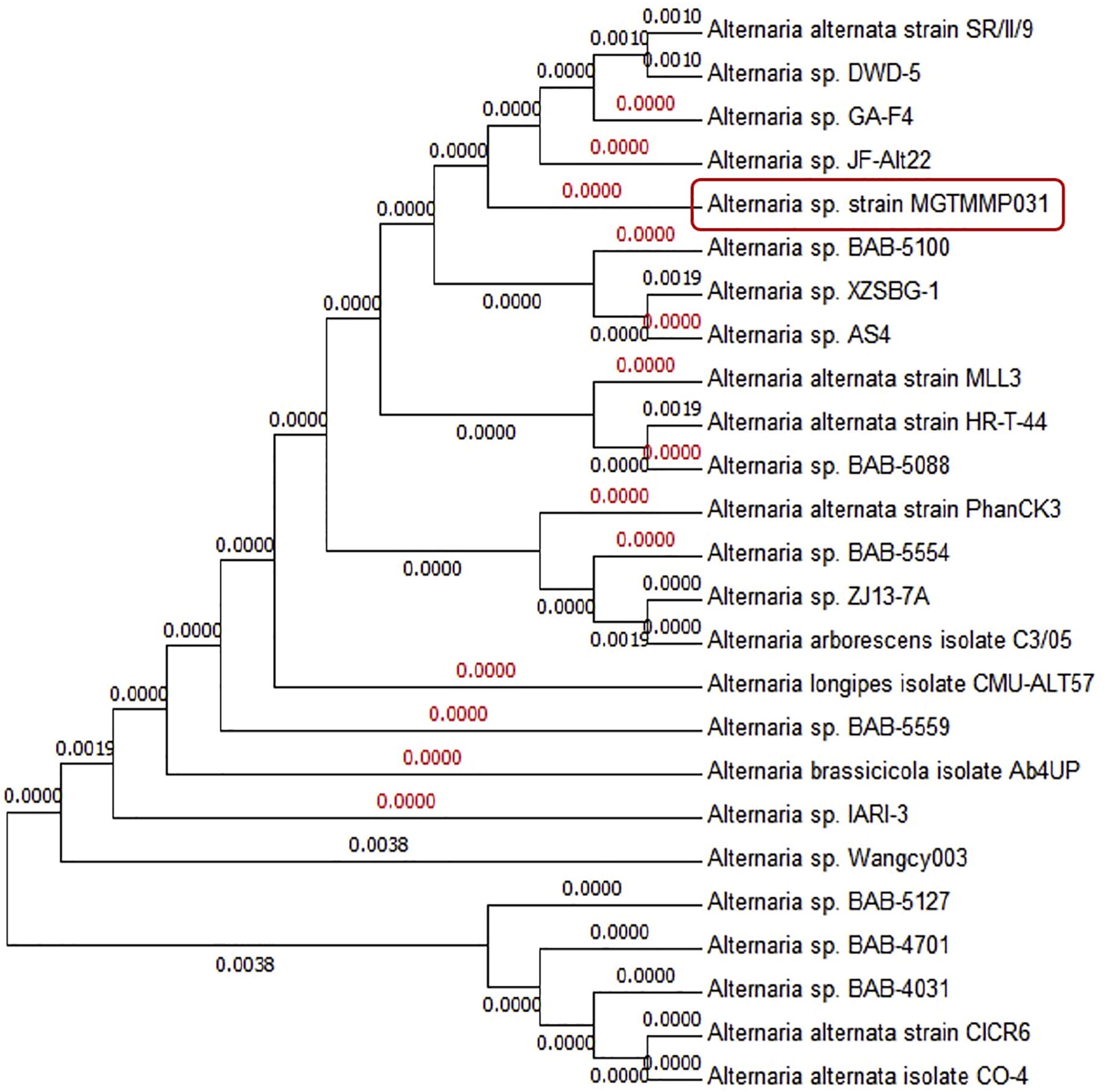
Fungal genomic DNA was isolated using STE buffer comprises of 10 mM Tris pH 8, 320 mM sucrose, 20 mM EDTA, 75 mM NaCl, 20% SDS, 5 mg of polyvinyl pyrrollidone for 50 ml [Citation22]. ITS region was amplified using specific universal primer ITS1 and ITS4 primers F (ITS1 – 5′ TCCGTAGGTGAACCTGCGG 3′), R (ITS4 – 5′ TCCTCCGCTTATTGATATGC 3′). Amplicon was sequenced and the sequences were compared with available sequences in the NCBI database by BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). High scored sequences were clustered using Molecular Evolutionary Genetics Analysis (MEGA 6.06). Sequences were aligned by ClustalW inbuilt in the MEGA 6.06 and the phylogenetic tree was constructed by neighbor joining method.
2.10 Statistics and data analysis
All experiments were carried out in triplicate to check for reproducibility. All the statistical data of this paper are the average of triplicate determinations ± SD.
3 Results
3.1 Isolation and identification of endophytic fungi
A total of 48 fungal strains were isolated from the medicinal plants and they exhibited characteristic colony and microscopic morphology that could be used to differentiate amongst the isolates. The high numbers of endophytic fungi (6 strains) were isolated from the plant Pleurostylia opposita. Out of the 48 primary fungal isolates, 42 morphologically dissimilar isolates were selected for biological activity screening. All the isolates were recognized based on spore morphology. Nearly half of the fungal strains (50% relative frequency) lacking sporulation were grouped together as mycelia sterilia (). Apart from them, MGTMMP002, MGTMMP017, MGTMMP039, MGTMMP042, MGTMMP013, MGTMMP031, MGTMMP033, MGTMMP001, MGTMMP010 and MGTMMP021 were found as Fusarium sp with 4 isolates (9.5% relative isolation frequency), Alternaria sp with 3 isolates and Phoma sp with 3 isolates (7.14%) respectively.
Table 1 Morphological identification of endophytic fungi from medicinal plants (L).
3.2 Assessment of antibacterial activity of fungal extracts
After fermentation with 14 days a total of 42 mycelium free culture supernatants were harvested and extracted with ethyl acetate. The extracts obtained were assessed for antibacterial activity by agar well diffusion method. Streptomycin was employed as a reference standard to evaluate the efficiency of fungal compounds. Antibacterial activity screening of secondary metabolites from selected 42 fungi that inhibited at least one of the bacteria studied, presenting MIC between 0.2 and 1.0 mg/ml were represented in . The crude extracts of fungus from Vitex negundo, Ocimum basilicum and Justicia gendarussa inhibited all the five pathogenic bacterial species. Differences were observed between strains from the same species with respect to their capability to yield active metabolites, as observed for the isolates MGTMMP008, MGTMMP021, MGTMMP022, MGTMMP033, and MGTMMP037 which showed broad spectrum of anti-bacterial activity against the tested pathogens. On the other hand, the extract from the strain MGTMMP006 was highly specific and showed antibacterial activity only against S. aureus. The fungal extract from strain MGTMMP031 resides in the plant Vitex negundo inhibited both Gram positive and Gram negative pathogens with superior activity ().
Table 2 Antibacterial activity of crude extracts from endophytic fungal isolates against human bacterial pathogens.
3.3 Molecular taxonomic characterization and phylogenetic analysis
Molecular and phylogenetic analysis was used for the taxonomical characterization of the potential secondary metabolite producing fungi. Using the ITS1 and ITS4 specific primers, the PCR product of 550 bp amplicon size was obtained. ITS1, ITS4 partial sequences of the isolate was matched to ITS sequences of organisms denoted in the Genbank. The highest score sequences were retrieved from Genbank, aligned, distance matrices were calculated and the phylogenetic tree was constructed (). Strain MGTMMP031 showed ITS sequence similarity with Alternaria species. The ITS sequence was submitted to the Genbank data base with the accession number KX198131.
3.4 Characterization of crude compound
The fungal extract was examined by chromatographic (TLC) and spectroscopic analyses (UV–VIS and FTIR). The solvent system of ethyl acetate and hexane in the ratio of 2:3 was used to separate the compounds in the TLC plate. Visualization of bands was done by UV illumination and the Rf values were found to be 0.83, 0.77, 0.66, 0.5, 0.35 (). The UV spectrum of crude extracts showed ʎ max at 224–342 nm. FT-IR spectrum of ethyl acetate extracts exhibited absorption at 3265 cm−1 which indicated acidic hydroxyl groups and the absorption in 1624 cm−1 which indicated carbonyl group and Also absorption observed at 1460 and 1263 cm−1, which indicated alkane and amine group stretching ().
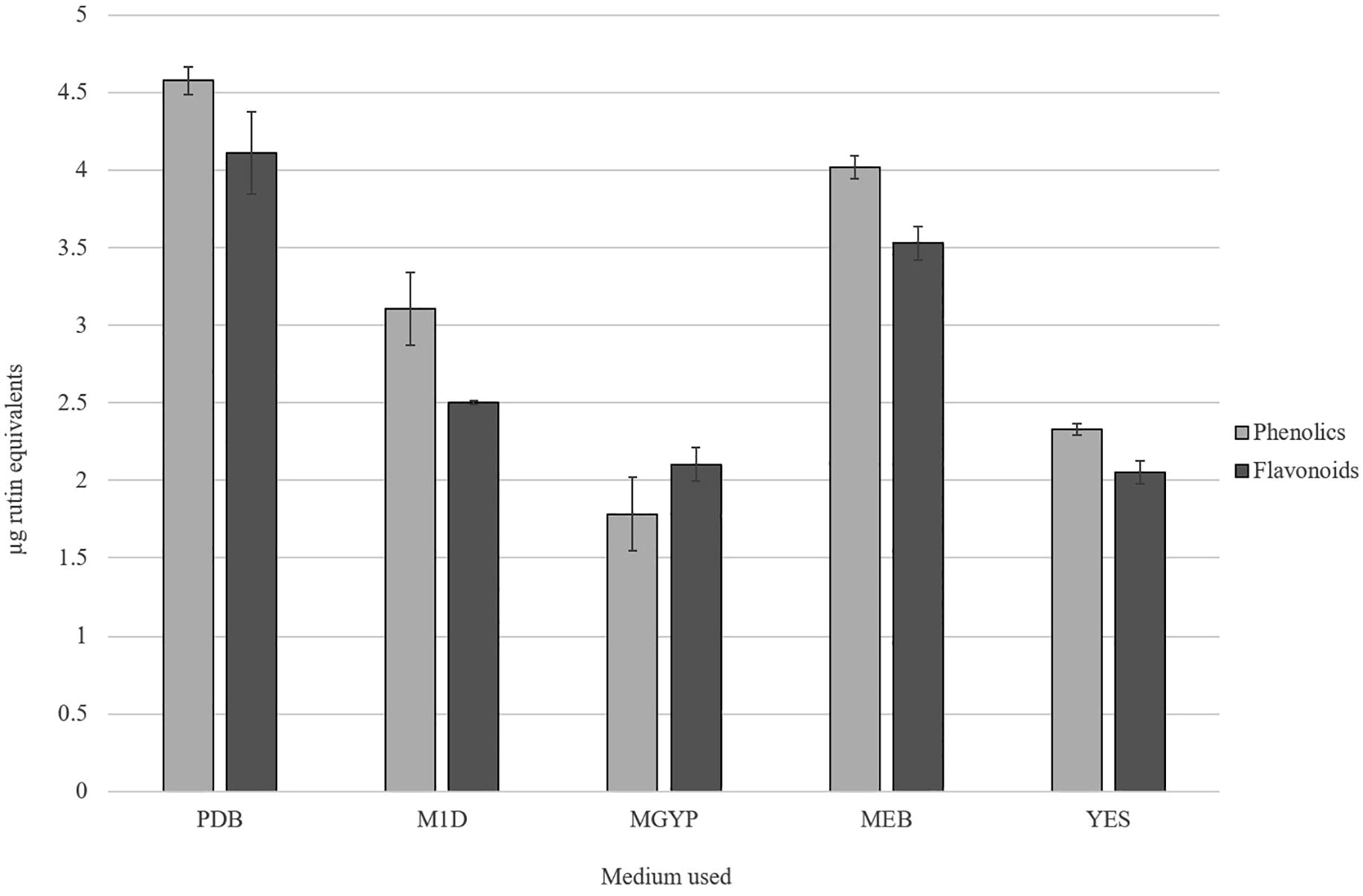
3.5 Total phenolic content (TPC) and total flavonoid content (TFC)
It was known that phenolic and flavonoid compounds are potential free radical-scavengers; hence, there is a close correlation was observed between the amount of phytochemical compounds, its antioxidant activity and lag in cancer progression. In the present study, the TPC and TFC of ethyl acetate extract from MGTMMP031 grown on five different fungal growth medium were investigated (). The TPC of the crude extracts varied from 1.781 to 4.577 µg rutin equivalents (RE) in 100 µg of extract. The extract with the highest TPC was observed in PDB extract (4.577 µg RE), followed by extract from MEB (4.021 µg RE). The TFC of the extracts ranged from 2.051 to 4.111 µg RE in 100 µg of extract. The PDB extract has the highest TFC (4.111 µg RE), followed by MEB extract (3.529 µg RE). It indicates that the fungal growth media have influence on secretion of phytochemicals in the fungi MGTMMP031. Among the extracts MGYP broth extract has very low TPC and YES broth has significantly low TFC.
3.6 Evaluation of in vitro antioxidant property
In the present study, ethyl acetate extracts from MGTMMP031 grown on five different fungal growth medium was investigated for the DPPH radical scavenging property (). The scavenging property of the extracts range from 13.818% to 66.162% inhibition on radical formation. The extract from PDB exerted the highest radical scavenging property (66.162%) and YES broth extract exhibited comparatively low DPPH radical scavenging property.
Fig. 6 Antioxidant activity of crude extracts from MGTMMP031 in five different medium. Activity was represented as percentage activity. Error bar indicates mean ± standard deviation.
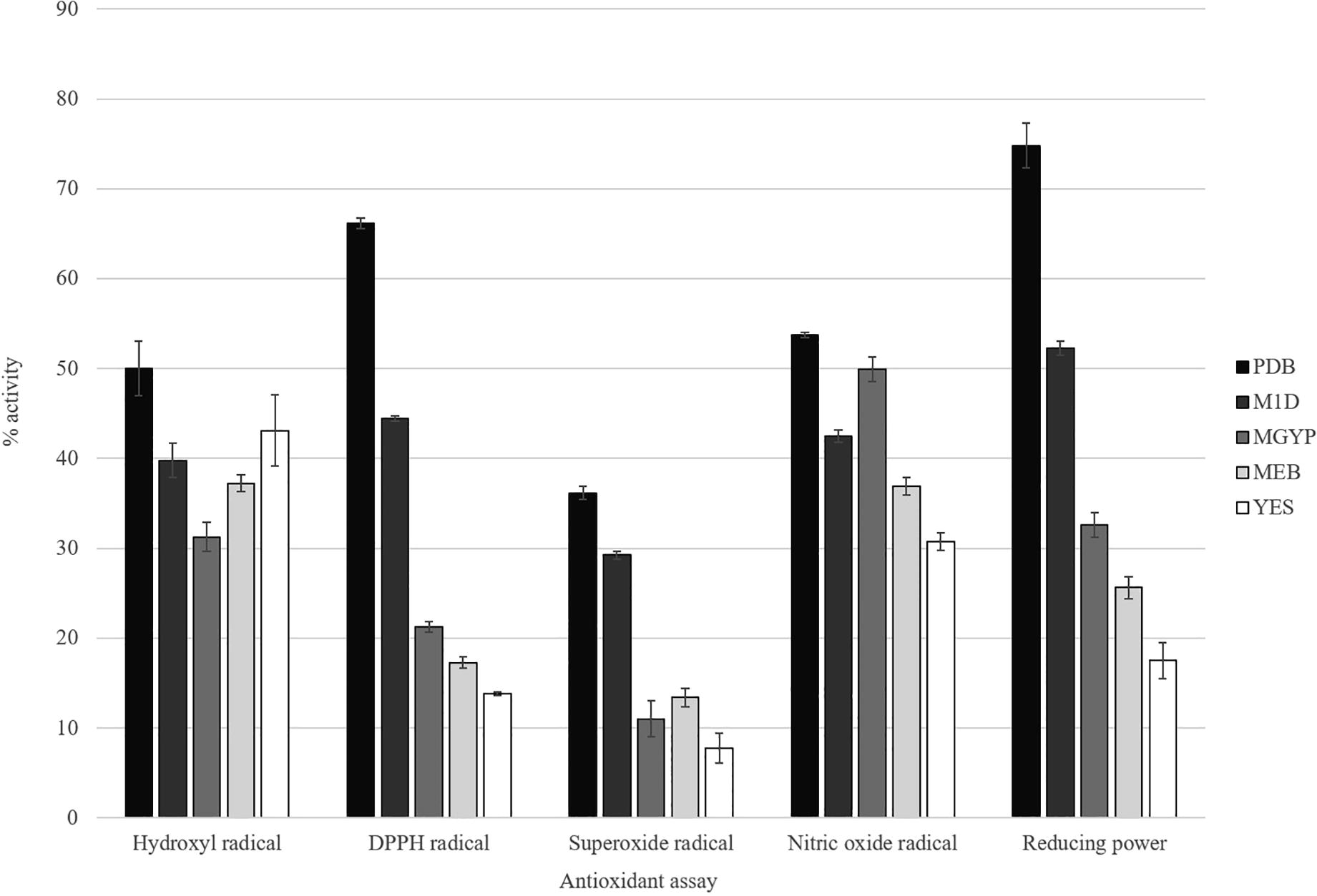
The superoxide radical is a powerful oxidizing agent that can react with biological membranes and induce tissue damage. It also acts as precursor to form singlet oxygen, hydroxyl radical, or hydrogen peroxide. In the present study, extracts were investigated for superoxide radical scavenging activity. The PDB extract exhibited highest radical scavenging activity (36.158%) followed by M1D broth (29.237%). The inhibition percentage values range between 7.768% and 36.158%. The YES broth showed lowest superoxide radical scavenging activity.
Hydroxyl radicals are the major active oxygen species that cause lipid peroxidation and enormous biological damage. The percentage of hydroxyl scavenging activity ranges from 31.262% to 50%. The extract from PDB (50%) exhibits higher radical scavenging activity followed by YES broth (43.085%). The extract from MGYP broth exhibits comparatively low hydroxyl radical scavenging activity.
In addition to reactive oxygen species, nitric oxide is also implicated in inflammation, cancer and other pathological conditions. In this study, extracts were subjected to compete with oxygen leading to reduce production of nitric oxide that can be measured by Greiss reagent. The extracts showed scavenging activity between 30.78% and 53.752%. The nitric oxide radical scavenging activity of the extracts decreased in the order of PDB > MGYP broth > M1D broth > MEB > YES broth.
In reducing power assay, the antioxidants present in the extracts reduced the ferric ions (Fe3+) into ferrous ions (Fe2+) by donating an electron. The amount of Fe2+ present can be monitored by measuring the formation of Prussian blue colour at 700 nm. Increasing absorbance at 700 nm indicates an increase in reducing ability. Reducing power of the extracts range from 17.5% to 74.81%. The PDB extract (74.81%) showed higher reducing power followed by M1D broth (52.3%) and the extract from YES broth exhibited comparatively low reducing power (17.5%).
3.7 Anti-proliferative effect of secondary metabolite against cancer cell lines
Cytotoxicity effect of fungal crude metabolite was evaluated against cancer cell lines by MTT assay. All the tested malignant cell lines showed significant response towards the fungal extract. The metabolites showed the strongest anti-proliferative activity against HUH-7 cell line, with the concentration of 75 µg/ml. In addition A549 and MCF-7 cell lines were treated with extracts and the changes in growth and morphology were observed by microscopy. The IC 50 values were calculated for all the strains. HUH-7 cells had 75 μg/ml. In the case of A549 it was found to be 50 μg/ml. MCF-7 breast cancer cells attained 50% cell death with the presence of 100 μg/ml of secondary metabolite. But the normal Vero cells have no selective toxicity against the extract up to 200 μg/ml concentration. These result specified selectivity of secondary metabolites towards abnormally proliferating cancer cells.
4 Discussion
All the 11 species of collected vascular plants were classified into 11 different families and found to have dissimilar endophytic fungi with different colonization rate. This is dependable with previous reports. The richness and diversity of the endophytic fungal strains associated with the medicinal plants is reliable with the other studies on isolation of endophytes from medicinal plants [Citation23,Citation24] . The colonization frequencies of endophytes were different among medicinal plants. The commonest fungus found in all the collected medicinal plants are non-spore producers comes under Mycelia sterilia which includes plant pathogens and endophytes. All the isolated fungal species effectively inhibits at least one of the bacterial pathogen. Secondary metabolite from MGTMMP008 which belongs to Mycelia sterilia showed moderate anti-pathogenic potential against E. coli, C. diphtheriae and P. mirabilis. The differences in the proportion of activities vary from the various metabolites, whereas the superior antibacterial activity was found in MGTMMP031 among the isolates. As a fact, the use of the phenotypic characteristics and spore morphology is the principal key to identify the fungus. The gross of the obtained colony characters, physiology as well as spore consistency clearly confirmed that the endophytic fungal isolate under study (MGTMMP031) is belonging to the genus Alternaria sp. This preliminary identification is supported by sequence analysis of the ITS region of selected strain. This inhibition is also likely being due to the production of bioactive metabolite rather than the competition for nutrients because of antibacterial compounds produced in the culture filtrate. So the treatment with the mycelia-free culture filtrate suppressed the bacterial growth [Citation5] recently proved that the diversity of metabolites isolated from endophytic fungi were synthesized via various metabolic pathways [Citation25] like polyketide and isoprenoid synthesis. Most of the Alternaria sp produces several biologically active secondary metabolites such as Altersetin, Equisetin, Hexahydroaltersetin, Dihydroaltersetin, Deprenylzinnimide, Zinnimide, Cichorine, HomodestruxinB, DesmethyldestruxinB, Alternariol, Alternariol 5-O-sulfate, and Macrosporin [Citation26]. The isolated secondary metabolite showed similar characteristic features with the alternariol methyl ether like compound with various characteristic analyses. The Rf value of 0.35 and 0.75 using ethyl acetate and hexane as a solvent system displayed a greater similarity with alternariol. Further the presence of alternariol in secondary metabolite was confirmed by UV, IR spectral data with published data for alternariol methyl ether (AME) previously reported from several Alternaria sp. It displayed UV absorbances at ʎ max 224, 256, 286, 332 and 340 nm having the typical pattern of alternariol derivatives. The IR spectrum showed absorption bands at 3269, 2964, 1710 and 1624 cm−1 revealed striking similarity with AME. Based on this observation, the active compound from crude secondary metabolite of Alternaria sp was identified as AME. Antioxidants are thought to be highly profitable in the management of free radical-mediated damages of biological systems [Citation27]. Naturally occurring phenolic phytochemicals have been reported to possess several biological properties in vitro like anti-inflammatory, antitumor, anti-mutagenic, anti-carcinogenic, antimicrobial and antiviral activities to a greater extent [Citation2]. Polyphenolic compounds are important for stabilizing lipid peroxidation [Citation28] and may directly contribute to its antioxidant property [Citation29]. The anti-oxidative property could be described by different mechanisms like ROS scavenging, inhibition of free radical generation, electron donation and metal chelation exerted by phenolic compounds and its synergistic activity with different free radical quenchers. Some of the compounds isolated from endophytic fungi were reported to be a potential antioxidant [Citation30]. The influence of five different fungal growth media on the production of antioxidants was analyzed. The result shows that PDB has significantly increased the production of phenolics and flavonoids. The relative high contents of phenols and flavonoids might explain the increased antioxidant activity exerted by PDB extracts. When comparing the other growth media, MGYP broth has the low phenolic content and YES broth has the low flavonoid content. The reduced phenolic content has significantly reduced the hydroxyl radical scavenging activity. The reduction in the amount of flavonoids in the YES broth influences the reducing power of the extract. It also reduces the antioxidant activity of the extract against DPPH, superoxide and nitric oxide radicals. The results provide support for the hypothesis that the presence of phenols and flavonoids are also responsible for the total antioxidant capacity and lag in cancer progression [Citation5,Citation30] . Several species of medicinal plants and their endophytic fungi have been found to be the sources of metabolites with anticancer and immune-stimulant activities. Antitumor and cytotoxic properties of these species belong to four structural types of secondary metabolites: polyketides, terpenes, nitrogen containing compounds and polysaccharides [Citation30]. Extract from PDB culture filtrate was used to analyze the anti-proliferative property against cancer cell lines in vitro. The extract influences the growth of cancer cells (A549, HUH-7 and MCF-7) in vitro but not the normal cells (Vero). Therefore, it may have potential as an anticancer agent. Our study demonstrates the fungal extract has antibacterial, anti-oxidant and anti-proliferative properties. However, further study is needed to determine whether the inhibitory effect of fungal extract on the growth of the cancer cells is due to the inhibition of cell proliferation or the induction of cell death. The mechanism behind the anti-proliferative effects of purified metabolite need to be studied. It has also been suggested that an in vivo study should be carried out in conjunction with the in vitro study, so that the results can be compared and more information regarding the anti-proliferative properties of the purified metabolite become clear.
5 Conclusion
The results of this study suggests that extract of these entophytic fungal sps could be used as an anti-oxidant to reduce the free radicals and also applied as a potential active biomolecule against disease causing pathogens. In future the active molecule (AME like compound) present in the secondary metabolite which is responsible for bioactivity will be purified and characterized.
Acknowledgement
Authors gratefully acknowledge the DBT – IPLS-INDIA, UGC – NRCBS-INDIA and CEGS for providing necessary instrumentation support.
Conflict of interest
The authors have no conflict of interest to declare.
References
- O.AkereleV.HeywoodH.SyngeConservation of medicinal plants1991Cambridge University Press Ltd.Cambridge, UK
- Y.CaiQ.LuoM.SunH.CorkeAntioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancerLife Sci7417200421572184
- B.SchulzC.BoyleS.DraegerA.K.RömmertK.KrohnEndophytic fungi: a source of novel biologically active secondary metabolitesMycol Res106920029961004
- K.ClayFungal endophytes of grasses: a defensive mutualism between plants and fungiEcology69119881016
- R.X.TanW.X.ZouEndophytes: a rich source of functional metabolitesNat Prod Rep1842001448459
- S.WiyakruttaN.SriubolmasW.PanphutN.ThongonK.DanwisetkanjanaN.Ruangrungsiet al.Endophytic fungi with anti-microbial, anti-cancer and anti-malarial activities isolated from Thai medicinal plantsWorld J Microbiol Biotechnol2032004265272
- T.S.SuryanarayananV.KumaresanJ.A.JohnsonFoliar fungal endophytes from two species of the mangrove RhizophoraCan J Microbiol4410199810031006
- H.L.BarnettB.B.HunterIllustrated genera of imperfect fungi1972Burgess Publishing Co.Broken Arrow, OK, USA
- R.R.M.PatersonP.D.BridgeBiochemical techniques for filamentous fungiIMI Technical Handbooks: No11994International Mycological InstituteU.K.
- F.PinkertonG.StrobelSerinol as an activator of toxin production in attenuated cultures of Helminthosporium sacchariProc Natl Acad Sci7311197640074011
- J.LouR.YuX.WangZ.MaoL.FuY.Liuet al.Alternariol 9-methyl ether from the endophytic fungus Alternaria sp. Samif01 and its bioactivitiesBraz J Microbiol471201696101
- C.PerezM.PauliP.BeziqueAn antibiotic assay by the agar well diffusion methodActa Biol Med Exp1511990113115
- V.L.SingletonJ.A.RossiColorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagentsAm J Enol Vitic1631965144158
- J.ZhishenT.MengchengW.JianmingThe determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicalsFood Chem6441999555559
- K.ShimadaK.FujikawaK.YaharaT.NakamuraAntioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsionJ Agric Food Chem4061992945948
- M.NishikimiN.A.RaoK.YagiThe occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygenBiochem Biophys Res Commun4621972849854
- S.M.KleinG.CohenA.I.CederbaumProduction of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systemsBiochem2021198160066012
- B.SangameswaranB.R.BalakrishnanDeshrajChumbhaleB.JayakarIn vitro antioxidant activity of roots of Thespesia lampas Dalz and GibsPak J Pharm Sci2242009368372
- M.OyaizuStudies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamineJpn J Nutr Diet4461986307315
- R.I.FreshneyCulture of animal cells: a manual of basic technique. Chapter 2: Biology of cultured cells2000Willey-Liss. IncNew York915
- Z.JubriN.N.N.NarayananN.A.KarimW.Z.W.NgahAntiproliferative activity and apoptosis induction by gelam honey on liver cancer cell lineInt J Appl Sci Technol242012135141
- U.RaederP.BrodaRapid preparation of DNA from filamentous fungiLett Appl Microbiol1119851720
- S.PhongpaichitN.RungjindamaiV.RukachaisirikulJ.SakayarojAntimicrobial activity in cultures of endophytic fungi isolated from Garcinia speciesFEMS Immunol Med Microbiol4832006367372
- M.L.VieiraA.F.HughesV.B.GilA.B.VazT.M.AlvesC.L.Zaniet al.Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae)Can J Microbiol58120115466
- J.S.TkaczPolyketide and peptide products of endophytic fungi: variations on two biosynthetic themes of secondary metabolismMicrobial Endophytes2000263294
- J.LouL.FuY.PengL.ZhouMetabolites from Alternaria fungi and their bioactivitiesMolecules185201358915935
- W.Y.HuangY.Z.CaiK.D.HydeH.CorkeM.SunEndophytic fungi from Nerium oleander L (Apocynaceae): main constituents and antioxidant activityWorld J Microbiol Biotechnol239200712531263
- G.C.YenP.D.DuhC.L.TsaiRelationship between antioxidant activity and maturity of peanut hullsJ Agric Food Chem41119936770
- P.D.DuhP.C.DuG.C.YenAction of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damageFood Chem Toxicol3711199910551061
- J.K.HarperA.M.ArifE.J.FordG.A.StrobelJ.A.PorcoD.P.Tomeret al.Pestacin: a 1, 3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activitiesTetrahedron5914200324712476