?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Bananas are globally used trade crops for nutrition and traditional medicine. The following research aims to study different polarity banana extracts from local and imported ripe bananas and determine the amount of phenols and flavonoids contents, and antioxidant potential by applying various spectroscopic methods. The banana extracts were prepared from the powdered banana samples of Pilipino (Cavendish) and Salalah ripe banana (Somali) by the maceration method with methanol. The methanol extracts were suspended separately in water and successively partitioned by using solvents of different polarity, namely n-hexane, chloroform, ethyl acetate, n-butanol, methanol and water in an increasing polarity pattern. The amount of total phenols and flavonoids contents and antioxidant potential were determined by using the Folin-Ciocalteu reagent, aluminum chloride and diphenyl-picrylhydrazyl methods. The maximum amount of total phenols in the Pilipino ripe banana was detected in ethyl acetate extract, and the minimum was in the n-butanol extract, but in the local Salalah ripe banana the maximum amount of phenols content was obtained in methanol extracts, and the minimum was in n-butanol extract.
Similarly, the maximum total flavonoids content in the Pilipino banana was obtained in chloroform extract and the minimum in n-hexane extract with a similar order for total flavonoids content in case of the Salalah ripe banana. Both types of ripe banana crude extracts showed significant antioxidant potential, with the maximum potential in chloroform and n-hexane extracts and the minimum in ethyl acetate crude extract. Therefore, the maximum potential extract of the bananas could be used as natural antioxidants.
1 Introduction
Antioxidants are an essential component which plays a vital role in maintaining good health. There is a growing interest amongst scientific researchers to further investigate and isolate the secondary metabolites compounds from a variety of plant sources with biological activities. Those isolated compounds are with higher potency and lower toxicities than synthetics that are currently available. Plants and other forms of vegetation have a broad range of bio potential due to the structural diversities that each species and variety of plants constituent. Many medicinal plants are known as viable sources of natural antioxidants; which mainly depend on various biologically active compounds [Citation1]. The isolation and characterization of individual active compounds are mandatory in the plant sources for improving the scientific extraction procedures, evaluating different biological potential and level of toxicity. Generally, plants contain several biologically active compounds such as pentacyclic triterpenes, steroidal saponins, flavonoids and derivatives of phenolic compounds [Citation2]. The therapeutic potential of plants or related products is associated with different biological effects brought on by the synergistic combination of various groups of chemical compounds rather than isolated single mixtures [Citation2]. Polyphenolic compounds are an essential group of plant metabolites which are essential for human diet and overall health. Also, the polyphenolic compounds display a board range of biological activities. The biological activities are directly related to involvement in various steps of cancer development [Citation3Citation[4]–Citation5] . The common banana is one of the most popular, cheapest and affordable fruits widely available worldwide. Even in Oman, several varieties of bananas are available and are considered as an important source of nutrition belonging to the plant classification, Musaceae. Bananas originate from the South-Western Pacific regions of the globe and are now distributed and cultivated globally across the world. About 100 tropical and sub-tropical countries currently commercially cultivated different species of bananas for medicinal and consumption purposes. Bananas are a common fruit source that provide instant energy that serve as an ideal source for potassium intake [Citation6,Citation7] . Bananas are considered the fourth most cultivated agricultural crops after rice, wheat, and maize [Citation8]. The plant’s height grows to about 6 to 8 m, and the leaves are arranged in a spiral formation. Every part of a banana possesses potential medicinal value and contains several chemical compounds such as fatty acids, sterols, steryl ester, linoleic, linolenic, fructose, xylose, galactose, glucose, mannose and oleic acids [Citation8,Citation9] . In addition, some other minor compounds such as vitamin B, oxalic acid, various vitamins, starch, potassium, calcium, tannin, glycosides, phenols derivatives, pentacyclic triterpenes, cyclicmusalenol, cyclomusalenone, cycloartanol, stigmast-cycloartanol, stigmast-7-en-3-ol, lanosterol, and α-amyrin are chemicals responsible for biological activities [Citation10Citation[11]–Citation12] . Traditionally, the stem juice is used for the treatment of epilepsy, hysteria and in dysentery and diarrhea [Citation8]. The extract of flowers are used for the treatment of bronchitis, dysentery, and ulcers and cough syrup from flowers is used for diabetes, epilepsy, leprosy, fevers, hemorrhages, acute dysentery and diarrhea [Citation12]. The paste formed from the leaves are used in the treatment of burns, skin afflictions, dysentery, diarrhea and malignant ulcers [Citation12] Traditionally; Omani people use bananas for the treatment of digestion, constipation, and diarrhea. However, the literature search has revealed that limited research conducted on the estimation of the total amount of phenols and flavonoids and antioxidant potential of Omani species of bananas. Therefore, it is important to recognize the biological activities of Omani bananas that are mainly grown in the region near Salalah. In the present study, the research has focused on the different polarity crude extracts of local and imported ripe bananas and aims to discover the amount of their antioxidant potential, total phenols, and flavonoids contents by applying established research methods.
2 Materials and methods
2.1 Chemicals and reagents
The different polarities of solvents used were n-hexane, ethyl acetate, n-butanol, chloroform and acetone (Sigma Aldrich Company, Germany). Methanol, Folin-Ciocalteu reagent, sodium carbonate, aluminum chloride, sodium hydroxide, sodium nitrate, quercetin, gallic acid and 1, 1-diphenyl-2-picrylhydrazyl (DPPH) were obtained from BDH, UK.
2.2 Instrument
All the spectrophotometric measurements were done using UV-visible spectroscopy (Model-1800, Japan) for the estimation of the number of phenols and flavonoids and antioxidant potential.
2.3 Sample collection
The local ripe banana samples were collected from Salalah, in the Dhofar Reion of the Sultantate of, Oman, and the imported Pilipino ripe bananas samples were collected from local supermarkets from the capital reion of, Muscat, Oman (). Both bananas were identified by the histological appearance, pictures and local people in that area. Only the best qualities of ripe bananas were used in the present experiment. The collected bananas sample was cleaned with water to eliminate foreign particles. The whole banana were sliced by a knife and then dried at an ambient temperature separately under sunlight for several days. The dried banana samples were then grounded into coarse powder. The powdered samples were stored in an amber color bottle for further analysis ().
2.4 Preparation of crude extract
The dried coarse powdered banana samples (500 gm) were extracted separately with methanol (2 L) by using the maceration method for several days. It was filtered and again reextracted by the same solvent for complete extraction. Both extracts were mixed and evaporated the methanol to give methanol extract (35.08 gm). It was dissolved in distilled water (300 ml) and transfer to the separatory funnel and portioned by n-hexane, chloroform, ethyl acetate and n-butanol solvents [Citation13]. Repeated the fractionation twice and combined. The different polarity fractions were dried by using a rotary evaporator to give n-hexane, chloroform, ethyl acetate, n-butanol and water crude extract. The obtained different extracts were used for the determination of the amount of phenols and flavonoids content and antioxidant potential.
2.5 Total phenols assay
2.5.1 Gallic acid standard
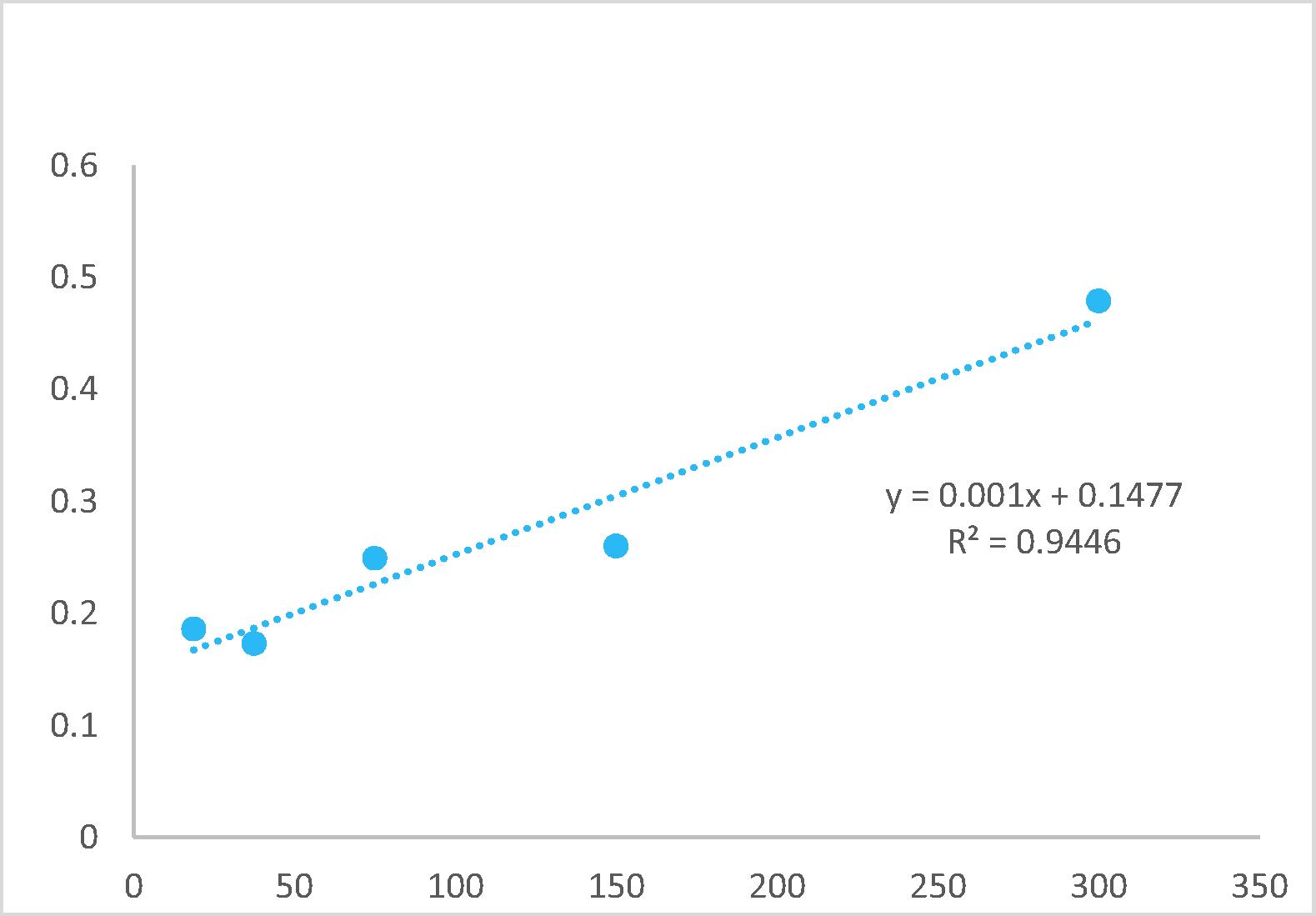
The amount of total phenols content was determined by the modified FCR method with a gallic acid standard [Citation13]. A stock solution was prepared by the addition of two milligrams of gallic acid with 10 ml of MeOH. The concentrations (100, 50, 25 and 12.5 μg/ml) were prepared from the stock solution. Two hundred microliters of each prepared concentration were taken in a test tube and then added 1.5 ml of 10% FCR solution. The solution was incubated for 5 min. Finally, added 1.5 ml of 6% Na2CO3 to each concentration test tube and incubated again for 2 h with covered by aluminum foil. UV–visible spectroscopy measured the absorbance of each incubated solution at 760 nm. The obtained data were used to prepare the standard curve.
2.5.2 Total phenols content
The amounts of phenols of different polarity extracts of local and imported ripe banana samples were determined according to the Folin-Ciocalteu modified procedure [Citation13]. Briefly, four milligrams of each crude extract of imported and local ripe bananas were dissolved in 4 ml of MeOH. From there 200 μl each extract sample was taken in other tubes and added to 1.5 ml of 10% FCR and incubated for 5 min. Finally, 1.5 ml of 6% Na2CO3 was added and incubated those tubes for 2 h with covered test tubes with aluminum foil. After 2 h incubation, UV–visible spectrophotometer at 760 nm was used to measure the absorbance. The amount of total phenols was calculated from the standard curve, and the results were expressed as mg gallic acid equivalents (GAE) 100 gm−1 dry powder weight of banana powder [Citation13].
2.6 Total flavonoids assay
2.6.1 Preparation of quercetin standard
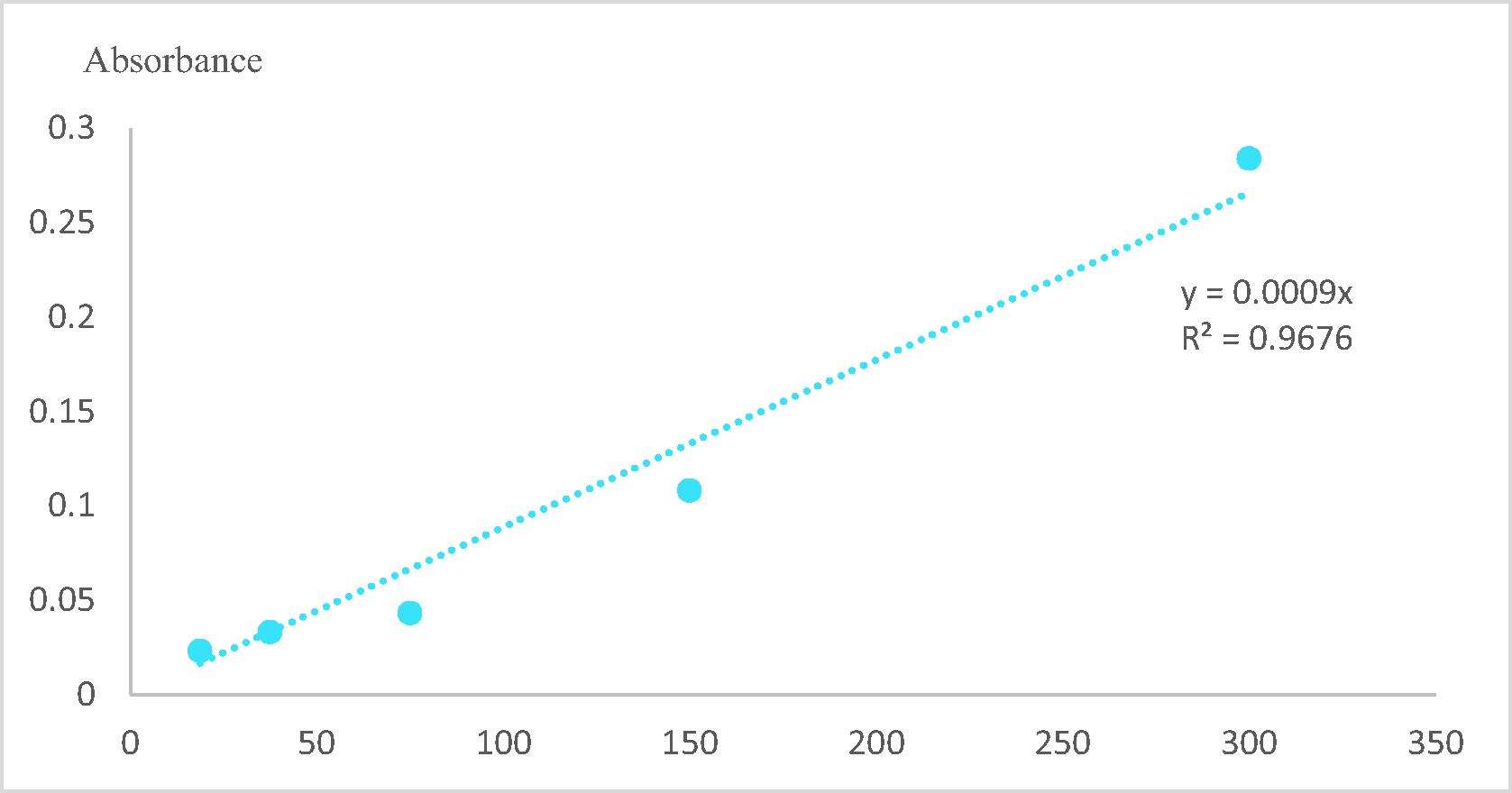
The amount of total flavonoids was determined by the modified colorimetric method with quercetin as standard [Citation14]. Briefly, two milligrams of quercetin standard was dissolved with methanol in a 10 ml. The serial dilution techniques were used to different prepared concentrations (100, 50, 25 and 12.5 μg/ml) by adding methanol solution. About two hundred and fifty microliters of each prepared concentration sample were placed in a separate test tube and added 125 μl and 75 μl of water and 5% NaNO3 solution. The solution was stirring and incubated for 6 min. After 6 min incubation at ambient temperature, 150 μl of 10% AlCl3 was added to the test tube and incubated for 5 min. Finally, 500 μl of 4% NaOH and 275 μl of water were added and recorded the absorbance against the blank at 510 nm wavelength with UV–visible spectroscopy.
2.6.2 Total flavonoids assay
The amount of total flavonoids was measured by using a modified colorimetric method according to Sheikha et al. [Citation14]. Each extract of both ripe bananas sample (4 mg extract/4 ml MeOH) was mixed in a separate test tube. Different concentrations (100, 50, 25 and 12.5 μg/ml) were prepared by adding methanol solution. Briefly, 200 μl of the different prepared concentration of each sample was mixed in a test tube and 125 μl and 75 μl of water and 5% NaNO3 solution were added. The solution was mixed and incubated for 6 min. After incubation at ambient temperature, 150 μl of 10% AlCl3 was added to the test tube and incubated for 5 min. Finally, 500 μl and 275 μl of 4% NaOH and water were added. The absorbance of the incubated samples was measured against black at 510 nm by using UV-visible spectroscopy. The amount of flavonoids content was determined concerning the standard curve quercetin [Citation13]. The calculated results are expressed in μg of QE/g of banana sample.
2.7 Antioxidant assays
The antioxidant potential of different prepared crude extracts of both bananas banana samples (local and imported) was examined spectrophotometrically by measuring the neutralization level of DPPH [Citation15]. The different extracts of both banana samples, which had previously been prepared according to Aziza & Hossain, 2015, were used to determine the scavenging potential of the banana samples. Different concentrations (200, 100, 50, 25 and 12.5 μg/ml) were prepared from both varieties banana samples by using the serial dilution technique with methanol solvent. DPPH (3.3%) was also prepared with methanol solvent. To 200 μl of each extract, 2.5 ml of DPPH and 2 ml of methanol solvent were added to the test tube, and this was incubated for an hour and a half. DPPH and methanol were used to measure the control. The incubated samples were determined by their absorbance against black at 517 nm by using UV-visible spectroscopy. Finally, scavenging potential of different extract samples was determined by using the formula below:
3 Results
3.1 Crude extract
The yields of different polarity extracts of Salalah and Pilipino ripe bananas are given in . The methanol and its successive prepared banana extract were used to determine the amount of phenols and flavonoids and antioxidant potential.
Table 1 Yield of crude extracts of Salalah and Pilipino ripe banana.
3.2 Total phenols content
The amount of phenols content of all prepared extracts of both bananas (Somali and Cavendish varieties) as determined by the Folin–Ciocalteu method with modification are reported as gallic acid equivalents [13; ; ). The experimental results indicated that the amount of phenol content of Salalah banana extract was the maximum amount in methanol extract (386.22 mg of GAE/100 g of powder extract) and the minimum was in water (150.13 mg of GAE/100 g of extract) followed by ethyl acetate extract (190.89), chloroform extract (175.89), n-hexane extract (173.44), water extract (150.13) and n-butanol extract (131.72). Similarly, the results of Pilipino banana extracts were obtained the maximum amount in ethyl acetate extract (370.13 mg of GAE/100 g of extract) and the minimum was in n-butanol extract (155.14 mg of GAE/100 g of powder crude extract) followed by chloroform extract (342.65), methanol extract (307.22), n-hexane extract (205.76) and water extract (163.98).
Table 2 Total phenols content of crude extracts of Salalah and Pilipino ripe banana.
3.3 Total flavonoids content
The amount of total flavonoids of different polarity crude extract of Salalah and Pilipino bananas are presented in and . The experimental results showed that the total flavonoids content of the Salalah ripe banana, the maximum amount was obtained in water (8.51 µg QE/g dry powder) and the minimum was n-butanol (1.38 µg QE/g dry powder). However, the maximum amount of total flavonoids content in the Pilipino banana was obtained in chloroform extract (1.58 µg QE/g dry powder) and the minimum was in n-hexane extract (0.16 µg QE/g dry powder).
Table 3 Total flavonoids contents of different crude extracts of Salalah and Pilipino ripe banana.
3.4 Antioxidant potential
All crude extracts of both the Salalah and the Pilipino ripe banana samples were determined their antioxidant potential by using well-established DPPH method [Citation15]. The inhibition as a percentage of each banana extract of both samples was determined by using an established formula. In our results indicated that the scavenging potential for methanol and its derived fractions from the Salalah ripe banana was the maximum in chloroform extract (98.1%) and the minimum in water extract (69.4%) (). However, the scavenging potential of different polarities crude extracts of the Pilipino banana was the maximum in n-hexane extract and the minimum in water extract followed in the direction of n-hexane > n-butanol > methanol > ethyl acetate > chloroform > water ().
Table 4 Antioxidant potential of hexane, ethyl acetate, chloroform, butanol, methanol and water crude extracts from Salalah ripe banana.
Table 5 Antioxidant potential of hexane, ethyl acetate, chloroform, butanol, methanol and water crude extracts from Pilipino ripe banana.
4 Discussion
Plant ingredients are broadly present in plant kingdoms and process foods. Sugars, minerals, organic acids, and dietary fiber are the most important source of plant metabolites. In addition, phenols, alkaloids, terpenoid, and flavonoids are the common bioactive compounds in all plant kingdoms and foods. They are mainly synthesized as secondary metabolites and constituents; those compounds are present in the plants as pharmacologically active forms. However, the other chemical components occur in bananas as inactive precursors, and they are inspired in response to different injury tissues or microbial attack [Citation16,Citation17] . All the mentioned secondary metabolites are producing by the plants along with a broad range of chemical, physical and biological activities. The bioactive compounds occur in the plants belong to different groups and families. Each family has specific structural and biological characteristics. Almost all aromatic amino acids and aliphatic amino acids are produced in the plant by different pathways. Most of the bioactive compounds obtained in plants are phenolics and polyphenols [Citation18]. Those secondary metabolites are also with the different diversified group. All the bioactive compounds which have been obtained in the banana sample to have different biological potential. Phenolics and their derivatives are also directly involved in many psychological and physiological activities. All of these biologically active compounds are generally soluble in H2O, hydroalcoholic and polar acetone solvent and gives sediments binding by proteins [Citation19]. Several scientific pieces of evidence showed that phenolics were traditionally used for prevention of inflammation of the mouth and to treat wounds, hemorrhoids, and diarrhea [Citation11,Citation20Citation[21]–Citation22] . Plants, animals, and microorganisms are considered as the sources of natural product. Among them, only plant and its formulated products are more reliable and safe. Therefore, the plant and its formulated products are considered as a catalyst for human welfare and used as a primary health care system worldwide. However, in the past few decades, interest has grown among researchers to find an active therapeutic agent from the plant sources. Serafinao et al. [Citation21] reported that plant crude extracts are more effective than the synthetic ones with no or insignificant side effects.
Both the nutritional values and biological properties are entirely depending on plant metabolites compounds as described by [Citation15]. The cyclic and acyclic phenols content, total phenols content among the six prepared banana extracts from Salalah ripe banana are given in (). The range of cyclic and acyclic phenols content of different banana extracts of both ripe bananas were obtained ranging from 131.72 to 386.22 and 155.14 to 370.13 mg/100 g dry plant extract (). On the other hand, the total polycyclic flavonoids content of the prepared banana extracts were calculated through the AlCl3 method, and the maximum flavonoids content in the Salalah banana extracts was in water extract, and the minimum was in the n-butanol extract (). The trend order of flavonoids content obtained in Salalah ripe banana was water > n-hexane > ethyl acetate > methanol > chloroform > n-butanol extract. Also, the flavonoids content of Pilipino banana (Cavendish variety) was the maximum in chloroform and the minimum in n-hexane followed by order of chloroform > methanol > ethyl acetate > n-butanol > water > n-hexane (). There are no reports available in the literature on phenols and flavonoids content to compare our results.
The scavenging potential of the six prepared banana extracts of each local and imported ripe banana samples which were collected from Salalah and local Supermarket was evaluated spectrophotometrically by measuring neutralization level of DPPH [Citation15]. The free radical scavenging potential of all prepared extracts of the Salalah and Pilipino ripe bananas through DPPH are presented in and . The chemical mechanism of scavenging potential is their interaction between the DPPH and crude antioxidants to produce free radicals. The antioxidants from the samples are reacted with the stable free radical. Then, the reagent DPPH is slowly converted to α,α-diphenyl-β-picrylhydrazine by neutralizing level of DPPH with the color change of the extract. The rate of color change of DPPH shows the free radical scavenging potential of the sample antioxidant. In our experiment, the prepared banana extracts from both banana samples at different concentrations were able to neutralize DPPH color due to natural antioxidants. The result of antioxidant potential of methanol and its derived fractions from Salalah banana samples was the maximum in chloroform extract and the minimum in water crude and followed by chloroform > methanol > n-butanol > ethyl acetate > n-hexane > water ().
On the other hand, in Pilipino ripe banana samples, the maximum potential was in n-hexane and minimum in water followed by order of n-hexane > n-butanol > methanol > ethyl acetate > chloroform > water (). From the literature, it has been obtained that the bananas crude extracts contain some primary active compound, e.g., tannin, glycosides, phenolic compounds, triterpenes, flavonoids, lanosterol, and α-amyrin [Citation3,Citation8Citation[9]Citation[10]–Citation11,Citation22] . These biologically active compounds can able to neutralize the level of DPPH with color change by their hydrogen donating ability [Citation15]. In our results showed that the maximum scavenging potential of Salalah banana was obtained in chloroform extract while in Pilipino ripe banana samples, the maximum scavenging potential was obtained in n-hexane extract. The difference in results obtained in our experiment might be due to the solvents and methods. Besides, it could be during the processing sample some of the responsible compounds decomposed or evaporated. In our future studies are designed for the characterization of each simple phenols, flavonoids, and antioxidant compounds and also in vivo studies are needed for their mechanism of action. Almost similar results have previously been reported between the relationship of free radical scavenging potential of bananas different extracts and their chemical screening [Citation23,Citation24,Citation11] .
5 Conclusion
Traditionally, the Omani people are used herbal therapy for the treatment of different diseases. Scientists/researchers are finding biologically new lead compounds including antibiotics and anticancer through the Omani medicinal plants. The data obtained from this study might result from invaluable tools that will help Oman to take its share of the burden and benefit in solving these present world health difficulties. The extracts of both Salalah and Pilipino ripe banana samples showed a maximum content of active polycyclic phenolic derivatives and both types of banana samples also showed significant scavenging potential against DPPH method. In conclusion, all polarity banana extracts could be used as a medicine to treat different diseases.
Acknowledgement
The authors are grateful to University of Nizwa, Nizwa, Sultanate of Oman for providing facilities to carry out this project. Thanks to Derek M. N. O’Connell, Director, the Writing Center and Learning Enhancement Center, University of Nizwa for his assistant to edit the manuscript.
References
- T.RiazM.A.AbbasiShahzadi T.Aziz-Ur-RehmanM.AjaibK.M.KhanPhytochemical screening, free radical scavenging, antioxidant potential and phenolic content of Dodonaea viscosaJ Serbian Chem Soc42012423431
- V.VeerapurK.PrabhakarV.PariharK.SrinivasanM.UnnikrishnanP.BansalAntidiabetic effect of Dodonaea viscosa aerial parts in high-fat diet and low dose streptozotocin-induced type 2 diabetic rats: a mechanistic approachInter J Phytomed481020106061
- B.AdinarayanaA.P.BabuAnti-oxidant potential and cytotoxicity of ethanolic extracts from the rhizome of Musa acuminate2011Research Gateway for BiosciencesVisakhapatnam, India
- S.P.KapadiaP.S.PudakalkattiS.ShivanaikarDetection of antimicrobial potential of banana peel (Musa paradisiaca L.) on Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans: an in vitro studyContemporary Clin Dent642015496500
- T.S.B.KhairBanana domestication on the Arabian Peninsula: a review of their domestication historyJ Hort Forest5112013194203
- A.PereiraM.MaraschinBanana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human healthJ Ethnopharma1602015149163
- I.M.ZafarA.SalehaM.M.E.HoqueR.M.SohelAntimicrobial and cytotoxic properties of different extracts of Musa sapientumL. subsp. sylvestrisInd J Experi Biol2820116266
- L.C.S.OliveiraA.J.FreireCordeiro N.SilvestreLipophilic extracts from banana fruit residues: a source of valuable phytosterolsJ Agri Food Chem5620200895209524
- M.DebabandyaM.SabyasachiS.NamrataBanana and its byproducts utilization: an overviewJ Sci Ind Res692010323329
- P.NatchareeK.R.SudipPhysical and antimicrobial properties of banana flour/chitosan biodegradable and self-sealing films used for preserving fresh-cut vegetablesLWT – Food Sci Technol4410201123102315
- M.A.E.AhmedA.S.ZeinabA.G.AlaaF.A.HananA.E.FatenA.A.HabibaIdentification of phenolic compounds from the banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agentsJ Chem Pharma Res8420164655
- N.M.RaoS.H.K.R.PrasadN.JyothirmayiEfficacy of ripened and unripened fruit extracts of Musa X paradisiaca L (Bontha cultivar) against human pathogensInter J Phar Pharmaceu Sci412012455460
- H.S.K.Al-JadidiM.A.HossainStudies on total phenolics, total flavonoids and antimicrobial potential from the leaves crude extracts of neem traditionally used for the treatment of cough and nauseaBeni-Suef Uni J Basic Appl Sci4220189398
- K.M.SheikhaN.S.W.RuqaiyaM.A.HossainIn vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorusPac Sci Rev A: Nat Sci Eng172015103108
- S.R.A.AzizaM.A.HossainStudy on total phenolics and antioxidant potential of leaves crude extracts of Annona squamosal traditionally used for the treatment of cancerous tumorsAsian Pac J Trop Dis5Suppl 12015S142S144
- S.A.DahanukarR.A.KulkarniN.N.RegePharmacology of medicinal plants and natural productsInd J Pharmacol322000S81S118
- A.E.OsbournPreformed antimicrobial compounds and plant defense against fungal attackPlant Cell8199618211831
- J.ShiH.NawazJ.PohorlyG.MittalY.KakudaY.M.JiangExtraction of polyphenolics from plant material for functional foods - Engineering and technologyFood Rev Inter212005139166
- D.F.BasriS.H.FanThe potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agentsInd J Pharmacol3720052629
- D.S.OgunleyeS.F.IbitoyeStudies of antimicrobial potential and chemical constituents of Ximenia AmericanaTrop J Pharma Res22003239241
- C.SpataforaC.TringaliValorization of vegetable waste: identification of bioactive compounds and their chemo-enzymatic optimizationOpen Agri J62012916
- M.RaoA.MuhammadS.M.KhamsahPhytochemical screening, total flavonoid and phenolic content assays of various solvent extracts of tepal of Musa paradisiacaMalaysian J Analyt Sci205201611811190
- A.Z.NessmaAntioxidant, antitumor, antimicrobial studies and quantitative phytochemical estimation of ethanolic extracts of selected fruit peelsInt J Curr Microb Appl Sci452015298309
- G.M.RafaelaM.L.GloriaG.MonicaAntioxidant potential in banana peel extracts: Testing extraction conditions and related bioactive compoundsFood Chem1193201010301039