Abstract
Vibrio alginolyticus, Vibrio parahemolyticus and Photobacterium damselae subsp damselae were isolated during recurrent episodes of mass mortalities among different stages of Gilthead sea bream (Sparus aurata) and European seabass (Dicentrarchus labrax). The pathogens were recovered from the external/internal lesions of a total of 320 seeds, juvenile and adult fishes from the period of February 2013 through August 2013. Two hundred and sixty four bacterial isolates were retrieved and presumptively identified using morpho-chemical characterization and API®20NE. However, definitive molecular confirmation of V. alginolyticus was obtained through implementing collagenase gene based regular PCR technique. The total prevalence of V. alginolyticus, V. parahemolyticus and Photobacterium damselae subsp damselae among naturally infected Gilthead seabream and European seabass was 82.19%, 87.28% 10.27%, 6.79% and 7.54%, 5.93% respectively. Antibiogram has revealed that isolates were sensitive to ciprofloxacin, chloramphenicol, enrofloxacin, nalidixic acid and oxolinic acid while resistant to ampicillin, amoxicillin, and lincomycin.
1 Introduction
The majority of mariculture activities are regularly depending on the collection of wild seeds from the Mediterranean Sea and some coastal northern lakes. Economy wise, Egyptian mariculture is remarkably incomparable to the freshwater aquaculture, where the total mariculture harvest does not exceed 5% of the entire Egyptian aquaculture harvest. The mariculture development is restrained by technical, economic and disease problems [Citation1].
Bacterial diseases mount the most influential sector of disease problems that has direct colossal impacts on Egyptian mariculture [Citation2]. Vibrios come on the top list of pathogens with direct jeopardy to mariculture development due to high mortalities associated with their invasion to fishes [Citation3]. It is crucial to know that, Vibrios are ubiquitous to marine environment, while clinical disease outbreaks only occur when a sharply stressed fish get exposed to the flaring up infectious agent [Citation3].
Septicemia induced by Vibriosis is characterized by haemorrhages on the base of pectoral fins, exophthalmia, loss of appetite and edematous lesions on the body surface [Citation4]. Vibrio alginolyticus (V. alginolyticus) and Vibrio parahemolyticus (V. parahemolyticus) are responsible for mass mortalities among fish stocks in many marine fish farms throughout the Mediterranean area and severe economic losses in aquaculture worldwide [Citation5–Citation7]. V. alginolyticus causes many epizootic outbreaks among the Gilthead seabream and European seabass populations, which possess high economic value at the Mediterranean communities [Citation8–Citation9].Morphologically, Vibrios are Gram negative, asporogenous rods that are straight or curved and are motile with a single polar flagellum when grown in liquid medium [Citation10].
Photobacterium damselae subsp. damselae (formerly Vibrio damsela) is a halophilic bacterium associated with marine environments that was initially isolated in 1981 as the causative agent of skin ulcers in damselfish [Citation11]. Photobacterium damselae subsp. damselae (P. damselae ssp. Damselae) has been repeatedly isolated from epizootic outbreaks affecting several cultured fish species especially Gilthead seabream, Sparus aurata and European seabass, Dicentrarchus labrax and new cultured marine fish species in southern Spain. Moreover, this pathogen has been reported to cause diseases in human, and for this reason, it may be considered as zoonotic pathogen [Citation12]. Photobacterium damselae subsp. damselae is a facultative anaerobic, Gram negative rod, weakly motile (by one or more unsheathed polar flagellum).
Hence, the present study investigates the possible bacterial causes behind the recurrent mass mortalities among different stages of Gilthead seabream and European seabass from Alexandria and Damietta provinces, Egypt. Further, adopts full identification/characterization scheme for the retrieved isolates.
2 Materials and methods
2.1 Fish sampling
Throughout the period from February 2013 to August 2013, a total of 320 moribund and freshly dead marine fishes were collected during some episodes of mass mortalities associated with disease outbreaks occurring at Alexandria and Damietta provinces, Egypt. Fish species, numbers of fishes, average body weights, average lengths and localities are shown in (). Clinical examination of naturally infected fish was carried out according to the method described by Austin and Austin [Citation3], Amlacher [Citation13]. Postmortem examination was performed on moribund sacrificed fish and/or freshly dead fish according to the method described by Conroy and Herman [Citation14].
Table 1 Fish species, geographical location, number, weight and length of examined fish.
2.2 Water sampling
Throughout the period from February 2013 to August 2013, two water samples were collected in sterile 500 ml glass bottles, during the disease outbreaks occurring at Alexandria and Damietta provinces, Egypt according to the standard methods described by Boyd [Citation15] and APHA [Citation16].Water parameters namely temperature, dissolved oxygen, salinity and pH were measured on spot using alcoholic thermometer, oxygen meter, salinometer and pH meter respectively.
2.3 Bacteriology
2.3.1 Sampling and sample processing
Loopfuls from kidney, liver, brain, spleen, gills and external lesions of examined fishes were spread onto Tryptic soy agar (TSA) (Lab M, Lancashire, UK), Tryptic soy broth (TSB) (Lab M, UK) and Brain heart infusion broth (BHI) (Lab M, UK) supplemented with 2% (w/v) sodium chloride, marine agar (MA) (Difco & BBL, MI, USA), and Thiosulphate citrate bile salt sucrose agar (TCBS, Lab M, UK). All inoculated media plates were incubated at 25 °C for up to 72 h.
In case of diseased seeds, sampling was carried out according to Grisez et al. [Citation17]. Briefly, seeds were disinfected externally by immersion in 0.1% (w/v) benzalkonium chloride prepared in 1.5% (w/v) saline for 10 s then washed three times in 0.85% (w/v) saline. Subsequently, 5seeds were transferred into 1 ml of sterile saline in a glass potter blender and were manually homogenized. 100 μl of the homogenates were aliquoted/spread onto the media plates before being incubated according to the aforementioned conditions.
2.3.2 Biochemical identification
Isolates were identified by biochemical characterization following the criteria described in Bergey’s Manual of Determinative Bacteriology [Citation18–Citation20]. Presumptive characterization of the retrieved isolates was achieved using commercial miniaturized API®20NE system (Biomerieux, France) according to manufacturer’s instructions. The isolates were identified according to diagnostic schemes described by Buller [Citation21], Costinar et al. [Citation22].
Growth in different concentration of sodium chloride was used as another presumptive criterion for identification of the retrieved isolates. Briefly, bacterial colonies were inoculated into peptone water supplemented with 0%, 2%, 3%, 4%, 6%, 8% and 10% sodium chloride.
2.3.3 Antibiogram
Antibiotic susceptibility of the retrieved bacterial isolates was determined using the Kirby Bauer disk diffusion method according to Bauer et al. [Citation23], Jorgensen and Turnidge [Citation24]. The following antimicrobial discs (Oxoid) were used: Ampicillin 10 μg (AML 10), Amoxicillin 25 μg (AX 25), Ciprofloxacin 5 μg (CIP 5), O/129 150 μg (129/150), Chloramphenicol 30 μg (C30), Enrofloxacin 5 μg (ENR 5), Erythromycin 15 μg (E 15), Gentamicin 10 μg (CN 10), Lincomycin 2 μg (L 2), Nalidixic acid 30 μg (NA 30), Nitrofurantoin 300 μg (F 300), Novobiocin 30 μg (NV 30), Oxolinic acid 2 μg (OA 2), Oxytetracycline 30 μg (TE 30), Rifampin 5 μg (RA 5), Streptomycin 10 μg (S 10), Tetracycline 30 μg (T 30), Trimethoprim/Sulfamethazole 25 μg (SXT 25). In vitro antimicrobial susceptibility was screened on Mueller-Hinton agar (MHA) (Oxoid, Hampshire, UK) supplemented with 1.5% (w/v) sodium chloride. At the end of incubation, inhibition zones were measured using measured caliber.
2.4 Molecular confirmation of V. alginolyticus by polymerase chain reaction (PCR)
2.4.1 Extraction of genomic DNA
Bacterial isolates were grown on TSA (Lab M, UK) supplemented with 2% sodium chloride and incubated at 25 °C for 24 h. Bacterial colonies were harvested then extraction of genomic DNA was carried out according to BioFlux DNA extraction kit manufacturer’s instructions.
2.4.2 Detection of collagenase gene by PCR
Two sets of oligonucleotide primers were used for identification of V. alginolyticus. The primer pairs were VA-F (5′-CGAGTACAGTCACTTGAAAGCC-3′) and VA-R (5′-CACAACAGAACTCGCGTTACC-3′) based on Collagenase gene producing 737-bp fragment [Citation25]. The PCR reaction was performed in a 50 μl reaction system consisting of 2 μl of purified genomic DNA (50 ng/μl), 5 μl of 10× PCR buffer, 1 μl each of the primers(50 pmol/μl),1 μl each of the 10 mM dNTPs, 0.2 μl (5 units/μl), 0.2 μl Taq DNA polymerase (5 units/μl) and the final volume was adjusted to50 μl with sterilized double distilled water [Citation25]. The tubes were then placed in the thermal cycler with program being set as follows: one cycle of initial denaturation step at 94 °C for 5 min followed by 35 cycles of94 °C for 60 s, 50 °C for 60 s and 72 °C (extension temp) for 60 s. The cycling was concluded by an additional final extension at 72 °C for 10 min and the reaction products were stored at 4 °C until further analysis [Citation25]. The amplicons were run into 1% agarose gel at 100 V for 30 min. The gel was stained with ethidium bromide then specific bands were detected under the ultraviolet (UV) trans-illuminator. The detected bands were photographed on gel documentation system using Digital camera.
3 Results
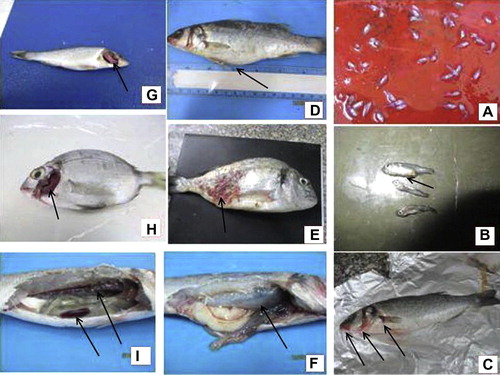
Clinically, diseased fish showed dark coloration of skin, detached scales, tail fin rot, skeletal deformity (lordosis), hemorrhage at base of pectoral fin, hemorrhages around the mouth opening and operculum, abdominal distension and superficial ulcerations (). The main post mortem lesions were, congested kidney, pale enlarged liver, congested spleen, congested brain, pale gills with excessive mucous (in some cases the gills were congested), enlarged gall bladder, ascitic fluid in the abdominal cavity and in some cases inflammation of swim bladder with thickening of its wall ().
The recorded water quality results throughout the study were indicating abrupt rise in un-ionized ammonia (0.07–0.09 mg/l), water temperature (24–30 °C), water salinity (32.60–67.20 g/l), nitrite (0.26–0.56 mg/l), iron (0.1–0.410 mg/l) and copper (0.12–0.149 mg/l) with concurrent decrease in dissolved oxygen (3.1–4.2 mg/l).
Biochemical and morphological characterization of bacterial isolates which were isolated from diseased Gilthead seabream and European seabass are shown in (). The results revealed that 264 bacterial isolates were recovered and characterized from 246 infected fish. One hundred and forty six isolates were recovered from 95 diseased seeds, 22 diseased juveniles and nineteen diseased adult fish of gilthead seabream, S. aurata. One hundred and eighteen isolates were recovered from 76 diseased seeds, 18 diseased juveniles and sixteen diseased adult fish of European seabass, D. labrax.
Table 2 Morphochemical characterization of bacterial isolates retrieved from diseased gilthead seabream and European seabass.
Also, the results revealed that the total prevalence of bacterial infections in this study was 76.57%. While the total prevalence among naturally infected Gilthead seabream, S. aurata was 95%, 88% and 76% for seabream seeds, fingerlings and fish respectively. Moreover, the reported total prevalence among naturally infected European seabass, D. labrax was 60%, 80%, 72% and 65% for seabass seeds1, seeds2, juveniles and adult fish. On the other side, the total prevalence of bacterial infections in Gilthead seabream, S. aurata and European seabass, D. labrax were 86.33% and 69.25% respectively. The prevalence of each bacterial pathogens and mixed infections among naturally infected Gilthead seabream, S. aurata and European seabass, D. labrax were described in ().
Table 3 The prevalence of each bacterial pathogens and mixed infections among naturally infected gilthead seabream, Sparus aurata and European seabass, Dicentrarchus labrax.
Moreover, the total prevalence of V. alginolyticus among naturally infected Gilthead seabream, S. aurata and European seabass, D. labrax, were 82.19% (120/146) and 87.28% (103/118) respectively, whiletotal prevalence of V. parahemolyticus among naturally infected Gilthead seabream, S. aurata and European seabass, D. labrax were 10.27% (15/146) and 6.79% (8/118) respectively. Further, the total prevalence of P. damselae ssp. Damselae among naturally infected Gilthead seabream, S. aurata and European seabass, D. labrax were 7.54% (11/146) and 5.93% (7/118) respectively, while the prevalence in adult Gilthead seabream and European seabass was 44% (11/25) and 28% (7/25) respectively.
In respect to intensity of infection, in case of Gilthead seabream, S. aurata, the highest intensities of V. alginolyticus, V. parahemolyticus and P. damselae ssp. Damselae were in liver (32%), kidney (33.3%) and liver (36.4%) respectively, while the lowest intensities were in skin (4%), gills (6.7%) and spleen, brain (18.2%) respectively (). In case of European seabass, D. labrax, the highest intensities of V. alginolyticus, V. parahemolyticus and P. damselae ssp. Damselae were in kidney (37%), kidney (38.5%) and liver, kidney (28.52%) respectively, while the lowest intensities were in gills (8.5%), gills (7.7%) and gills, spleen (12%) respectively ().
Table 4 Intensities of bacterial infection in different organs of naturally infected gilthead seabream, Sparus aurata and European seabass, Dicentrarchus labrax fingerlings and juveniles.
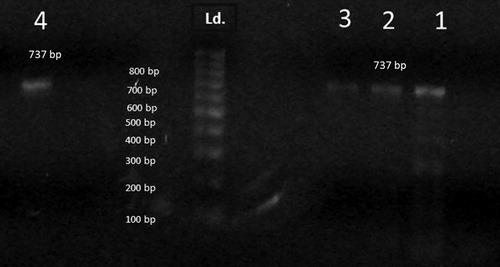
Species specific polymerase chain reaction (PCR) was carried out to V. alginolyticus isolates using collagenase gene primers. Results have indicated that all tested isolates showed the appearance of 737-pb size bands characteristic for V. alginolyticus ().
Antibiogram of retrieved has revealed that V. alginolyticus was sensitive to ciprofloxacin, chloramphenicol, gentamicin, enrofloxacin, nitrofurantoin, oxytetracycline, tetracycline, streptomycin, nalidixic acid, oxolinic and vibriostat (O/129), while resistant to ampicillin, amoxycillin andlincomycin. Moderate sensitivities against trimethoprim–sulfamethoxazole, novobiocin and erythromycin were reported. V. parahemolyticus isolates were sensitive to ciprofloxacin, chloramphenicol, enrofloxacin, oxytetracycline, tetracycline, trimethoprim–sulfamethoxazole, novobiocin, nalidixic acid, oxolinic and vibriostat (O/129), while resistant to ampicillin, amoxycillin, gentamycin, lincomycin and streptomycin. Intermediate sensitive was found to nitrofurantoin and erythromycin (Table 5). P. damselae ssp. Damselae isolates were sensitive to ciprofloxacin, chloramphenicol, enrofloxacin, nitrofurantoin, oxytetracycline, trimethoprim–sulfamethoxazole, novobiocin, nalidixic acid, oxolinic and vibriostat (O/129), while resistant to ampicillin, amoxycillin, tetracycline, gentamycin, streptomycin and lincomycin. Intermediate sensitive was found to erythromycin.
4 Discussion
V. alginolyticus is considered one of the most dangerous pathogens in aquaculture, causing serious damage in finfish, shellfish and crustaceans [Citation26]. Its zoonotic importance has been increasing through the past few decades. V. alginolyticus has been sporadically associated with episodes of human infection such as wound infection, cellulitis, seawater-related otitis media [Citation27–Citation29]. V. parahemolyticus is compatible with marine / brackish aquatic environment adjusting well to the broad range of salinities. It is commonly found on shellfishes and all varieties of finfish that are traditionally taken from marine and shore areas [Citation30]. P. damselae ssp. Damselae has been isolated from aquatic environments and as causative agents of diseases in a variety of aquatic animals and humans [Citation31]. P. damselae ssp. Damselae is a systemic bacterial infection affecting more than 48 fish species in widely distributed regions [Citation32].
In the current study, clinical signs and post mortem lesions recorded among naturally infected Gilthead seabream and European seabass similar to clinical signs and post mortem lesions reported by Labella et al. [Citation12], Moustafa et al. [Citation34]. The onset of outbreaks of fish diseases may be attributed to the suppression of fish immune system. Such immune-suppression is positively linked to the abrupt increase in water temperature, un-ionized ammonia, iron, copper, nitrite, salinity associated with uprising depletion of dissolved oxygen. Immunologically, the above mentioned water quality results are well known to have direct impact on adaptive, innate and cellular immune responses [Citation33,Citation35]. These results were in complete accordance with those reported by Chen et al. [Citation36], Suomalainen et al. [Citation37] who stated that sharp increase in ammonia, decreased dissolved oxygen, high water salinity and temperature and ability to scavenge iron from iron-binding proteins are the most possible triggering factors for initiation, establishment and spread of infections with consequent jeopardy to the fish immune system.
The total number of bacterial isolates recovered (prevalence of infection) from infected seabream and seabass were relatively higher than those previously reported by Zorrilla et al. [Citation38], which might be attributed to the difference in geographical localities (each geographical zone has each specific bacterial flora which completely differs from other zones even within the same sea pool), diverse size and age of investigated fish (seeds, juveniles and adult fish). Locally, the overall prevalence of bacterial infections in Gilthead seabream and European seabass exceeded those recorded by El-Gendy [Citation39]. Such increase could be accredited to the difference in site/time of sampling, immune status of fish and disease resistance especially for seeds and juveniles, which were the most critical stages in the production of marine fish according to Grisez et al. [Citation17].
Species wise, the total prevalence of V. alginolyticus was 82.19% and 87.28% for seabream and seabass, which were relatively higher than those reported by Zorrilla et al. [Citation38]. This can be explained by the difference in normal bacterial load of V. alginolyticus in the Egyptian coastal sea water, sediment from the localities reported by Zorrilla et al. [Citation38]. Moreover, the high levels of agricultural/municipal wastes in the Damietta and Alexandria sampling sites could be another enriching factor to the uprising prevalence of V. alginolyticus and most probably other zoonotic vibrios. Further, the difference in intestinal bacterial flora of the Egyptian marine fish from those of the cited study together with difference in sampling seasons could be another reliable explanation. Interestingly, the highest prevalence of V. alginolyticus had occurred in September followed by July and June then October and August perfectly coincide with similar data reported by Yiagnisis and Athanassopoulou [Citation40], Vandenberghe et al. [Citation41].
The largest bundle of strains (203/223) has expressed typical morpho-chemical characteristics such as colony color on TCBS, carbohydrate utilization and salt tolerance profiles that were completely consistent with the standard criteria of V. alginolyticus. A definitive confirmation of strains was obtained by PCR detection of collegenase gene of V. alginolyitcus where the entire bundle of retrieved isolates has amplified a 737-pb size fragment simulating that of Pinto et al [Citation25]. Insight analysis of the morpho-chemical characteristics and molecular patterns of the retrieved isolates coincided with the V. alginolyticus profiles reported by Buller [Citation21], Costinar et al. [Citation22], Zorrilla et al. [Citation38].
In respect to the total prevalence of V. parahemolyticus, it was 10.27% and 6.79% for seabream and seabass respectively, which were nearly similar tothose reported by Yiagnisis and Athanassopoulou [Citation40]. Different localities of and season of sampling would explain the slight difference between our extracted data and the previous study [Citation40] as the highest prevalence of this bacterium occurred in May, June, October, September, August and July. Locally, the localities of fish sampling could have played a greater role in elevating the prevalence of such pathogen in examined seabream than seabass. This difference could be accredited to difference in feeding habits/type of food used by seabream juveniles and adult collected from earthen pond farms which mainly depend on natural feed and live prey (tilapia juveniles and tilapia zilli) in contrast to seabass juveniles collected from the same localities. Definitively, the insight analysis of the morpho-chemical characteristics (API 20 NE and conventional biochemical tests) for the majority of retrieved isolates (20/23) coincided with the V. parahemolyticus profiles reported by Buller [Citation21], Yiagnisis and Athanassopoulou [Citation40].
The total prevalence of P. damselae ssp. damselae was 7.54% and 5.93% for seabream and seabass respectively, which were in slight accordance with those recorded by Balebona et al. [Citation35]. This can be correlated to difference in localities of isolation and season of sampling. It is imperative to know that P. damselae ssp. damselae predominant in summer. The biochemical profile of a large portion of the strains (13/18) was in accordance with Austin and Austin [Citation3], Labella et al. [Citation42].
Tissue wise, liver and kidney were found to be the main target organs for isolation of V. alginolyticus, V. parahemolyticus and P. damselae ssp. damselae in both Gilthead seabream and European seabass. Further, intensity of infection with the three pathogens were found to be very remarkable in the above mentioned three organs which run parallel to similar results reported by Zorilla et al. [Citation38], Botella et al. [Citation43]. On the pathophysiological level, this tissue preference could be related to some of the virulence determinants owned by the three pathogens which augment their septicemic nature with final predisposition into the toxin neutralizing vessel (liver) and main immune worrier (kidney).
Insight analysis of the achieved the antibiogram of V. alginolyticus and V. parahemolyticus has indicated that examined fishes were not or very scarcely exposed to previous treatments with the tested antibiotics. This was very obvious from the extending sensitivity of all isolates to ciprofloxacin, chloramphenicol, enrofloxacin, oxytetracycline, tetracycline, nalidixic acid and oxolinic acid. These results were concordant with Labella et al. [Citation12], Julian et al. [Citation44]. Similarly, P. damselae ssp. damselae showed wider range of sensitivities to ciprofloxacin, chloramphenicol, enrofloxacin, nitrofurantoin, oxytetracycline, trimethoprim-sulfamethoxazole, novobiocin, nalidixic acid and oxolinic acid, which was in relative agreement with Labella et al. [Citation12], Zorilla et al. [Citation38].
However, some sort of resistance to ampicillin, amoxycillin and lincomycin has been observed for all V. alginolyticus and V. parahemolyticus isolates, while P. damselae ssp. damselae were resistant to tetracycline and streptomycin. This could be attributed to the repetitive usage of these antibiotics in controlling previous outbreaks throughout the sampling localities or consistent presence of such antibiotics in coastal waters receiving agricultural/municipal wastes, which could contain poultry, large animals and human antibiotic wastes of the same types mentioned before.
In conclusion the mortality episodes among seabream and seabass fish were an end result of multi-factorial process in which, poor water quality, seasonal factor, host susceptibility, age susceptibility and pathogen virulence have interacted to produce such catastrophic mass mortalities among different stages of examined fishes. Also, insight analysis of the achieved epidemiological data is very alarming to possible future recurrent outbreaks and mass kills among other fish species inhabiting such vulnerable areas in Alexandria and Damietta. Further, antibiogram profiles are very elaborative of erratic usage of antibiotics in different livestocks neighboring these sampled coastal areas. This could encourage breeders to use alternative biological control or competent vaccination strategies to avoid antibiotic resistance uprising catastrophe. Finally, a future pathogenicity study should be implemented on the retrieved bacterial isolates and further molecular characterization of V. parahemolyticus and P. damselae ssp. damselae isolates should be adopted.
Acknowledgments
The authors would like to express their gratitude to Dr. Nermeen Moustafa, Lecturer at the Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Cairo University, for her valuable assistance during the molecular confirmation procedures of the V. alginolyticus isolates.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- General Authority for Fish Resources Development (GAFRD)Statistics of fish production of year 20092010GAFRD, Ministry of Agriculture and Land Reclamation 104 pp.
- L.GrisezF.Ollevieribrio (Listonella) anguillarum infections in marine fish larvicultureP.LavensE.JaspersI.RoelandsFish and crustacean larviculture symposium1995European Aquaculture SocietyGent Special Publication No. 24, 497 pp,
- B.AustinA.D.AustinBacterial fish pathogens: diseases of farmed and wild fish5th ed.2012Springer/Prazis PublishingChichester, UK
- A.E.ToranzoB.MagariñosJ.L.RomaldeA review of the main bacterial fish diseases in mariculture systemsAquaculture24620053761
- L.A.ActisM.E.TolmaskyJ.H.CrosaVibriosisP.T.KWooD.W.BrunoFish diseases and disorders. Viral, bacterial and fungal infectionsvol. 31999CABI publishingWallingford, Oxon, UK523557
- A.Ben Kahla-NakbiK.ChaiebA.BesbesT.ZmantarA.BakhroufVirulence and enteriobacterial repetitive intergenic consensus PCR of Vibrio alginolyticus strains isolated from Tunisian cultured gilthead sea bream and sea bass outbreaksVet Microbiol1172–42006321327
- M.SnoussiH.HajlaouiE.NoumiS.ZanettiA.BakhroufPhenotypic and molecular characterization of Vibrio alginolyticus strains recovered from juveniles and older Sparus aurata reared in a Tunisian marine farmAnn Microbiol582008141146
- A.BakhroufM.JeddiH.Ben OuadaEssai de traitement des vibrioses du loupDicentrarchuslabrax dans une zone de Pisciculture, à Monastir, TunisieMarine Life5219954754
- I.ZorrillaM.A.MoriñigoD.CastroM.C.BalebonaJ.J.BorregoIntraspecific characterization of Vibrio alginolyticus isolates recovered from cultured fish in SpainJ Appl Microbiol95200311061116
- Kaysner C, Depaola A.J. US: food and drugs administration: bacteriological analytical manual, methods for specific pathogens. Chapter 9 Vibrio spp. <http://www.fda.gov/Food/scienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM>; 2004.
- M.LoveD.Teebken-FisherJ.E.HoseJ.J.FarmerF.W.HickmanR.FanningVibrio damsela, a marine bacterium, causes skin ulcers on the damselfish (Chromis punctipinnis)Science214198111391140
- A.LabellaC.BerbelM.ManchadoD.CastroJ.J.BorregoPhotobacterium damselae subsp. damselae, an emerging pathogen affecting new cultured marine fish species in southern SpainRecent Adv Fish Farm92011135152
- E.AmlacherTextbook of fish diseases1970T.F.H. PublicationsNeatune city, NJp.117–135
- D.A.ConroyL.R.HermanTextbook of fish diseases1981T. F. H. PublicationWest Sylvania
- C.E.BoydWater quality in ponds for aquaculture1990Auburn UniversityAlabama 482 pp
- APHA, American Public Health AssociationStandard Methods for the Examination of Water and Wastewater20th ed.1998APHAWashington, DC, USA
- L.GrisezJ.ReyniersL.VerdonckJ.SwingsF.OllevierDominant intestinal micro-flora of seabream and seabass larvae, from two hatcheries, during larvae developmentAquaculture1551997387399
- J.G.HoltN.R.KriegP.H.A.SueathJ.T.SatleyS.T.WilliamsBergey’s manual of determinative bacteriology9th ed.1993Williams and WilkinsBaltimore
- M.AlsinaA.R.BlanchImprovement and update of a set of keys for biochemical identification of Vibrio speciesJ Appl Bacteriol771994719721
- M.OrtigosaE.GarayM.J.PujalteNumerical taxonomy of Vibrionaceae isolated from oysters and seawater along an annual cycleSyst Appl Microbiol171994216225
- N.B.BullerBacteria from fish and other aquatic animals: a practical identification manual2004CABI PublishingCambridge
- Costinar L, Herman V, Pascu C, Marcu AD, Marcu A, Faur B. Isolation and characterization of Vibrio alginolyticus and Pasteurella species from Siberian sturgeon (Acipenser baerii) in the south–west region of Romania, Preliminary Report, Scientific Papers. LUCRĂRI ŞTIINłIFICE MEDICINĂ VETERINARĂ 2010; vol. XLIII (1): 125–127.
- A.W.BauerW.M.KirbyJ.C.SherrisM.TurckAntibiotic susceptibility testing by a standardized single disc methodAm J Clin Pathol451966493496
- J.H.JorgensenJ.D.TurnidgeSusceptibility test methods: dilution and disk diffusion methodsP.R.MurrayE.J.BaronJ.H.JorgensenM.L.LandryM.A.PfallerManual of clinical microbiology2007ASM PressWashington, DC, USA11521172
- A.Di PintoG.CiccareseG.TantilloD.CatalanoV.T.ForteA collagenase-targeted multiplex PCR assay for identification of Vibrio alginolyticus, Vibrio cholera and Vibrio parahemolyticusJ. Food Protection682005150153
- S.HormansdorferH.WentgesK.Neugebaur-BuchlerJ.BauerIsolation of Vibrio alginolyticus from seawater aquariaInt J Hygiene Environ Health2032000169175
- P.L.HoW.M.TangK.S.LoK.Y.YuenNecrotizing fasciitis due to Vibrio alginolyticus following an injury inflicted by a stingrayScand J Infect Dis301998192193
- P.Matsiota-BernardC.NaucielVibrio alginolyticus wound infection after exposure to sea water in an air crashEur J Clin Microbiol Infect Dis121993474475
- A.MukherjiS.SchroederC.DeylingG.W.ProcopAn unusual source of Vibrio alginolyticus-associated otitis: prolonged colonization or fresh-water exposureArch Otolaryngol Head Neck Surgery1262000790791
- M.Nithya QuintoilK.PorteenA.K.PramanikStudies on occurrence of Vibrio parahemolyticus in fin fishes and shellfishes from different ecosystem of West BengalLivestock Res Rural Develop1912007
- C.R.OsorioM.L.LemosPhotobacteriumD.LiuMolecular detection of human bacterial pathogens2011CRC Press Inc.Boca Raton, FL959968
- F.G.SantiagoM.J.KrugM.E.NielsenY.SantosD.R.CallSimultaneous Detection of Marine fish pathogens by using multiplex PCR and a DNA MicroarrayJ Clin Microbiol42200414141419
- Khalil RH, Aly SH. Photobacteriosis in some wild and cultured fresh water fish in Egypt. In: 8th international symposium of tilapia in Aquaculture, Egypt, 2008. pp. 1211–1228.
- M.MoustafaA.M.LailaM.A.MahmoudW.S.SolimanA.E.EissaM.Y.EL-GendyBacterial infections affecting marine fishes in EgyptJ Am Sci6122010603612
- M.C.BalebonaI.ZorrillaM.A.MoriñigoJ.J.BorregoSurvey of bacterial pathologies affecting farmed gilthead seabream (Sparus aurata) in southern Spain from 1990 to 1996Aquaculture16619981935
- M.ChenG.LiT.ZhengDistribution of Vibrio alginolyticus like species in Shenzhen coastal waters, ChinaBrazil J Microbiol422011884896
- L.R.SuomalainenM.TiirolaE.T.ValtonenFlavobacterium columnare infection of rainbow trout (Oncorhynchus mykiss) (Walbaum)J Fish Dis282005271277
- M.A.ZorrillaA.S.ChabrillonP.D.RosalesE.M.ManzanaresM.C.BalebonaM.A.MorinigoBacteria recovered from diseased cultured gilthead seabream (Sparus aurata) in southwestern SpainAquaculture21820031120
- El-Gendy MY. Epizootiological and molecular studies on the common septicemic bacterial infections of some salt water fishes. PhD thesis, fish diseases and management, Faculty of Veterinary Medicine, Cairo University; 2013, 211pp.
- M.YiagnisisF.AthanassopoulouBacteria isolated from diseased wild and farmed marine fish in GreeceRecent Adv Fish Farms2011 Dr. Faruk Aral Editor
- J.VandenbergheF.L.ThompsonB.Gomez-GilJ.SwingsPhenotypic diversity amongst Vibrio isolates from marine aquaculture systemsAquaculture2192003920
- A.LabellaM.ManchadoM.C.AlonsoD.CastroJ.L.RomaldeJ.J.BorregoMolecular intraspecific characterization of P. damselae subsp. damselae strains affecting cultured marine fishJ Appl Microbiol1086201021222132
- S.BotellaM.J.PujalteM.C.MaciànM.A.FerrúsJ.HernándezAmplified fragment length polymorphism (AFLP) and biochemical typing of Photobacterium damselae subsp. damselaeJ Appl Microbiol932002681688
- R.JulianM.TamrinH.AhmedCharacterization and experimental infection of Vibrio harveyi isolated from diseased Asian seabass (Lates calcarifer)Malaysian J Microbiol822012104115