Abstract
A number of broiler flocks with respiratory disease and high mortality in five broiler farms in Libya were sampled for detection of infectious bronchitis virus (IBV). Twelve IBV strains from these farms were detected by reverse transcription polymerase chain reaction (RT-PCR) and differentiated by nucleotide sequencing of the hypervariable region of the S1 gene. A pair-wise comparison of the sequences showed two distinctive patterns. Those from farms 1, 2, 4 and 5, formed a separate cluster with 94–99% relatedness to the Egyptian IBV strains CK/Eg/BSU-2/2011, CK/Eg/BSU-3/2011 and Eg/1212B. Sequences from the farm 3 formed another cluster with 100% relatedness to Eg/CLEVB-2/IBV/012 and IS/1494/06. This appears to be the first report on the co-circulation these variant IBVs in Libya.
1 Introduction
Avian infectious bronchitis virus (IBV) causes a highly contagious disease in chicken. It mainly affects the respiratory tract, and frequently causes damage to the kidneys and reproductive systems [Citation1]. Although vaccination is commonly adopted, outbreaks continue to occur worldwide with significant economic consequences due to a substantial decrease in production performances [Citation1,Citation2]. Different genotypes of IBV have been identified worldwide, and new variants continue to emerge [Citation3]. A number of IBV variant genotypes have been reported in the Middle East, including Iran/793B/19/08, Iraq/Sul/01/09, Israel/720/99, Israel/885/00, IS/1494/06, Egypt/Beni-Seuf/01, Egypt/F/03, Egypt/D/89, CK/CH/LDL/97I, and CK/CH/SCYA/10I [Citation4–Citation9]. Some of these genotypes in particular IS/885/00 and IS/1494/06, have become dominant in the majority of farms in the Middle East countries, causing respiratory and renal diseases [Citation4,Citation10,Citation11]. To date, there is no information available on the circulation of variant IBVs in Libya. In the Middle East, the vaccination against IBV is performed with vaccines that contain live-attenuated or killed viruses belonging to the Massachusetts serotype [Citation10]. In the past few years, vaccine strains belonging to 793B and D274 serotypes are also widely used. In spite of this, IBV infection is considered endemic and widely spread both in vaccinated and unvaccinated poultry farms generally associated with kidney damages [Citation4]. The aim of this study is to provide information on the molecular characteristic and the phylogenetic relationship of strains in Libya in comparison to other strains reported in the Middle East.
2 Materials and methods
2.1 Case history and clinical samples
In July 2012, a number of broiler flocks in five different farms with respiratory disease and high mortality at East Libya were visited. The flocks had no vaccination against IBV but were vaccinated against Newcastle disease (ND) and infectious bursal disease (IBD) (). All flocks showed clinical signs of respiratory distress, manifested by sneezing, tracheal râles, gasping, nasal discharge, head swelling, conjunctival congestion and frothy eyes. Post-mortem examination revealed lesions of inflamed trachea, cheesy exudate in airsacs and swelling of the kidneys. Mortality on the day of sampling ranged from 1.4% to 3.7% ().
Table 1 Flock details, RT-PCR and genotype results.
From each of the farms, oropharyngeal swabs (OP) were collected from a total of 40 chicks. These swabs were divided into sets of 10 and were dipped into bijou tubes containing 2 ml of sterile water. After vigorous shaking, 100 μl of the mixture was spotted onto the Flinders Technology Associates (FTA) cards. Ten to twenty diseased birds per farm were killed and tissues of turbinates, trachea, lungs and kidneys were collected. The like-tissues were rubbed gently onto matrix areas of the FTA cards. These cards were air-dried and transported to the poultry virology laboratory at the University of Liverpool for analysis.
2.2 RNA extraction
The FTA cards were processed as described by the manufacturer with some modification. Briefly, the spotted or imprint area of the FTA card were cut using sterile scissors and forceps, each sample was placed into bijou tubes containing 2 ml of guanidinium thiocyanate and stored at −20 °C until required. RNA was extracted using guanidinium thiocyanate–phenol chloroform method as described [Citation12]. Three hundred microliters of the mixtures above were placed in 1.5 ml eppendorf tube containing 300 μl guanidinium thiocyanate and stored at −20 °C for few hours. After thawing, this mixture was transferred into eppendorf tube and 50 μl of 2 M sodium acetate and 650 μl of phenol–chloroform were added. The suspensions were vortexed and centrifuged at 13,000g for 5 min. The aqueous phase containing the RNA was mixed with 500 μl isopropanol and stored at −20 °C overnight for precipitation of the RNA. The supernatant was carefully removed, and the precipitated RNA was pelleted at 13,000g for 15 min and washed twice with 100% ethanol. The pellet was dried and resuspended in 30 μl of treated water and used for RT-PCR.
2.3 RT-PCR and DNA sequencing
Procedures for the IBV RT-PCR have been described by [Citation13]. Briefly, detection of the IBV genome and molecular characterization were achieved by identifying (380) base pairs of the S1 region of the S protein gene. The RT-PCR procedure including the primers used were as described [Citation14]. For sequencing, the nested-PCR products corresponding to the spike glycoprotein were purified with 0.15 μl Exonuclease 1 (EXO) and 0.99 μl shrimp alkaline phosphatase (SAP) at 37 °C for 30 min, followed by 80 °C for 10 min to remove any extraneous material. The purified product, together with positive sense primer (forward direction using primer SX3+), were submitted to external laboratory for analysis of the partial S1 gene sequences.
2.4 Phylogenetic analysis and nucleotide comparison
Multiple sequence alignments were carried out with Clustal W [Citation15], and phylogenic tree was constructed with MEGA 5 software [Citation16], using the Neighbor-joining tree method with 1000 bootstrap replicates to assign confidence levels to branches. The IBV sequences were aligned and compared with reference and vaccine strains that were found or used in the Middle East. The sequences were retrieved from GenBank (National Centre of Biotechnology Information) and BLAST search was carried out. The other S1 gene sequences used for comparison or phylogenetic analysis were CK/Eg/BSU-2/2011 (JX174185), CK/Eg/BSU-3/2011 (JX174186), Eg/1212B (JQ839287), Eg/CLEVB-2/IBV/012 (JX173488), IS/885/00 (AY279533) and IS/1494/06 (EU780077), M41 (GQ219712), H120 (GU393335), D274 (X15832), 4/91 (JN600614), CR88 (JN542567) and 793B (Z83979).
3 Results and discussion
The daily mortality on the day sampling is given in the . At necropsy, the main lesions found were tracheitis, lung congestion, air-sacculitis and enlarged kidneys. IBV was detected in samples obtained from all the farms. The nucleotide sequences of these IBVs were submitted to the GenBank and the assigned accession numbers are shown in the . and shows the relatedness between the Libyan IBV sequences in comparison to those found in the Middle East and the reference IBVs. A pair-wise comparison of the IBVs, showed two distinctive patterns. The Libyan IBVs, from farm 1, 2, 4, and 5, formed a separate cluster, with 94–99% homology to CK/Eg/BSU-2/2011, CK/Eg/BSU-3/2011 and Eg/1212B. The percent similarity to another regionally important IBV (IS/885/00), which was first detected in Israel ranged from 85–89% (). The Egyptian IBV strains CK/Eg/BSU-2(also 3)/2011 were associated with high mortality, respiratory and renal pathology [Citation17]. The IS/885/00 strain was isolated in 2000 from broiler chickens in Israel. This strain was reported to cause acute renal disease, severe morbidity and high mortality ranging from 15% to 25% [Citation4].
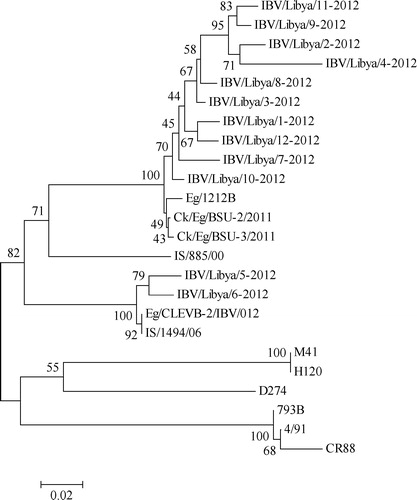
Table 2 Nucleotide and amino acid identity of the part-S1 glycoprotein gene of the Libyan in comparison to other IBV strains.
Those IBVs detected in farm 3 (IBV/Chicken/Libya/05/2012 and IBV/ Chicken/Libya/06/2012) formed another cluster, with 100% relatedness to Eg/CLEVB-2/IBV/012 and IS/1494/06 (). The IS/1494/06 was first identified in Israel in 2006, was recognised as a nephropathogenic IBV, and later classified as variant 2. It has been reported that birds vaccinated with H120 were poorly protected when challenged with these strains [Citation4,Citation9].
These findings show both, IS/885/00-like and IS/1494/06-like IBVs, are now circulating in Libya. Not much is known about the mode of IBV spread between the countries in the Middle East, however, cross-border movements of poultry and poultry-related products are likely an important factor. These finding shows that high mortality and severe respiratory diseases in Libyan chicken farms is likely contributed by these variant IBVs. The role of other co-infections and other exacerbating factors (e.g. immunosuppression, poor ventilation, stocking density, poor management) need to be examined. In North Africa, both classical and variant IBVs have been reported in Egypt, Tunisia and Morocco [Citation6,Citation17–Citation19]. In this study, even though only small numbers of farms were sampled, our findings have highlighted the circulation of variant IBVs in Libya for the first time. Further studies should include sero-surveillance, isolation and serotyping/genotyping of IBVs in the region.
Acknowledgement
The authors wish to thanks Anne Forrester and Emma Newsham for technical assistance and help with the molecular analysis respectively.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- D.CavanaghCoronavirus avian infectious bronchitis virusVet Res382007281297
- D.CavanaghCoronaviruses in poultry and other birdsAvian Pathol342005439448
- Y.A.BochkovG.V.BatchenkoL.O.ShcherbakovaA.V.BorisovV.V.DryginMolecular epizootiology of avian infectious bronchitis in RussiaAvian Pathol352006379393
- R.MeirE.RosenblutS.PerlN.KassG.AyaliS.PerkIdentification of a novel nephropathogenic infectious bronchitis virus in IsraelAvian Dis482004635641
- J.GelbJr.Y.WeismanB.S.LadmanR.MeirS1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996–2000)Avian Pathol342005194203
- A.S.Abdel-MoneimM.F.El-KadyB.S.LadmanJ.GelbJr.S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in EgyptVirol J3200678
- M.W.JackwoodReview of infectious bronchitis virus around the worldAvian Dis562012634641
- Ababneh M, Dalab A, Alsaad S, Al-Zghoul M. Presence of infectious bronchitis virus strain CK/CH/LDL/97I in the Middle East. ISRN Vet Sci 2012, 2012. Article ID 201721, 6 pages. http://dx.doi.org/10.5402/2012/201721.
- S.KahyaF.CovenS.TemelliA.EyigorK.CarliPresence of IS/1494/06 genotype-related infectious bronchitis virus in breeder and broiler flocks in TurkeyAnkara Üniv Vet Fak Derg6020132731
- S.S.El-MahdyS.EkramA.AhmedEfficacy of some living classical and variant infectious bronchitis vaccines against local variant isolated from EgyptNat Sci102012292299
- K.SelimA.S.ArafaH.A.HusseinA.A.El-SanousiMolecular characterization of infectious bronchitis viruses isolated from broiler and layer chicken farms in Egypt during 2012IJVSM12013102108
- P.ChomczynskiN.SacchiThe single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years onNat Protoc12006581585
- K.J.WorthingtonR.J.W.CurrieR.C.JonesA reverse transcriptase polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006Avian Pathol2008372002247257
- R.C.JonesK.J.WorthingtonI.CapuaC.J.NaylorEfficacy of live infectious bronchitis vaccines against a novel European genotype, Italy 02Vet Rec1562005646647
- J.D.ThompsonD.G.HigginsT.J.GibsonCLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choiceNucleic Acids Res22199446734680
- K.TamuraD.PetersonN.PetersonG.StecherM.NeiS.KumarMEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methodsMol Biol Evol28201127312739
- Abdel-Moneim, A, Afifi M, El-Kady M. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch Virol; 20121–20125.
- H.BourogaaK.MiledL.GribaaI.El BehiA.GhramCharacterization of new variants of avian infectious bronchitis virus in TunisiaAvian Dis532009426433
- M.El-HouadfiR.C.JonesJ.K.CookAGA.G.AmbaliThe isolation and characterisation of six avian infectious bronchitis viruses isolated in MoroccoAvian Pathol15198693105
